Abstract
Pseudomonas fluorescens has the ability to produce the siderophore pyoverdine, a biotechnologically significant iron chelator, which has a wide range of potential applications, such as in agriculture (iron fertilizers) and medicine (development of antibiotics). The present work aimed to evaluate the influence of culture medium composition on the production of siderophores by P. fluorescens DSM 50090, an industrial relevant strain. It was found that the bacterium grown in minimal medium succinate (MMS) had a higher siderophore production than in King B medium. The replacement of succinate by glycerol or dextrose, in minimal medium, originated lower siderophore production. The increase of succinate concentration, the addition of amino acids or the reduction of phosphate in the culture medium did not improve siderophore production by P. fluorescens. The results obtained strongly suggest that (i) MMS is more appropriate than King B for large-scale production of siderophores; (ii) the modification of the culture medium composition, particularly the type of carbon source, influences the level of siderophore secreted; (iii) the production of siderophore by P. fluorescens seems to be a tightly regulated process; once a maximum siderophore concentration has been reached in the culture medium, the bacterium seems to be unable to produce more compound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ubiquity of iron in the earth’s soil and the possibility of existing in two oxidation states led to its establishment as a central player in a wide range of metabolic processes, either in prokaryotes and eukaryotes [1]. Fe3+ is the prevailing species in soils and water due to the oxidizing presence of O2. However, ferric iron (Fe3+) is far less soluble than its ferrous counterpart (Fe2+), which leads to iron shortage [1]. The limitation in the availability of iron is long known to be mitigated by bacteria, fungi, and plants through the secretion of siderophores (from the Greek: “iron carrier”). Siderophores are a heterogeneous group of organic molecules (500–1500 Da) that strongly chelate Fe3+ (Kf > 1030) and thus act as iron scavengers [2]. These molecules are classified according to the groups available to coordinate with iron; the three major groups are hydroxamate, catecholate, and α–hydroxycarboxylate [3].
Siderophores are produced by Gram-negative and Gram-positive bacteria, inhabiting both soil and aqueous environments. Pseudomonas is a genus of Gram-negative bacteria, which comprises several siderophore-producing species [4]; among them, Pseudomonas fluorescens is an example of a non-pathogenic bacterium that shares with other Pseudomonas the ability to produce pyoverdines (PVDs) [5]. These are a family of similar siderophores, comprising moderately distinct structures, which are also designated by yellow-green fluorescent pigment due to the color exhibited under neutral to alkaline pH [6].
Pyoverdines contain one catecholate and two hydroxamate groups, being constituted by three main domains: (i) a 2,3-diamino-6,7-dihydroxiquinoline (the chromophore responsible by PVDs spectral characteristics), (ii) an acyl side chain linked to the 3-amino group of the chromophore, and (iii) a short peptide sequence (6-14 amino acids) bound to the C1-carboxyl group of the chromophore [5, 7]. The synthesis of PVD begins in cytoplasm, by the assembly of an initially acylated ferribactin (the peptide precursor of PVD) through the action of non-ribosomal peptide synthetases, such as PvdL, Pvdl, or PvdD [5]. The first three amino acids of ferribactins are always L-glutamic acid (L-Glu) which is coupled via its γ-carboxy group to D-tyrosine (D-Tyr) and L-diaminobutyrate (L-Dab) [5]. The acylated ferribactin is translocated across the plasma membrane into the periplasm, being deacylated after transport. After deacylation, the fluorescent dihydroxyquinoline ring is formed, which transforms the ferribactin into a PVD [5]. While the D-Tyr/L-Dab portion leads to the formation of the fluorophore, the L-Glu residue is transformed being the responsible of a wide range of PVDs produced by the different strains [5]. Thus, the assembly of ferribactin occurs in the cytoplasm and the maturation to the fluorescent PVD and the tailoring of the initial L-Glu residue are periplasmic processes [5].
