Abstract
Hyaluronic acid (HA) is a high value glycosaminoglycan mostly used in health and cosmetic applications. Commercial HA is produced from animal tissues or in toxigenic bacteria of the genus Streptococcus grown in complex media, which are expensive and raise environmental concerns due to the disposal of large amounts of broth with high organic loads. Other microorganisms were proposed as hosts for the heterologous production of HA, but the methods are still costly. The extraordinary capacity of this biopolymer to bind and retain water attracts interest for large-scale applications where biodegradable materials are needed, but its high cost and safety concerns are barriers for its adoption. Bacillus subtilis 3NA strain is prototrophic, amenable for genetic manipulation, GRAS, and can rapidly reach high cell densities in salt-based media. These phenotypic traits were exploited to create a platform for biomolecule production using HA as a proof of concept. First, the 3NA strain was engineered to produce HA; second, a chemically defined medium was formulated using commodity-priced inorganic salts combined at the stoichiometric ratios needed to build the necessary quantities of biomass and HA; and third, a scalable fermentation process, where HA can be produced at the maximum volumetric productivity (VP), was designed. A comparative economic analysis against other methods indicates that the new process may increase the operating profit of a manufacturing plant by more than 100%. The host, the culture medium, and the rationale employed to develop the fermentation process described here, introduce an IP-free platform that could be adaptable for production of other biomolecules.
Key points
• A biomolecule production platform based on B. subtilis 3NA strain and a synthetic medium was tested for hyaluronic acid biosynthesis
• A fermentation process with the maximum volumetric productivity was designed
• A techno-economic analysis forecasts a significant reduction in the manufacturing cost compared to the current methods
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
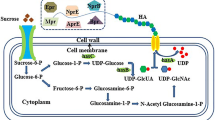
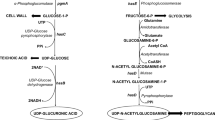
Hyaluronic acid (HA), also termed hyaluronan, is a linear glycan chain consisting of successive units of a disaccharide formed by β-1,3-D-glucuronic acid (GlcUA) and β-1,4-N-acetyl-D-glucosamine (GlcNAc) (Fig. 1a) with molecular weights of up to 8 MDa (Weissmann and Meyer 1954). This polysaccharide, which is ubiquitous in vertebrates and in human tissues, is found in the extracellular matrix playing structural and physiological functions. It is also present in few selected microorganisms, forming the capsule of some pathogenic bacteria. Due to the presence of abundant polar groups, which bind water to form a hydrated gel, HA is an excellent moisturizing factor. HA diverse properties, along with its lack of immunogenicity, made this polysaccharide attractive for cosmetic and pharmaceutical applications, including skin moisturizers, osteoarthritis treatment, wound healing, adhesion prevention after surgeries, and artificial tissue engineering (Dicker et al. 2014; Fallacara et al. 2018; Lou et al. 2018).
HA was traditionally obtained from rooster combs, and today is mostly prepared by fermentation of Streptococci attenuated strains, such as Lancefield group A and C (DeAngelis et al. 1993; Kumari and Weigel 1997). In these microorganisms, HA is produced by a hyaluronan synthase (HasA), as a result of the alternative addition of GlcNAc and GlcUA units from their respective nucleotide sugars, UDP-GlcNAc and UDP-GlcUA, while the growing chain is exported outside the cell (Fig. 1b).
The Streptococci-based HA raise environmental concerns since they grow in complex culture media. These media are contamination-prone, generate waste with high organic loads, multiple operations are needed to isolate high-quality HA, and may contain impurities not acceptable by regulatory agencies (Boeriu et al. 2013).
The HA global market for the cosmetic and pharmaceutical industries is estimated to be above 15 billion US$ in 2025 (Fallacara et al. 2018). Aside from these applications, HA and derivatives have extraordinary water binding capacities and are biodegradable, and are attractive candidates to be used in spill control, or in water absorbent pads, including diapers (Kurane and Nohata 1994). A large opportunity arises in the food industry, where the use of HA and derivatives is being explored as a component of functional foods and to build 3D frames along with collagen for cultivated animal-free meat, projected to be a trillion US$ market in 2040 (Ben-Arye and Levenberg 2019; de Souza et al. 2019; Oe et al. 2017; Zając et al. 2017).
To seize these opportunities, the cost effectiveness, safety, and sustainability of the current manufacturing processes need to be improved.
In the last two decades, several attempts have been made to produce HA in safe microorganisms (Chien and Lee 2007b; Hoffmann and Altenbuchner 2014; Widner et al. 2005). B. subtilis, classified as a generally recognized as safe (GRAS) bacterium, is one of the most explored (Chien and Lee 2007a; Jia et al. 2013; Jin et al. 2016; Li et al. 2020; Westbrook et al. 2018; Widner et al. 2005; Zhang et al. 2016). Widner et al. (2005) reported for the first time the heterologous production of HA in B. subtilis. They reached the upper limit of viscosity for stirred vessel fermentations, equivalent to ~ 7 g/L of HA, after 25 h using an engineered strain growing in a chemically defined salt-based medium (CDM). The medium composition was not disclosed, but a final cell density of 15 g DCW/L was informed. This is in agreement with reports indicating that most of the B. subtilis strains deficient in sporulation-specific sigma factors can only reach DCWs between 5 and 15 g/L in CDM (Chen et al. 2013; Huang et al. 2003; Huang et al. 2004; Martínez et al. 1998; Pierce et al. 1992; Reuß et al. 2015; Wu et al. 2013; Yao et al. 2010). However, the production of HA in this host still faces many cost-related challenges. Only a few reports describe HA production in GRAS heterotrophic strains using CDM, but the VPs are in all the cases much lower than those reported for Streptococci-based commercial processes (de Oliveira et al. 2016).
