Abstract
Under scarce iron conditions, several bacteria, fungi and plants secrete ferric iron-specific ligands, generically termed as siderophores that are able to bind with insoluble ferric ion thereby making them available to the host organisms. Siderophore producing bacteria were isolated from the rhizospheric soil of Eragrostis cynosuroides by CAS agar screening and CAS shuttle assay method. Among five positive isolates, DR2 produced a relatively high level of siderophore (69.81 SU%) and was identified as catecholate type. Further, it was identified as Bacillus subtilis DR2 (KP455653) based on 16S rRNA gene sequencing and phylogenetic analysis. Media optimization revealed that the strain B. subtilis DR2 showed maximum siderophore yield (80.60 SU%) under optimized condition of 72 h incubation at 35°C in succinate media at pH 8, supplemented with sucrose as carbon and NaNO3 as nitrogen sources. It was further tested as seed inoculants under pot culture conditions and was found to be very efficient in seed germination and growth promotion of Coriandrum sativum. Thus, the present study signifies that B. subtilis DR2 may be a promising candidate with potential of plant growth promotion to be used as biofertilizer for various crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The rhizosphere is a dynamic environment, harbouring diverse array of microorganims. It is a rich repository of beneficial bacteria commonly known as plant growth promoting rhizobacteria (PGPR) that enhances crop productivity and maintains soil health in a sustainable way [1]. PGPR must be able to colonize the plant rhizosphere, promote growth with its multi-spectrum mode of action, good viability, be ecofriendly, and show tolerance to temperature, ultraviolet (UV) radiation, oxidizing agents and various other unfavourable conditions [2]. PGPR promote plant growth via different mechanisms to make the respective elements available, like nitrogen fixation (N2), siderophore production (Fe), IAA production, phosphate solubilisation (PO\(_{4}^{{ - 3}}\)), etc. [3]. All of them have their own vital roles in plant growth promotion.
Iron is a vital trace element for living organisms. But due to very low solubility of Fe3+ in the earth’s crust, its availability is limited and it cannot easily be utilized by them. This element is predominantly present as oxide and hydroxide forms with their characteristic very low solubility. In response to this, microorganisms have evolved a strategy for acquiring iron through siderophore production [4, 5].
Siderophore is a low-molecular weight, iron chelating compound having high affinity for ferric iron, converting them into ferrous form, which is to be further utilized in the metabolic processes of plants [6]. Rhizospheric bacteria capable of producing siderophores increase the bioavailability of iron near the root to promote plant growth. Based on the ligands used to chelate ferric iron, bacterial siderophores are categorized into four types: catecholate, hydroxamate, carboxylate and salicylate. Various bacterial species of genera Bacillus, Aeromonas, Aerobacter, Enterobacter, Escherichia, Mycobacterium, Klebsiella, Vibrio, Salmonella and Yersinia have been reported to produce siderophores [7–9]. Bacillus spp. are known to be excellent siderophore producers and stimulate plant growth through enhanced phosphate nutrition, iron, potassium and nitrogen uptake [10] and have additional tolerance property in any adverse condition and protect plant from phytopathogens infection [11] hence they are better for several crops. Various PGP attributes of Bacillus species involved in increased productivity of rice, maize, wheat, cucumber, soybean, potato, apple, tomato and ornamental plants have been validated through green house as well as field trials [12]. In the present scenario, the natal approaches are generally used as an alternative to chemical fertilizers for enhancing crop productivity apart from including plant nutrient management systems. Eragrostis cynosuroides known as kusha or dharbham grass is a medicinal plant and has extensive properties such as antimicrobial, antioxidant and anticancerous [13]. This plant has ability to absorb ultraviolet radiation and grow independently in natural condition without exogenous supply of any chemical fertilizer. In the view of aforesaid benefits this grass has been selected to explore its rhizospheric bacteria.
Coriandrum sativum (Coriander), a member of family Apiaceae is an important herbal spice crop, generally grown in the winter season of India. Its different parts are valued for its culinary and medicinal properties. The green herb being sink of various vitamins like, vitamin A, vitamin C, and vitamin B2 [14] is used as flavouring agents in preparation of delicacies (sauces, soup, breads, cakes, confectionery and meat products), while the seeds act as tonic, stomachic, diuretic, carminative and aphrodisiac. So keeping in mind, the immersing importance of coriander crop, the present study is aimed at the production and estimation of siderophores produced by Bacillus subtilis and their role in vegetative growth promotion of Coriandrum sativum seedlings under in vitro pot culture experiment.