Iron shortage is the main factor governing PVD secretion [6]. Ferric iron uptake regulation (Fur) protein plays a key role in the regulation of the production of the pigment; the presence of ferrous ions leads Fur to repress genes involved in iron uptake [5]. In P. aeruginosa, the regulation of PVD production is more complex and probably it is not only driven by the quantity of iron in medium [5], since molecular genetics studies reported the regulation by other factors, such as biofilm formation [5], cell density [8], quorum signalling molecules [5] [8], phosphate starvation [9] [10], or sulfur availability [11]. In the case of P. fluorescens, the type of carbon [6] or nitrogen source [12], as well as the supplementation of culture medium with amino acids [12] [13], have been appointed as influencing PVD production.
The application of siderophores for bioengineering purposes has been gradually explored during the last decades, particularly in environmental, agronomic, and medical fields [14]. The high affinity of siderophores for ferric iron makes it useful for several applications, such as bioremediation of heavy metals, fertilization of crops, or the development of antibiotics [15]. Pyoverdine has been explored as a tool for all the applications described above, being tested for the removal of metal nanoparticles from aquatic environments, in the production of fertilizers (for iron-induced chlorosis) or the making of Trojan horse–like approaches [15] [16] [7].
The increase of possible PVDs use, and consequent commercialization, makes its large-scale production an important issue. Chemical synthesis can be a possibility for less complex siderophores. However, in the case of PVDs, due to their molecular complexity and the presence of a side chain with a high diversity of residues, the chemical synthesis presents low yields and leads to unnecessary waste of reactants, which decreases the feasibility of their production by this route [17]. The microbiological route emerges as the best option for PVD production, through the culture of biologically safe microorganisms (group 1 biological agents), like P. fluorescens. In this context, it is increasingly important to know more deeply how cultural conditions can influence the levels of PVD secreted. While P. fluorescens has been widely used as a tool for PVD production, in the context of several applications, there is a lack of research regarding the specific factors governing the regulation of PVD release, contributing to a better explanation of the production levels, and to understand what is the role of the own molecule in the process, such has been made for other strains [18].
This work proposes to track back the nutritional factors driving the production of PVD by P. fluorescens, in a standardized and comparable way, by benchmarking classic and less explored approaches on medium optimization, using a commercial strain (DSM 50090, also known as ATCC 13525 [19]) widely applied in both fundamental [12, 20, 21] and applied [16, 22, 23] PVD-related research as bacterial model. More specifically, two media (minimal medium succinate—MMS and King B) were evaluated for growth and siderophore production by P. fluorescens. In addition, the type and concentration of carbon source, the supplementation of the culture medium with amino acids, and the effect of phosphate concentration on siderophore production were also studied. The influence of culture medium composition on siderophore production by P. fluorescens, in a biotechnological context, is discussed.
Material and Methods
Strain, Media, and Cultural Conditions
Pseudomonas fluorescens DSM 50090 was originally obtained from DSMZ, German Collection of Microorganisms and Cell Cultures, GmbH and maintained at 4 °C on nutrient agar (Merck, Darmstadt, Germany) plates.
The strain was cultured in King B medium (Peptone (BD-Difco, Waltham, MA, EUA), 20 g/L; dipotassium hydrogen phosphate (Merck), 1.5 g/L; magnesium sulfate heptahydrate (Merck), 3.0 g/L; and glycerol (Merck), 10 mL/L), minimal medium succinate (MMS) (dipotassium hydrogen phosphate (Merck), 6 g/L; potassium dihydrogen phosphate (Sigma-Aldrich, St. Louis, Missouri, EUA), 3 g/L; magnesium sulfate heptahydrate (Merck), 0.2 g/L; ammonium sulfate (Merck), 1 g/L; and succinic acid (Merck), 4 g/L (unless stated otherwise)) without or with modifications. The alterations made in the formula of MMS medium led to replacement of succinate by glycerol (MMG) (4 mL/L) or by dextrose (MMD) (10 g/L, Merck); variation of succinate and phosphate concentration at 4, 6, and 8 g/L or 0.1, 2.4, and 5.4 g/L, respectively; supplementation with L-glutamic acid (MMS + Glu) (2 g/L), L-arginine (MMS + Arg) (2.9 g/L), and L-tyrosine (MMS + Tyr) (0.4 g/L); L-glutamic acid, L-arginine, and L-tyrosine were obtained from Sigma-Aldrich. In all cases, the initial pH of culture media was adjusted to 7.0 ± 0.1.