The heterotrophic B. subtilis 3NA is a non-sporulating strain described in 2015 by Reuß and co-workers as a hybrid between B. subtilis 168 and its sub-species the W23 strain (Reuß et al. 2015). The strain shows a high growth rate in CDM, where it can reach cell densities of up to 75 g DCW/L in fed-batch cultures. This distinctive phenotype makes the 3NA strain an excellent candidate to be used as a host for the industrial production of biomolecules.
In modern fermentation, the use of CDM, containing only inorganic low-cost compounds, is preferred (Stanbury et al. 2017). Based on the elemental composition and balance mass equations, the components can be precisely dosed at quantities sufficient to obtain the product. In this way, cost reductions of raw materials in up to 80% can be obtained with a concomitant fall in the process waste (Dahod et al. 2010). In CDM, contamination events are reduced, fewer downstream operations are required, and the productivity is significantly more consistent, avoiding lot to lot deviations caused by regional or seasonal quality changes in the complex ingredients (Dahod et al. 2010; Reisman 2019; Stanbury et al. 2017).
The purpose of this study was to test a platform for the low cost and sustainable production of biomolecules. As a proof of concept, the robust B. subtilis 3NA strain was engineered to produce HA, a CDM formulated according to the elemental composition of B. subtilis, and fermentation process with the maximum VP designed. A techno-economic analysis indicates that producing HA using this platform has remarkable benefits compared to the methods reported so far.
Materials and methods
Strains, plasmids, and growth conditions
The Escherichia coli Top10 (Invitrogen) strain was used for general cloning purposes. E. coli strains were cultivated in Luria-Bertani (LB) medium (tryptone, 10 g/L; yeast extract, 5 g/L; and NaCl 5 g/L) supplemented with 100 mg/L ampicillin or 50 mg/L kanamycin at 200 rpm and 37 °C.
B. subtilis 3NA strain is available from the Bacillus Genetic Stock Center as BGSCID 1S1. The cells were made competent by the method of Anagnostopoulos and Spizizen (Anagnostopoulos and Spizizen 1961). The B. subtilis 3NA strains carrying different constructions (Table S1) were used for HA production. For general purposes, these strains were grown in LB medium or Spizizen minimal medium at 200 rpm and 37 °C.
DNA preparation, cloning, and transformation
In order to obtain synthetic operons containing different combinations of genes of the HA biosynthetic pathway enzymes, all relevant genes, i.e., hasA, tuaD, gtaB, and gcaB, were PCR amplified using the primers listed in the Electronic Supplementary Material ESM_1, Table S2, and genomic DNA from Streptococcus zooepidemicus ATCC6580, when amplifying hasA, or B. subtilis 3NA when amplifying tuaD, gtaB, and gcaB. Primers were designed in order to add a XbaI and/or NdeI restriction sites on the 5′ end and SpeI in the 3′ end of all genes in order to facilitate sequential cloning into the pTRG5 vector.
In order to provide strong ribosomal binding sites (RBS), the optimal consensus sequence of B. subtilis RBS 5′-AAAGGAGG-3′ (Rocha et al. 1999) was included as an extension of the upper primer for tuaD gene amplification and then added in one cloning step to each pathway gene swapping tuaD by the corresponding ORF using NdeI-SpeI restriction sites. The construction of the different operons is depicted in Figure S1. For the proof of principle, the IPTG-inducible Phyper-spank promoter was used to control the expression of the different operons. The constructed operons, in the pTGR5-derived plasmids, were digested with XbaI and SpeI and cloned into the NheI site of the pDR111 vector. The correct orientation was checked via PCR using a primers annealing in the inserted fragments and in the lacI sequence present in the plasmid (Table S1). The resulting plasmid was integrated into the amyE locus of competent 3NA cells. Selection for spectinomycin resistance (50 mg/L) was used to isolate the recombinant clones and in all the cases the insertion was confirmed by PCR.
High cell density fermentation process
Fed-batch fermentations were carried out in 3 L (New Brunswick Bio Flo 115, USA) fermenters. The temperature, stirring, and pH were maintained at 37 °C, 1200 rpm, and pH 7.0 (by addition of 30% NH4OH), respectively. The level of dissolved oxygen was controlled at 30% of air saturation by changing the gas inflow (0.1 to 1 vvm) and adding pure oxygen when necessary. The seed culture was grown in batch mode with 40 g/L of glycerol to a final cell mass of ~ 17 g DCW/L. A total of 10% V/V of the seed culture was diluted in a bioreactor containing the same medium supplemented with 1 g/L of glycerol and 0.1 mM IPTG. The feeding process was initiated when the glycerol present in the medium was exhausted.