MATERIALS AND METHODS
Sample Collection and Isolation of Rhizospheric Bacteria
Rhizospheric soil sample of Eragrostis cynosuroides plant was collected from road side (devoid of any fertilizer) of Danapur, Patna, Bihar, India (25°34′56.2″ N, 85°2′37.06″ E). The plant was uprooted with the help of trowel for the collection of rhizospheric soil. The soil sample was collected and transferred in sterile ziplock polythene bags to the laboratory (Microbial Biodiversity Lab, Department of Botany, Patna) for further study and processed within three hours. The soil suspension was prepared by adding 1 g of soil sample to 10 mL sterile distilled water and diluted up to 10–6 dilution and spread on to nutrient agar media (NAM) and incubated at 30 ± 2°C for 24 h. Bacterial colonies appeared on plates were purified by sub culturing repeatedly to get axenic culture and preserved at 4°C in NAM for further use. The isolates were designated as DR1, DR2, DR3, DR4, DR5, DR6 and DR7. All the experiments were carried out in triplicates.
Iron Decontamination
All glasswares used in the present study was soaked overnight in 6 M hydrochloric acid (HCl) and thoroughly rinsed with distilled water (DW) to remove any traces of iron.
Qualitative screening of Siderophore Production
Siderophore production by plant growth promoting microorganisms was tested qualitatively by modified Chrome Azural Sulfonate (CAS) plate assay [15]. 0.06 g CAS was dissolved in 50 mL of distilled water and mixed with 10 mL of iron (III) solution (1 mM FeCl3·6H2O in 10 mM HCl). This was added to 0.073 g of hexa decyl trimethyl ammonium bromide (HDTMA) in 40 mL of distilled water. The dark blue colored CAS reagent was then autoclaved for 15 min. This reagent was added to succinate agar medium (succinic acid—4 g, K2HPO4—3.0 g, (NH4)2SO4·7H2O—0.2 g, DDW—1000 mL at pH 7.0). After that 24 h old bacterial isolates were spot inoculated on succinate agar medium and incubated at 30 ± 2°C for 24–72 h. Formation of an orange halo zone from dark blue color around the colonies was indicative for siderophore production.
Quantitative Assay of Siderophore Production (CAS Shuttle Assay)
Quantitative estimation of siderophore production was carried out by CAS shuttle assay [15]. The isolates were inoculated in succinate broth medium and incubated on shaker incubator at 120 rpm and 30 ± 2°C for 24 h. The broth was centrifuged at 10 000 rpm for 10 min at 4°C. An aliquot of 0.5 mL of supernatant (cell-free extract) was mixed with 0.5 mL of CAS solution. The resulting color obtained was measured after 20 min of incubation at room temperature at the wave length of 630 nm, using the UV-VIS spectrophotometer (Systronics, Ahmedabad, India), referring the uninoculated CAS solution as blank. The percentage of siderophore units (SU%) was estimated as the proportion of CAS color shifted using the formula:
Whereas,
SU%—percentage of siderophore units,
Ar—absorbance of reference (CAS assay solution + uninoculated media) and
As—absorbance of the sample (CAS assay solu-tion + cell-free supernatant).
Phenotypic and Genotypic Characterization of Isolates
All the isolates were further characterized on the basis of its morphological, cultural and biochemical characteristics as per the Bergey’s Manual of Systematic Bacteriology. The promising strain was identified by 16S rRNA gene sequence analysis and sequence was submitted to the National Centre for Biotechnology Information (NCBI) for accession number. The phylogenetic tree was constructed with similar sequences available on BlastN tool of NCBI (http://www.ncbi.nlm.nih.gov/ BLAST) by using MEGA 10 software. PCR based 16S rRNA gene amplification and sequencing of the promising bacterial isolate was carried out using universal primers at Xcelris lab Ltd, Ahmedabad, Gujarat, India.
Characterization of Siderophores
The characterization of the siderophore as catechol or hydroxamate types was carried out as follows:
Hydroxamate type of Siderophore (Tetrazolium salt test). 0.1 g of tetrazolium salt and 1–2 drops of 2 N NaOH was added to 0.1 mL supernatant of the test culture. Instant appearance of a red to deep-red color was indicative of presence of hydroxamate siderophores [4].