The starter cultures were obtained by inoculating 2 loops of bacterium in 20 mL of the corresponding liquid culture medium, containing 1 mg/L of FeCl3.6H2O (Merck) (~ 3.7 μmol/L Fe), in a 100 mL Erlenmeyer flask; the bacterium was incubated at 30 °C, at 150 rpm, for ~ 8 h. The pre-cultures were prepared in 250 mL Erlenmeyer flasks containing 100 mL of the same culture medium (but with 0.37 μmol/L Fe), by inoculating an appropriate volume of the starter culture. Then, bacterium was incubated overnight (15–16 h) to obtain cells in exponential phase of growth (OD600 ∼ 1.0), under the same conditions described above. Experimental cultures for siderophore production were prepared in 500 mL Erlenmeyer flasks containing 200 mL of the same culture medium, without iron, by inoculating an appropriate volume of the pre-culture in order to obtain an initial OD600 ∼ 0.1; subsequently, bacterium was incubated for 48 h under the same conditions described above. Under the conditions used, the iron carry-over was theoretically lesser that 0.08 μmol/L Fe, which is 45 times lower than the iron concentration described (> 3.6 μmol/L Fe) that inhibits pyoverdine production in P. fluorescens [6].
Evaluation of Bacterial Growth, Culture Media pH, and Siderophore Production
Bacterial growth, culture media pH, and siderophore production were monitored in distinct time points, indicated in the figures, in order to have an indication of the physiological and metabolic state of the culture. For this purpose, samples (5–10 mL) were withdrawn and bacterial growth was quantified spectrophotometrically, by measuring the optical density at 600 nm, after appropriate dilution of the samples; bacterial dry-weight biomass was calculated using a calibration curve (OD600 versus dry-weight biomass (g/L)) previously performed. The pH of the culture medium was monitored using a pH probe, which combines glass and reference electrodes and a temperature probe.
For estimate of siderophore production, samples were centrifuged (3000×g, during 15 min, at 25 °C), filtered using a 0.45 μm cellulose nitrate membrane filter (Sartorius, Göttingen, Germany), and stored at − 20 °C, until quantification. Siderophore (PVD) production was evaluated by UV-Vis spectroscopy, since the chromophore is common to all PVDs produced by Pseudomonas genera [24]. The maximum absorbance value corresponding to the presence of PVD can be found at 380–400 nm [6]; thus, the spectra of culture supernatants collected at different time points were obtained, the values corresponding to maximum absorbance were registered and PVD concentrations calculated through Lambert Beer law, using the molar extinction coefficient (ε) of 16,500 M−1 cm−1 [6].
Reproducibility of the Results and Statistical Analysis
The assays were repeated independently three times. In each assay, the values of biomass produced and PVD concentration obtained in each time point were further measured in triplicate and duplicate, respectively. The results are presented as the average of the total of determinations. The standard deviation associated with each average value was represented in the form of error bars. The mean values of siderophore concentration were further subjected to one-way ANOVA analysis, followed by Tukey-Kramer multiple comparison method; P values < 0.05 were considered statistically significant.
Results
Influence of Nutritional Enrichment of the Culture Media in Siderophore Production
The first attempt to evaluate the importance of culture media composition on the siderophore production by P. fluorescens consisted in the use of two standard media with distinct degrees of nutrient abundance and diversity, in terms of carbon and nitrogen sources. Thus, the following were tested: (i) MMS, a minimal-chemically defined (synthetic) medium, containing inorganic salts and succinate as a source of carbon and energy, [24] and (ii) King B medium, a rich-medium, containing mineral nutrients, peptone, and glycerol [25].