Two different feeding strategies were used using a solution containing 800 g/L glycerol and 20 g/L MgSO4·7H2O. In the first one, the feeding rate (F, g/Lh) was determined by Eq. 1 (Lee 1996) in order to maintain the indicated specific growth rate (μ):
where X0 is the initial biomass concentration (g/L), V0 is the initial volume (L), μ is the desired specific growth rate (h−1), S0 is the glycerol concentration in the feeding solution (g/L), and YX/S is the substrate yield (g/g). The second one consisted in an increase of glycerol from 2.5 to 9 g/L of the feeding solution in 2 h, maintaining a constant feeding of 9 g/L·h until the end of the fed-batch fermentation process.
In both cases, production of HA was induced at the beginning of the fed-batch by adding IPTG at a final concentration of 0.1 mM. The supernatant was used to determine HA at the indicated points.
After the fermentation process, the cell culture was diluted in half in NaCl solution (final concentration of 2 M), and centrifuged at 5000g for 10 min or the cells separated by microfiltration with a hollow fiber unit followed by ultrafiltration in a 750 KDa UF unit. For final formulation, HA was separated by ultrafiltration using a 0.1 μm MWCO hollow fiber cartridge followed by diafiltration to formulate the HA in pure water.
Hyaluronic acid concentration and MW determination
HA samples were extracted from the fermentation broth at the times indicated. The cultures were centrifuged at 5000g for 5 min. Amberlite IR120 (Sigma) was added to the supernatants until pH < 4. The mixture was then centrifuged for 10 min at 8000g and NaOH was used to reach pH 7.0 of the resulting supernatants.
HA was precipitated with two volumes of ethanol and incubated at 4 °C for 1 h. The precipitate was collected by centrifugation at 5000g for 10 min, and then redissolved in an equal volume of distilled water to measure the HA concentration via a modified Blumenkrantz method (Blumenkrantz and Asboe-Hansen 1973). Briefly, a 200 μL sample was mixed with 1.2 mL of 2.5 g sodium borate in 1 L H2SO4 solution on ice. The mixture was boiled for 5 min and 12 μL methahydroxydiphenil 0.15% in 0.5% NaOH was added while vortexing the samples. Uronic content was determined by measuring absorbance at 520 nm. Commercial glucuronic acid 1 mg/mL was used as standard.
For molecular mass determination, further purification of the samples was performed by re-precipitating the samples with two volumes of ethanol. The samples were next resuspended, and dialyzed against water using a 100 KDa cutoff membrane (Sigma).
The weight average molar mass and MW distributions of HA were determined by high-performance size-exclusion chromatography (HPSEC) with online multi-angle light scattering (MALS), fitted with a K5 cell and a laser wavelength of 658 nm, and a refractive index detector. One hundred microliters of a 2 g/L sample was injected in the column (SB 806 M HQ columns (Shodex)) and eluted with 0.1 M NaNO3 containing 0.03% NaN3 at 0.5 mL/min. Solvent and samples were filtered through 0.1 μm and 0.2 μm filter units (Millipore), respectively.
Techno-economic analysis
The simulation was made using the following assumptions:
The plant is located in the region of Rosario, Argentina. The total cost to start the business is 130 million dollars (US$) and includes the land, the facility with the building, all capital expenditures, working capital, and all other expenses required to start the business (Electronic Supplementary Material, ESM_2.xlsx). The plant operates continuously for 330 days per year. Other infrastructures, like pilot plants, research laboratories, and sales offices space, are not considered here. Financing was not included, and annual depreciation costs were equally distributed in 10 years. Annual maintenance rate of equipment was set as 2% of the direct fixed capital cost (DFC).
Four production fermenters with a working capacity of 100,000 L each are used for HA production. A seed train is employed where the last seed fermenter transfers 10,000 L of an inoculum grown in batch mode to a final cell density of 15 g DCW/L.
At the end of the fermentations, the cultures are transferred to an auxiliary tank, 5% of the broth is kept in the fermentor, and fresh medium from reservoir tanks fed with a continuous sterilization system is added to start a new fermentation cycle. After 3 days of semi continuous work, the fermentor is cleaned and steam sterilized. This turnaround takes 24 h; to calculate the duration of each fermentation cycle, the turnaround time is divided by the number of runs and proportionally assigned to the row “average fermentation cycle.” The turnaround time for the fermentor with complex media is assumed 10% longer because more time is required for cleaning than when using a CDM.
It is assumed that 96% of fermentations are successful for CDM and 93% success is obtained for fermentations with complex media due to higher frequency of contaminations or other problems associated to complex media. HA titers are 6.8 kg/m3 and the recovery yield of the downstream processing is 92%. The final product is HA with an average MW ~ 1 MDa.
Prices of the chemicals and consumables were obtained from local suppliers for quantities of at least 1 ton and salaries from a local survey. Technical grade glycerol was used, since it is locally available at large quantities from biodiesel producers and is 30% cheaper than glycerol pharma grade.
Results
B. subtilis 3NA was engineered for HA production
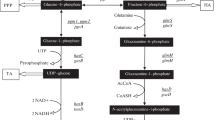
Bacteria naturally synthesize UDP-GlcUA and UDP-GlcNAc, a basic component of the peptidoglycan, also called murein, as intermediates for the assembly of cell wall structures (de Oliveira et al. 2016). Heterologous expression of hasA in the B. subtilis 3NA strain is necessary and sufficient to confer to this host the capacity to produce and secrete HA. In addition, it has been shown that the over-expression of some of the native enzymes involved in the production of murein, and HA precursors, along with hasA, increases the productivity (Fig. 1b) (Jin et al. 2016; Widner et al. 2005).