A difference in the growth of the strain in the two media was observed (Fig. 1a). A maximum biomass was achieved in King B medium (~ 2 g/L), while in MMS, less than 1 g/L was obtained. In King B medium, the maximum siderophore concentration was observed at 24–48 h, while in MMS medium, it was observed at 32-48 h (Fig. 1b). The comparative analysis of the pyoverdine production profile associated with both media indicated that the concentrations reached at 32 and 48 h in MMS and King B medium were not significantly different (Fig. 1b). However, the normalization of siderophore production considering the bacterial biomass, which gives a measure of the quantity of molecules produced by cell in each media, revealed a higher production of PVD in MMS (Fig. 1c). Thus, taking into account that MMS yielded higher levels of PVD than King B medium, this was the medium selected to be used in the subsequent studies.
Evolution of growth and PVD secretion by P. fluorescens cultured in minimal medium succinate (MMS) or King B medium. a Growth of bacterium. b Concentration of PVD, expressed as quantity of siderophore per liter; the means with different letters are significantly different (P < 0.05). c PVD normalized, expressed as the quantity of siderophore per gram of dry-weight biomass at 32 h of culture time. The error bars represent the SD
Effect of Carbon Source and Its Concentration
The study progressed with the evaluation of the influence of carbon source on PVD production. Thus, succinate was substituted by dextrose (MMD) or glycerol (MMG) in the backbone of MMS. These carbon sources represent three groups of molecules (an organic acid, a simple sugar, and a polyol, respectively), which are catabolized by Pseudomonas genera through different metabolic pathways and therefore may be associated with distinct siderophore production profiles [26].
The growth profiles of the strain in the media containing the above mentioned carbon sources were similar (Supplementary Fig. S1). However, there are pronounced differences in what concerns siderophore production (Fig. 2a). The PVD concentration was significantly higher, at 32 h, when P. fluorescens was grown in a culture medium containing succinate instead of dextrose or glycerol (Fig. 2a). Similar results were observed when the siderophore level was normalized considering bacterial growth (Fig. 2b).
PVD secretion by P. fluorescens in minimal medium containing different carbon sources. a Concentration of PVD expressed as quantity of siderophore per liter; the means with different letters are significantly different (P < 0.05). b PVD normalized, expressed as the quantity of siderophore per gram of dry-weight biomass at 32 h of culture time. The error bars represent the SD
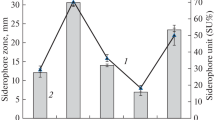
Following the observation that succinate is a better source of carbon than the other two tested in the formulation of minimal medium, the effect of its concentration was evaluated. For this purpose, P. fluorescens was grown in the presence of succinate in a range between 4 and 8 g/L. The increase of succinate concentration resulted in higher values of biomass produced (Supplementary Fig. S2), since the cultures with 6 g/L and 8 g/L continued to grow after 32 h, the time point in which maximum biomass was reached by cultures grown with 4 g/L (Fig. 1a). Consistent with these results, it was observed a maximum siderophore concentration at 32 h, for cells incubated with 4 g/L succinate, while in the case of cells cultured with 6 or 8 g/L succinate reached the maximum siderophore concentration at 48 or 56 h, respectively (Fig. 3a). The siderophore concentration, corresponding to the lower value of succinate (4 g/L), at 32 h, was not significantly different than those obtained for higher values (6 and 8 g/L), at 48 or 56 h, respectively (Fig. 3a). However, the values of PVD normalized per biomass were higher when the culture medium contained 4 g/L of succinate (Fig. 3b), suggesting that the excess of carbon source was canalized for bacterial growth instead of siderophore production.
Influence of succinate concentration on siderophore secretion by P. fluorescens. a Concentration of PVD expressed as the quantity of siderophore per liter; the means with different letters are significantly different (P < 0.05). b PVD normalized, expressed as the quantity of siderophore per gram of dry-weight biomass at 32 h (all succinate concentrations) and 48 h (6 g/L and 8 g/L succinate) of culture time. The error bars represent the SD
Repercussion of the Supplementation of MMS Medium with Amino Acids
The MMS medium was further supplemented with the amino acids arginine, glutamic acid, and tyrosine, giving origin to MMS + Arg, MMS + Glu, and MMS + Tyr media, respectively. Arginine was chosen to supplement MMS since it has already been associated with an enhancement in pyoverdine production [12, 27]. On the other hand, glutamic acid and tyrosine are central building blocks in the biosynthetic route of pyoverdine [5], constituting a reasonable option for the media optimization attempt.