To the best of our knowledge, HA has not been produced before in B. subtilis 3NA. Thus, derivative strains carrying hasA from S. zooepidemicus (hasASz) and increase copy number of the native tuaD, gcaD, and gtaB genes naturally present in the host were engineered (Fig. 1b and Figure S1). Three cell lines containing different IPTG-inducible expression cassettes inserted into the amyE locus were constructed. The first one (KCNHA8) expresses only hasAsz; the second contains a bicistronic operon formed by hasAsz and an additional copy of the native tuaD gene (KCNHA9); and a third one (KCNHA10) with a four-gene operon (hasAsz-tuaD-gcaD-gtaB genes) (Electronic Supplementary Material ESM_1; Figure S1, Table S1).
The HA production of the three strains was tested in shake flask cultures using Spizizen minimal medium supplemented with 10 g/L of glycerol. Under the tested conditions, the co-expression of hasAsz with the three auxiliary genes in the KCNHA10 strain gave the highest HA titer, producing more than twice the quantity obtained with KCNHA9 and four times the amount of the KCNHA8 cultures (Fig. 2). No further productivity improvements were observed when the remaining genes of the pathways for the synthesis of UDP-GlcUA and UDP-GlcNAc (pgi, pgm, glmM, and glmS) were over-expressed (data not shown).
Titers of HA in batch cultures of B. subtilis 3NA-derived strains grown in salt-based medium. KCNHA8 expresses hasASz KCNHA9, expresses hasASz, and expresses an extra copy of tuaD; KCNHA10 expresses hasASz and extra copies of tuaD-gcaD-gtaB. The results are expressed as means and standard deviations of at least three independent experiments
Culture medium formulation
The efforts for lowering the manufacturing cost presented in this section were addressed to design a culture medium fulfilling the following constraints: (i) the medium should have the least possible amount of the cheapest raw materials, (ii) it should be based on inorganic salts to facilitate the purification of high-quality HA and avoid effluents with a high load of organic matter, and (iii) the producing strain should grow at a high μ (specific growth rate, g/g·h) and the cells should produce HA at high qP (specific productivity, mg/g of cells·h), to shorten the process time, in order to increase the VP (g of HA/L h).
To build a given mass of cells, the quantities for each medium component were calculated based on the elemental composition of B. subtilis reported for carbon-limited chemostat cultures (Dahod et al. 2010; Dauner et al. 2001).
The empirical formula for B. subtilis cell growing at 0.4 h−1 is C1N0.221P0.020S0.006 (Dauner et al. 2001), where carbon represents 48% of the DCW, N is 12.3%, P is 2.6%, and S is 0.6%. Because of its local availability at low prices, technical grade glycerol was used as a carbon and energy source. The yield coefficient (Y) for this carbon source, obtained from fed-batch fermentations in our laboratory, is 0.41 g/g, which means that 2.44 g of glycerol (1 g/0.41) are required to provide all the carbon contained to obtain 1 g of dry B. subtilis cellular mass (DCW). Such amount of glycerol contains 0.94 g of C, from which 0.48 g (48 % × 1 g) is converted, under aerobic conditions, in organic compounds that are part of the biomass, and the remaining C generates CO2 and energy (Dahod et al. 2010).
Because of their low cost, industrial grade H3PO4 and NH4OH were chosen as P and N sources, respectively. According to the mass ratio in the elemental formula, 0.026 g of P and 0.123 g of N are needed for 1 g DCW of biomass; thus, 0.082 g of H3PO4 and 0.29 g of NH4OH were listed in the medium recipe.
Since NH4OH is toxic at high concentrations, 0.04 g/L was added in the form 0.1 mL of 28% water solution. This quantity was calculated to accommodate the exact amount of potassium required to build 1 g DCW of biomass. K was provided by adding 0.023 g of KOH, the amount of base necessary to equilibrate the pH to 7.0. The remaining 0.99 mL of NH4OH was gradually fed during the course of the fermentation to maintain pH 7, since this compound is both the nitrogen source and the base used for pH control. In the same way, the quantities of sulfur and magnesium indicated in the elemental composition of B. subtilis biomass were provided as MgSO4 7H2O. For the minor elements, low-cost salts with high availability were used, also in amounts sufficient to provide the quantities reported in the biomass elemental composition analysis (Dahod et al. 2010). Ethylenediaminetetraacetic acid (EDTA), a non-metabolizable chelating agent, was used instead of citrate; since this compound is used as a carbon source by the 3NA strain, it causes precipitation of divalent cations, which resulted in early growth interruption. The formula of this medium, CDM-1, designed to produce 1 g of DCW is shown in Table 1.
On top of the components added to build the wished cell mass, extra amounts of C and N must be added for the synthesis of HA. For this, a yield coefficient of 0.31 g/g, determined by Don and Shoparwe (2010), was used for glycerol, since the synthesis of HA requires significant amounts of energy. Thus, to obtain 7 g of HA, 22.1 g of glycerol (7/0.31) must be added to the medium. Based on the HA empirical formula, 0.24 g of extra N is needed to produce 7 g of HA, equivalent to 0.54 g of NH4OH.
Fermentation process design
Having a CDM formulation defined, the goal of the fermentation process development described in this section was to achieve the highest possible VP. In this particular case, the titer for high MW HA (> 1 MDa) is limited by the viscosity of the broth to ~ 7 g/L, which is unfortunately quite low for a biomolecule intended to be used as a biomaterial. Thus, the only way to increase the VP was to reduce as much as possible the process time required to reach such a limiting titer.