In general, the growth profile of the cultures without (MMS) or supplemented with amino acids were similar (Supplementary Fig. S3). The supplementation of MMS with Arg, Glu, or Tyr did not enhance the PVD concentration secreted by P. fluorescens (Fig. 4a). In general, a similar tendency was obtained when the siderophore production was normalized considering the biomass reached at 32 h (Fig. 4b).
Effect of the presence of amino acids on siderophore secretion by P. fluorescens. a PVD in minimal medium supplemented with L-arginine (MMS + Arg), L-glutamic acid (MMS + Glu), or L-tyrosine (MMS + Tyr), expressed as the quantity of siderophore per liter; the means with different letters are significantly different (P < 0.05). b PVD normalized, expressed as the quantity of siderophore per gram of dry-weight biomass at 32 h of culture time. The error bars represent the SD
Phosphate Concentration and Siderophore Secretion
Phosphorus is an important element in the composition of culture media, since it is fundamental in cell metabolism and can act as a buffer through phosphate buffering system. It was described that phosphate concentration can affect PVD production in the Pseudomonas genus [9] [10]. Therefore, the influence of phosphate concentration on siderophore production was assessed by cultivating the bacteria in MMS medium containing decreasing amounts of this chemical species.
As it can be seen in Fig. 5a, the reduction of phosphate concentration (in the range values tested) did not modify, significantly, the concentration of siderophore secreted by P. fluorescens; a similar tendency was achieved when PVD was normalized (Fig. 5b). In addition, a comparable growth profile (Supplementary Fig. S4A) and buffering capacity of the medium (assessed through the evolution of the pH of the medium (Supplementary Fig. S4B)) was observed.
Impact of phosphate concentration on siderophore secretion by P. fluorescens. The bacterium was grown in MMS medium containing different phosphate concentrations. a Concentration of PVD expressed as the quantity of siderophore per liter; the means with different letters are significantly different (P < 0.05). b PVD normalized, expressed as the quantity of siderophore produced per gram of dry-weight biomass at 32 h of culture time. The error bars represent the SD
Discussion
In the estimation of operating costs associated with a biotechnological process, such as siderophore production, two factors are fundamental: product yields (productivity) and the price of raw material associated with culture medium ingredients. Typically, in most biotechnological-based products, the medium constituents are the main cost factors, contributing for 10–60% of operating costs [28]. Taking these aspects in mind, it was compared the production of siderophore by P. fluorescens, in a batch mode (Erlenmeyer flasks), using two culture media: MMS and King B. Although King B medium is nutritionally richer than MMS and originates higher biomass values, the production of siderophore (normalized considering bacterial biomass) in this medium was lower than that reached in MMS (Fig. 1c). These results, associated with the lower cost of MMS formulation (due to the absence of expensive ingredients, such as peptone, present in King B), point it as the best option (between the two media tested) for the siderophore production. The results here obtained also indicate that the production of siderophore in this strain (and by extension in P. fluorescens) is not mainly dependent on cell growth or the abundance and diversity of carbon sources but rather on the specific composition of culture medium; in fact, King B medium has glycerol and possibly peptone as carbon sources in higher amounts than in MMS (only succinate).
Within culture medium ingredients, the selection of carbon source is of paramount importance [28, 29]. While dextrose was previously reported to originate intermediate amounts of PVD in other strains of P. fluorescens [27], in the present work, using a minimal medium, it was found that the levels of siderophore were significantly higher with succinate (MMS) than with dextrose (MMD) or glycerol (MMG) (Fig. 2a, b). Our results are in agreement with those which describe that succinate and other organic acids constitute a better carbon source for siderophore production in P. fluorescens than other candidate nutrients [27] [30].
Interestingly, the increase of succinate concentration, in culture medium, to 6 or 8 g/L was not accompanied by an enhancement in PVD maximum production (Fig. 3a, b), although it resulted in a rise of the biomass produced (Supplementary Fig. S2); a comparable tendency was previously found for different media formulation, where similar PVD production values were obtained when testing 5 and 10 g/L succinate [30]. Probably, P. fluorescens has a limited capacity to channel the carbon source for the production of PVD.