The two time-dependent variables that govern the VP in this case are (i) μ, which indicates how fast the cell factories can be created, and (ii) qP, which indicates how efficient those factories to produce HA.
Thus, a set of experiments was designed to determine how μ and qP influence each other, to determine if the production of HA is growth associated or not. For this, the qP value of cultures grown at different specific rates was determined. In all the cases, culture conditions feasible to be reproduced at large scale were used.
The fermentation time was set in less than 12 h, since that is the time employed to achieve the maximum VP reported for high molecular weight HA for Streptococcus equi (Kim et al. 1996). A fed-batch culture grown at a constant μ = 0.25 (~ 50 % μ max) and with a starting cell density of 5 OD600, equivalent to 1.7 g DCW/L, is expected to be near 30 g DCW/L after 11 h of exponential feeding. Under the mentioned constraints, and assuming an average qP of 70 mg/g DCW h could be maintained throughout the fermentation, a process time of 11 h should provide more than 7 g/L of HA.
A seed culture of the KCNHA10 was grown in batch mode with 40 g/L of glycerol. When glycerol was consumed, the final cell mass was ~ 17 g DCW/L. The seed culture was added to reach a final 10% V/V in CDM-30 (designed to produce 30 g of DCW) with 1 g/L of glycerol and 0.1 mM IPTG to twin bioreactors. After 15 min, glycerol was exponentially fed to keep μ at 0.25 h−1 in the first reactor and at 0.35 h−1 in the second one.
Figure 3 a and b show that in both experiments, the final cell density was near 30 g DCW/L, indicating that the medium formulation was sufficient to build the expected mass of cells, but none of the experiments reached the expected HA titer. The culture grown at 0.25 h−1 produced ~ 3 g/L of HA and qP was 23.03 ± 1.04 mg of HA/gDCW h (Fig. 3a). The culture grown at 0.35 h−1 was stopped when keeping the dissolved oxygen above 20% was no longer possible and excessive foam was formed, indicating a progressive cell lysis. At that point, 3 g/L of HA was accumulated and qP was 52.67 mg ± 2.54 mg of HA/gDCW·h (Fig. 3b). We observed in batch experiments qP values above 80 mg of HA/gDCW·h when the cells were grown in excess of glycerol at μ max. Thus, a positive correlation between qP and μ exists, at least in the range between 0.25 h−1 and μ max.
Based on the result of the analysis, the preferred fermentation strategy was to maximize μ, since this variable in a CDM with limited carbon can be precisely controlled by increasing the feeding rate of the carbon source. A feeding of 9 mL/L·h was chosen, since this is the maximum value that can be later reproduced in large-scale fermentation, and 1 h later, cells were growing at 0.53 h−1, the value for μmax observed in batch cultures with excess of glycerol. As expected, qP reached ~ 80 mg/g·h and the two variables converged to give the highest possible value VP for the system. The cells grew at 0.53 h−1 until the point where the glycerol uptake by the biomass present in 1 L of the culture was large enough to consume all the glycerol and the feeding rate matched the uptake by the cell mass. From that point and to the end of the fermentation, μ and qP started to decrease and the biomass showed the expected linear growth.
Figure 3c shows that HA average titers near 7 g/L were consistently obtained after feeding glycerol for 10.5–11 h and that the final average cell density of 29.4 g DCW/L was achieved.
The HA obtained in CDM-30 medium was easily purified by (i) separating the cells and large cell debris with microfiltration, and (ii) separating trace of protein and DNA fragments from lysed cells and other low MW compounds using ultra and diafiltration steps. HPSEC-MALS analysis of purified HA obtained from fed-batch fermentation process performed at constant feeding rate of 9 g/L·h of glycerol showed a main peak of 9.53 105 Da (± 3.53%) with a dispersion of 1.19 (± 4.54%) (Figure S2).
Cost analysis
The techno-economic analysis presented below illustrates the potential impact of the process described in this work on the HA market. As a reference, the expected costs of two other methods extracted from the literature are included; when data is not provided, the values were assumed to be the same as the process described here:
Table 2 models the operating profit for the large-scale HA manufacturing in a plant with a working capacity of 400 m3, and compares the process described here with two others. The first one, using S. equi grown in a complex medium, was chosen as an example of a typical Streptococci-based process. In this case, the final HA titer and fermentation time are similar to those of the method described here, which is reflected in the VPs. However, the cost of the culture media is more than five times higher than the CDM designed here, mostly due to the cost of tryptone required by the auxotrophic strain, and the HA purification.
For the second comparison, part of the data was obtained from the work of Widner et al. (2005), reporting for the production of HA in a prototrophic B. subtilis strain grown in a salt-based medium. The culture was incubated for 25 h, but the medium composition is not described and they report a HA final titer “in the multigram range.” Even assuming that the process is terminated when titers reached ~ 7 g/L, the technical limit for high MW HA production, and that the cost of the medium used is as low as the one designed for this work, the expected VP would be 56% lower than the one projected for the method described here (Table 2). The difference is caused by the extended process time, which directly reduces the VP and therefore increases the cost (Ferreira et al. 2018). The sensitivity analysis of Fig. 4 is based on the techno-economic analysis of Table 2. At the current HA bulk market price of 200 US$/kg, the process presented here is 110% more profitable than the Streptococci-based processes used today. If the price drops to 150 US$, the operating profit would be 230% higher, while at prices below 112 US$, the S. equi process would not even reach the breakeven point. In the former scenario, producing HA with the 3NA strain would triplicate the operating profit obtained with the B. subtilis A164Δ5 strain in 25 h (Widner et al. 2005).
Discussion
As a part of a program to produce biomolecules at low cost, the goal of this work was to design from the ground a bioprocess to manufacture high molecular weight HA in a GRAS microorganism, analyzing every step where savings can contribute to achieve the lowest possible cost. For high MW HA, titers above 7 g/L cannot be obtained due to the constraint imposed by the viscosity of the broth and the consequent rapid drop of the oxygen transfer capacity (Boeriu et al. 2013; Fallacara et al. 2018). Thus, efforts were focused on
-
i.
Engineering a prototrophic, fast growing, and GRAS HA-producing strain.
-
ii.
Designing a CDM with the least possible cost.
-
iii.
Finding process conditions where the producing strain cultured in the designed medium reaches the maximum VP.
The B. subtilis 3NA strain was reported to grow to high cell densities in CDM (Reuß et al. 2015). In our laboratory, a notable capacity to grow fast in CDM was observed. These features make the 3NA an ideal host to be explored for the development of high VP fermentations using inorganic salts as raw materials, the type of bioprocess needed for manufacturing bio-commodities like HA.
Strains harboring the heterologous hasASz gene produced more HA when additional copies of three native genes involved in the production of HA precursors were over-expressed. Similar results were shown in other Bacillus stains (Jin et al. 2016; Widner et al. 2005), suggesting that the carbon flow through biosynthetic pathways of GlcNAC and GlcUA is at least similar. This is not surprising considering that the gene clusters are highly conserved.
For microorganisms with a long history of industrial use, the culture media were empirically shaped throughout decades, but contain a large excess of the elements required to build the required mass of cells and products. CDMs facilitate downstream processing with the consequent cost reduction, minimize contamination events, and improve the reproducibility of the fermentations (Dahod et al. 2010; Stanbury et al. 2017).
Here, for a rational design of a CDM, the amount of carbon source to be added was first calculated based on the yield coefficient. Oxygen is provided by the air flow and limited by the oxygen transfer rate (OTR) of the bioreactor. For all the other elements, the quantity of each inorganic salt to be added was calculated according to the elemental formula of a B. subtilis to build 1 g DCW of cells (Table 1). To reach the least possible cost, the cheapest inorganic salts and carbon source were chosen. The design of the CDM medium is flexible and allows the users to customize the formulation by keeping the relative ratio of the components. According to the final cell density of B. subtilis 3NA required for a given bioprocess, the amounts of each component can be determined using the Electronic Supplementary Material, ESM_2, adding in each case the amounts of C and other elements needed to support the production of the target bioproducts.
Rigorous comparisons of bioprocess performances supported by costs analysis are scarce in the literature. Often, a fermentation process is considered successful based on an isolated piece of information, like the product titer or the use of some waste as a low cost material, ignoring industrial needs, like operating parameters and even the process time. The VP, where the process time is taken into account, along with the cost of all the raw materials employed, are the metrics that define the success of a fermentation process.
For the rational design of a fermentation process, the interactions between μ and qP, the variables that define the VP for a given volume, were studied. The two variables may have a positive correlation, like in the case of proteins expressed using the GAP promoter in P. pastoris, or β-galactosidase from E. coli in B. subtilis, where the expression of the target proteins is growth associated. In other cases, the product can be non-growth associated like certain secondary metabolites in the genus Streptomyces, mixed growth associated, or the correlation can be negative as well (Looser et al. 2017; Martínez et al. 1998; Sakthiselvan et al. 2019).
In B. subtilis 3NA, a positive correlation between μ and qP was found, in the range of μ between 0.25 and 0.53; similar results were found in S. zooepidemicus grown in complex media in batch experiments (Don and Shoparwe 2010). The culture conditions that maximized the values of μ and qP were determined, limited by the constraints that guarantee a smooth transfer to large-scale bioreactors. To the best of our knowledge, the VP achieved is the highest reported for HA with MW ~ 1 MDa using a GRAS host.
In summary, the CDM medium, the capacity of the B. subtilis 3NA strain to reach high cell densities and the designed fermentation process, provide the basis to obtain a remarkable cost reduction of high MW HA manufacturing at large scale.
-
First, the prototrophic phenotype of the B. subtilis 3NA allows for the use of CDM with a cost estimated to be 80% lower than the media used for HA production in Streptococci.
-
Second, reaching high cell density in CDM provides the 3NA strain an advantage over any other B. subtilis strain described so far, since more producing cells shorten the process time to reach the limiting titer of HA and therefore increasing the VP. Widener and co-workers (Widner et al. 2005) produced in a derived B. subtilis A164Δ5 strain 7 g/L of HA in 25 h, while the data presented here showed that the 3NA strain produced the same amount in 11 h, resulting in a VP more than 100% higher (Table 2 and Electronic Supplementary Material ESM_2)
HA is a biodegradable polymer with remarkable properties to be used in many industrial applications, but the current price of this biomaterial is a barrier for its widespread adoption (Fallacara et al. 2018). As the patents protecting production methods expire, the price of bioproducts naturally tends to decrease due to the arrival of new competitors. The advantages expected from the platform described in this work are exposed in Table 2 and in the sensitivity model of Fig. 4. The platform presented here dramatically reduce the current manufacturing prices in 80% making available industrial quantities of HA as a food ingredient among other uses.