Another attempt to enhance PVD production by P. fluorescens consisted in the supplementation of MMS with amino acids, a strategy highlighted in the literature to optimize PVD production [12] [27]. For this purpose, besides arginine (which has recently been described as able to modulate the molecular pathway leading to PVD production, even if it does not enter in their structure) [31], glutamic acid, and tyrosine were tested, since they are two central building blocks in the biosynthetic route of PVD [5]. The enrichment of MMS culture medium with these amino acids could lead to an increase of siderophore production. However, the results obtained do not confirm the efficiency of this approach (Fig. 4a, b). The fact that other media were used as backbone for amino acid supplementation [12] [13], and the strain used here is different from those reported in previous studies [13] [27], possibly exhibiting a distinct PVD structure [20] [32] [33], may have a role in the different experimental outcomes.
Phosphate concentration is reported to influence siderophore production, in liquid cultures, of Pseudomonas genus [34]. In this context, phosphate starvation (as low as approximately 0.01 g/L phosphate) in P. aeruginosa has been linked with a stimulatory effect in PVD secretion [9] [10]. However, the opposite was also described: the presence of high phosphate concentration (5.4 g/L) had a positive effect on PVD production by a different strain of P. aeruginosa [29]. In a way or another, there was not much previous knowledge about the effect of phosphate concentration in the production of PVD by P. fluorescens. In the present work, it was found that a reduction (from 5.4 g/L to 0.1 g/L) of phosphate concentration did not originate an increase in PVD produced by P. fluorescens (Fig. 5a, b). These results suggest that in P. fluorescens the level of siderophore produced does not seem to be significantly dependent on the concentration of phosphate used to supplement the medium. It is also important to notice that pH-based degradation of the siderophore (as consequence of phosphate reduction) should be ruled out by two reasons: (i) neutral to mild alkaline conditions (at around pH 9.0) as those here observed (Supplementary Fig. S4B) do not result in significant siderophore degradation [6] [27]; (ii) when lower concentrations of phosphate are added, a similar pH profile of the culture medium is obtained, indicating that smaller amounts of phosphate are able to equally buffer the pH of the medium.
It was described that a clampdown in the release of siderophore by P. fluorescens was associated with an ability to use external siderophores, from either plants or other bacteria [35]. This possibility should be dismissed in liquid pure cultures, under iron stress conditions, as those verified in the set of experiments here presented; in alternative, a strict regulation of genes directly involved in PVD biosynthesis is proposed, as a factor responsible for the limitation of PVD released [18] [26].
In conclusion, the results obtained indicate that under iron-deprived conditions and using batch cultures, the modification of the culture medium composition, particularly the type of carbon source, influences the production of PVD by P. fluorescens DSM 50090. It was also found that MMS appears to be more appropriate than King B for the production of PVD, using this strain in a batch process, since it (i) originates higher siderophore values; (ii) is a chemical defined medium (allowing a reproducible formulation); and (iii) presents lower operating costs due the absence of expensive nutrients like peptones. The results obtained also indicate that the production of PVD by P. fluorescens seems to be a process tightly regulated since once reached a defined level of siderophore, the wild type strain, in normal physiological conditions (besides iron limitation) seems to be unable to originate more PVD; by other words, after having reached the maximum capacity of siderophore production, the modification of culture medium composition (under the conditions tested) was not able to induce more siderophore production by P. fluorescens. This work contributes for the understanding of the role of nutrients on siderophore production by P. fluorescens. The information here obtained could be important in the development of future strategies related to the large-scale production of PVDs.
References
Chu, B. C., Garcia-Herrero, A., Johanson, T. H., Krewulak, K. D., Lau, C. K., Peacock, R. S., & Vogel, H. J. (2010). Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. BioMetals, 23(4), 601–611.
Hider, R. C., & Kong, X. (2010). Chemistry and biology of siderophores. Natural Product Reports, 27(5), 637–657.
Ahmed, E., & Holmstrom, S. J. M. (2014). Siderophores in environmental research: roles and applications. Microbial Biotechnology, 7(3), 196–208.