The B. subtilis 3NA strain, the CDM medium, and the fermentation process compose a freely available platform that could be used in principle for the safe and environmentally friendly production of other biomaterials in a cost-effective manner. The guidelines of Electronic Supplementary Material (ESM_2.xls) can be used as a starting point and the materials are freely available.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Change history
23 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00253-021-11506-5
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81(5):741–746. https://doi.org/10.1128/JB.81.5.741-746.1961
Ben-Arye T, Levenberg S (2019) Tissue engineering for clean meat production. Front Sustain Food Syst 3(46). https://doi.org/10.3389/fsufs.2019.00046
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Boeriu CG, Springer J, Kooy FK, van den Broek LAM, Eggink G (2013) Production methods for hyaluronan. Int J Carbohydr Chem 2013:624967–624914. https://doi.org/10.1155/2013/624967
Chen X, Zhang C, Cheng J, Shi X, Li L, Zhang Z, Bai J, Chen Y, Li S, Ying H (2013) Enhancement of adenosine production by Bacillus subtilis CGMCC 4484 through metabolic flux analysis and simplified feeding strategies. Bioproc Biosyst Eng 36(12):1851–1859. https://doi.org/10.1007/s00449-013-0959-6
Chien LJ, Lee CK (2007a) Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin. Biotechnol Prog 23(5):1017–1022. https://doi.org/10.1021/bp070036w
Chien LJ, Lee CK (2007b) Hyaluronic acid production by recombinant Lactococcus lactis. Appl Microbiol Biotechnol 77(2):339–346. https://doi.org/10.1007/s00253-007-1153-z
Dahod SK, Greasham R, Kennedy M (2010) Raw materials selection and medium development for industrial fermentation processes. In: Bull AT, Junker B, Katz L, Lynd LR, Masurekar P, Reeves CD, Zhao H (eds) Manual of Industrial Microbiology and Biotechnology 3rd edn. Am Soc Microbiol
Dauner M, Storni T, Sauer U (2001) Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J Bacteriol 183(24):7308–7317. https://doi.org/10.1128/jb.183.24.7308-7317.2001
de Oliveira JD, Carvalho LS, Gomes AM, Queiroz LR, Magalhães BS, Parachin NS (2016) Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb Cell Factories 15(1):119. https://doi.org/10.1186/s12934-016-0517-4
de Souza AB, Chaud MV, Santana MHA (2019) Hyaluronic acid behavior in oral administration and perspectives for nanotechnology-based formulations: a review. Carbohydr Polym 222:115001. https://doi.org/10.1016/j.carbpol.2019.115001
DeAngelis PL, Papaconstantinou J, Weigel PH (1993) Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem 268(26):19181–19184
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10(4):1558–1570. https://doi.org/10.1016/j.actbio.2013.12.019
Don MM, Shoparwe NF (2010) Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem Eng J 49(1):95–103. https://doi.org/10.1016/j.bej.2009.12.001
Fallacara A, Baldini E, Manfredini S, Vertuani S (2018) Hyaluronic acid in the third millennium. Polymers 10(7). https://doi.org/10.3390/polym10070701
Ferreira RG, Azzoni AR, Freitas S (2018) Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: the case of recombinant β-glucosidase. Biotechnol Biofuels 11(1):81. https://doi.org/10.1186/s13068-018-1077-0
Hoffmann J, Altenbuchner J (2014) Hyaluronic acid production with Corynebacterium glutamicum: effect of media composition on yield and molecular weight. J Appl Microbiol 117(3):663–678. https://doi.org/10.1111/jam.12553
Huang H, Ridgway D, Gu T, Moo-Young M (2003) A segregated model for heterologous amylase production by Bacillus subtilis. Enzym Microb Technol 32(3):407–413. https://doi.org/10.1016/S0141-0229(02)00312-5
Huang H, Ridgway D, Gu T, Moo-Young M (2004) Enhanced amylase production by Bacillus subtilis using a dual exponential feeding strategy. Bioproc Biosyst Eng 27(1):63–69. https://doi.org/10.1007/s00449-004-0391-z
Jia Y, Zhu J, Chen X, Tang D, Su D, Yao W, Gao X (2013) Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. Bioresour Technol 132:427–431. https://doi.org/10.1016/j.biortech.2012.12.150
Jin P, Kang Z, Yuan P, Du G, Chen J (2016) Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168. Metab Eng 35:21–30. https://doi.org/10.1016/j.ymben.2016.01.008
Kim J-H, Yoo S-J, Oh D-K, Kweon Y-G, Park D-W, Lee C-H, Gil G-H (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzym Microb Technol 19(6):440–445. https://doi.org/10.1016/S0141-0229(96)00019-1
Kumari K, Weigel PH (1997) Molecular cloning, expression, and characterization of the authentic hyaluronan synthase from group C Streptococcus equisimilis. J Biol Chem 272(51):32539–32546. https://doi.org/10.1074/jbc.272.51.32539
Kurane R, Nohata Y (1994) A new water-absorbing polysaccharide from Alcaligenes latus. Biosci Biotechnol Biochem 58(2):235–238. https://doi.org/10.1271/bbb.58.235