Cornelis, P. &, & Matthijs, S. (2007). Pseudomonas siderophores and their biological significance. In A. Varma & S. B. Chincholkar (Eds.), Microbial Siderophores (pp. 193–203). Springer: Berlin Heidelberg.
Ringel, M. T., & Brueser, T. (2018). The biosynthesis of pyoverdines. Microbial Cell, 5(10), 424–437.
Meyer, J. M., & Abdallah, M. A. (1978). The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. Microbiology, 107(2), 319–328.
Cezard, C., Farvacques, N., & Sonnet, P. (2015). Chemistry and biology of pyoverdines, Pseudomonas primary siderophores. Current Medicinal Chemistry, 22(2), 165–186.
Stintzi, A., Evans, K., Meyer, J. M., & Poole, K. (1998). Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiology Letters, 166(2), 341–345.
Zaborin, A., Romanowski, K., Gerdes, S., Holbrook, C., Lepine, F., Long, J., & Alverdy, J. C. (2009). Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proceedings of the National Academy of Sciences, 106(15), 6327–6332.
Zaborin, A., Gerdes, S., Holbrook, C., Liu, D. C., Zaborina, O. Y., & Alverdy, J. C. (2012). Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. PLoS One, 7(4), e34883.
Imperi, F., Tiburzi, F., Fimia, G. M., & Visca, P. (2010). Transcriptional control of the pvdS iron starvation sigma factor gene by the master regulator of sulfur metabolism CysB in Pseudomonas aeruginosa. Environmental Microbiology, 12(6), 1630–1642.
Albesa, I., Barberis, L., Pajaro, M., & Alberto, E. (1985). Pyoverdine production by Pseudomonas fluorescens in synthetic media with various sources of nitrogen. Microbiology-sgm, 131(12), 3251–3254.
Kisaalita, W. S., Slininger, P. J., & Bothast, R. J. (1993). Defined media for optimal pyoverdine production by Pseudomonas fluorescens 2-79. Applied Microbiology and Biotechnology, 39(6), 750–755.
Saha, M., Sarkar, S., Sarkar, B., Sharma, B. K., Bhattacharjee, S., & Tribedi, P. (2016). Microbial siderophores and their potential applications: a review. Environmental Science and Pollution Research, 23(5), 3984–3999.
Poirier, I., Kuhn, L., Demortiere, A., Mirvaux, B., Hammann, P., Chicher, J., & Bertrand, M. (2016). Ability of the marine bacterium Pseudomonas fluorescens BA3SM1 to counteract the toxicity of CdSe nanoparticles. Journal of Proteomics, 148, 213–227.
Nagata, T., Oobo, T., & Aozasa, O. (2013). Efficacy of a bacterial siderophore, pyoverdine, to supply iron to Solanum lycopersicum plants. Journal of Bioscience and Bioengineering, 115(6), 686–690.
Martins, J. G., Clara Martin, L. A., Barros, M. T., Soares, H. M. V. M., & Lucena, J. J. (2018). Azotochelin and N-dihydroxy-N,N’-diisopropylhexanediamide as Fe sources to cucumber plants in hydroponic cultures. Emirates Journal of Food and Agriculture, 30(1), 65–76.
Ambrosi, C., Leoni, L., & Visca, P. (2002). Different responses of pyoverdine genes to autoinduction in Pseudomonas aeruginosa and the group Pseudomonas fluorescens-Pseudomonas putida. Applied and Environmental Microbiology, 68(8), 4122–4126.
Environment Canada & Health Canada. (2015). Final screening assessment for Pseudomonas fluorescens ATCC 13525. Environment Canada & Health Canada
Linget, C., Stylianou, D. G., Dell, A., Wolff, R. E., Piémont, Y., & Abdallah, M. A. (1992). Bacterial siderophores: the structure of a desferriferribactin produced by Pseudomonas fluorescens ATCC 13525. Tetrahedron Letters, 33(27), 3851–3854.
Philson, S. B., & Llinás, M. (1982). Siderochromes from Pseudomonas fluorescens. I. Isolation and characterization. Journal of Biological Chemistry, 257(14), 8081–8085.
Chiadò, A., Varani, L., Bosco, F., & Marmo, L. (2013). Opening study on the development of a new biosensor for metal toxicity based on Pseudomonas fluorescens pyoverdine. Biosensors, 3(4), 385–399.
Pahlow, S., Stöckel, S., Pollok, S., Cialla-May, D., Rösch, P., Weber, K., & Popp, J. (2016). Rapid identification of Pseudomonas spp. via raman spectroscopy using pyoverdine as capture probe. Analytical Chemistry, 88(3), 1570–1577.
Hoegy, F., Mislin, G. L. A., & Schalk, I. J. (2014). Pyoverdine and pyochelin measurements. In A. Filloux & J.-L. Ramos (Eds.), Pseudomonas Methods and Protocols, Methods in Molecular Microbiology, 1149 (pp. 293–301). Springer: New York.
King, E. O., Ward, M. K., & Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescin. The Journal of Laboratory and Clinical Medicine, 44(2), 301–307.
Rojo, F. (2010). Carbon catabolite repression in Pseudomonas : optimizing metabolic versatility and interactions with the environment. FEMS Microbiology Reviews, 34(5), 658–684.
Sayyed, R., Badgujar, M. D., Sonawane, H. M., Mhaske, M. M., & Chincholkar, S. B. (2005). Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads. Indian Journal of Biotechnology, 4(4), 484–490.
Kristiansen, B. (2001). Process economics. In C. Ratledge & B. Kristiansen (Eds.), Basic Biotechnology (pp. 239–252). Cambridge University Press: Cambridge.
Fallahzadeh, V., Ahmadzadeh, M., & Sharifi, R. (2010). Growth and pyoverdine production kinetics of Pseudomonas aeruginosa 7NSK2 in an experimental fermentor. Journal of Agricultural Technology, 6(1), 107–115.
Gouda, S., & Greppin, H. (1965). Biosynthèse pigmentaire chez Pseudomonas fluorescens en fonction de la concentration du substrat hydrocarboné ou aminé. Archives des sciences de Genève, 18, 716–721.
Barrientos-Moreno, L., Molina-Henares, M. A., Pastor-Garcia, M., Ramos-González, M. I., & Espinosa-Urgel, M. (2019). Arginine biosynthesis modulates pyoverdine production and release in Pseudomonas putida as part of the mechanism of adaptation to oxidative stress. Journal of Bacteriology, 201(22), e00454–e00419.
Hohlneicher, U., Hartmann, R., Taraz, K., & Budzikiewicz, H. (1995). Pyoverdin, ferribactin, azotobactin - a new triade of siderophores from Pseudomonas chlororaphis ATCC 9446 and its relation to Pseudomonas fluorescens ATCC 13525. Zeitschrift für Naturforschung. Section C, 50(5–6), 337–344.
Hohnadel, D., Haas, D., & Meyer, J.-M. (1986). Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiology Letters, 36(2–3), 195–199.
Elena, M., & de Villegas, D. (2007). Biotechnological production of siderophores. In A. Varma & S. B. Chincholkar (Eds.), Microbial siderophores (pp. 219–231). Springer: Berlin Heidelberg.
Marschner, P., & Crowley, D. E. (1998). Phytosiderophores decrease iron stress and pyoverdine production of Pseudomonas. Soil Biology and Biochemistry, 30(10), 1275–1280.
Funding
João M. Vindeirinho received the grant from the project PTDC-AGR-TEC/0458/2014—POCI-01-0145-FEDER-016681. This work is financed by the FEDER funds through the Operational Competitiveness Factors Program—COMPETE and by national funds through FCT—Foundation for Science and Technology within the scope of the project PTDC-AGR-TEC/0458/2014—POCI-01-0145-FEDER-016681.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 132 kb)
Rights and permissions
About this article
Cite this article
Vindeirinho, J.M., Soares, H.M. & Soares, E.V. Modulation of Siderophore Production by Pseudomonas fluorescens Through the Manipulation of the Culture Medium Composition. Appl Biochem Biotechnol 193, 607–618 (2021). https://doi.org/10.1007/s12010-020-03349-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03349-z