Lee SY (1996) High cell-density culture of Escherichia coli. Trends Biotechnol 14(3):98–105. https://doi.org/10.1016/0167-7799(96)80930-9
Li Y, Shi Z, Shao Y, Wu M, Li G, Ma T (2020) Temperature-controlled molecular weight of hyaluronic acid produced by engineered Bacillus subtilis. Biotechnol Lett 43:271–277. https://doi.org/10.1007/s10529-020-03001-0
Looser V, Lüthy D, Straumann M, Hecht K, Melzoch K, Kovar K (2017) Effects of glycerol supply and specific growth rate on methanol-free production of CALB by P. pastoris: functional characterisation of a novel promoter. Appl Microbiol Biotechnol 101(8):3163–3176. https://doi.org/10.1007/s00253-017-8123-x
Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O (2018) Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154:213–222. https://doi.org/10.1016/j.biomaterials.2017.11.004
Martínez A, Ramírez OT, Valle F (1998) Effect of growth rate on the production of β-galactosidase from Escherichia coli in Bacillus subtilis using glucose-limited exponentially fedbatch cultures. Enzym Microb Technol 22(6):520–526. https://doi.org/10.1016/S0141-0229(97)00248-2
Oe M, Sakai S, Yoshida H, Okado N, Kaneda H, Masuda Y, Urushibata O (2017) Oral hyaluronan relieves wrinkles: a double-blinded, placebo-controlled study over a 12-week period. Clin Cosmet Investig Dermatol 10:267–273. https://doi.org/10.2147/ccid.S141845
Pierce JA, Robertson CR, Leighton TJ (1992) Physiological and genetic strategies for enhanced subtilisin production by Bacillus subtilis. Biotechnol Prog 8(3):211–218. https://doi.org/10.1021/bp00015a006
Reisman HB (2019) Economic analysis of fermentation processes. CRC Press
Reuß DR, Schuldes J, Daniel R, Altenbuchner J (2015) Complete genome sequence of Bacillus subtilis subsp. subtilis strain 3NA. Genome Announc 3(2):e00084–e00015. https://doi.org/10.1128/genomeA.00084-15
Rocha EPC, Danchin A, Viari A (1999) Translation in Bacillus subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res 27(17):3567–3576. https://doi.org/10.1093/nar/27.17.3567
Sakthiselvan P, Meenambiga SS, Madhumathi R (2019) Kinetic studies on cell growth. In: Vikas B (ed) Cell Growth. IntechOpen
Stanbury PF, Whitaker A, Hall SJ (2017) Chapter 4 - media for industrial fermentations. In: Stanbury PF, Whitaker A, Hall SJ (eds) Principles of fermentation technology, 3rd edn. Butterworth-Heinemann, Oxford, pp 213–272
Weissmann B, Meyer K (1954) The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord 1,2. J Am Chem Soc 76(7):1753–1757. https://doi.org/10.1021/ja01636a010
Westbrook AW, Ren X, Oh J, Moo-Young M, Chou CP (2018) Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab Eng 47:401–413. https://doi.org/10.1016/j.ymben.2018.04.016
Widner B, Behr R, Von Dollen S, Tang M, Heu T, Sloma A, Sternberg D, Deangelis PL, Weigel PH, Brown S (2005) Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol 71(7):3747–3752. https://doi.org/10.1128/aem.71.7.3747-3752.2005
Wu F-C, Chou S-Z, Shih I-L (2013) Factors affecting the production and molecular weight of levan of Bacillus subtilis natto in batch and fed-batch culture in fermenter. J Taiwan Inst Chem Eng 44(6):846–853. https://doi.org/10.1016/j.jtice.2013.03.009
Yao J, Xu H, Shi N, Cao X, Feng X, Li S, Ouyang P (2010) Analysis of carbon metabolism and improvement of γ-polyglutamic acid production from Bacillus subtilis NX-2. Appl Biochem Biotechnol 160(8):2332–2341. https://doi.org/10.1007/s12010-009-8798-2
Zając M, Kulawik P, Tkaczewska J, Migdał W, Filipczak-Fiutak M, Fiutak G (2017) The effect of hyaluronic acid addition on the properties of smoked homogenised sausages. J Sci Food Agric 97(8):2316–2326. https://doi.org/10.1002/jsfa.8041
Zhang L, Huang H, Wang H, Chen J, Du G, Kang Z (2016) Rapid evolution of hyaluronan synthase to improve hyaluronan production and molecular mass in Bacillus subtilis. Biotechnol Lett 38(12):2103–2108. https://doi.org/10.1007/s10529-016-2193-1
Acknowledgements
The authors want to acknowledge Juan Diego All, Mauro Nicolás Cerchi, and Claudio Exequiel Cansino Quispe for their technical assistance.
Funding
Part of this work was supported by Glyco@Alps (ANR-15-IDEX-02).
Author information
Authors and Affiliations
Contributions
SC, HM, and BP conceived and designed research. SC, ML, SP, and PA conducted experiments. BP and JCA contributed new reagents or analytical tools. SC and HM analyzed data. SC and HM wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cerminati, S., Leroux, M., Anselmi, P. et al. Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain. Appl Microbiol Biotechnol 105, 3075–3086 (2021). https://doi.org/10.1007/s00253-021-11246-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11246-6