Catecholate type of Siderophore (Arnow’s Test). In this assay 1 mL of cell-free supernatant was mixed with 1 mL of 0.5 M HCl and 1 mL of nitrate molybdate resulted in yellow color. Further, 1 mL of 1 M NaOH was added, mixed and incubated for 5 min at room temperature, resulting in red color formation indicative of presence of catecholate siderophores. The color was stable for 1 h and the absorbance was measured at 510 nm using a UV-VIS spectrophotometer [16].
Detection of siderophores by Thin Layer Chromatography (TLC)
The culture supernatant of siderophore producer strain was spotted on 10 × 20 mm silica gel plates and allowed to dry. The plates were run in an n-butanol: acetic acid: distilled water (12:3:5) solvent system until the solvent front reached the top. Thereafter it was dried and 0.1 M FeCl3 (prepared in 0.1 N HCl) was sprayed. Appearance of a wine-colored spot indicated a hydroxamate-type siderophore, while that of a dark gray spot indicated catechol-type siderophore [7].
Optimization of Physicochemical Parameters for Siderophore Production
The biological production of siderophores is governed by several environmental factors like growth medium, temperature, pH, incubation time, carbon sources, nitrogen sources etc. In the present study, the optimization experiments were initiated by evaluating the optimum nutrient medium for siderophore production. The three types of nutrient media tested in the current study were Nutrient broth, JNFb– broth and Succinate broth. The siderophore production was monitored by using 50 mL medium each, separately inoculated with 0.25 mL of 24 h old culture, incubated at 37°C in shaker incubator (120 rpm for 24 h).
The optimization of other physicochemical parameters for production of siderophores was studied by varying one parameter at a time, while keeping the others constant. These varying parameters included, incubation time (24, 48, 72, 96, 120 h), temperature (25, 30, 35, 40°C), and pH (5, 6, 7, 8, 9, 10). In addition, the effect of 0.1% solution of different carbon sources (glucose, sucrose, fructose, lactose, mannitol) and nitrogen sources (urea, sodium nitrate, ammonium sulphate) were also studied on siderophore production. The bacterial isolates were inoculated in the succinate medium and the estimation was done on the above mentioned quantitative assay.
Pot Experiment
In having experimental microbial status under controlled settings, pot culture is a good supplement to field monitoring. The use of this method facilitates the transferability of experimental results to real-world situations. In order to evaluate the potential of selected isolates with coriander plant, a pot experiment was conducted in a growth chamber at the Department of Botany, Patna University, Bihar, India. The coriander seeds purchased from a local market (Bakarganj, Patna), were surface sterilized by exposing to 2–3% of NaOCl followed by 70% ethanol solution for 3 min followed by rinsing with autoclaved DW at least for three times. Sterilized seeds were soaked in autoclaved DW for 24 h at room temperature inside closed petri dishes. Further seeds were transferred in bacterial suspension (108 cfu mL–1) at 30°C for 6 h and sown in the pot having sterile soil (by autoclaving at 15 lbs/121°C for 3 h) to a depth of 5 mm as a test (inoculated seeds) and control (uninoculated seeds) [17]. Sterile water was used for maintaining moisture of soil in the pots as per requirements and observed for seed germination, root length and shoot length with respect to control. The germination percentage was calculated. After one week, seedling vigour was recorded in terms of root and shoot length with the help of a measuring scale. Fifteen days after sowing of seeds, plants were harvested, roots were washed free of soil and shoot and root lengths were measured. Each treatment was carried out in three replications. Germination percentage was determined by the following formula.
The entire plant was dried in an oven at 72°C for 48 h and fresh weight and dry weight were recorded as seedling growth parameter. Total biomass was calculated after deducting the dry weight from wet weight. The plants involved in our study comply with institutional guidelines.
Statistical Analysis
The data obtained were statistically analyzed by using software (SPSS 16.0).
RESULTS AND DISCUSSION
Sample Collection and Isolation of Rhizospheric Bacteria
Choice for soil sample was guided by the luxuriant growth of E. cynosuroides on roadside, which are practically zero chemical fertilizer zones. Seven isolates (DR1-DR7) appeared on solid NAM, which upon repeated sub culturing retained their growth and preserved on NAM slant at 4°C.
Qualitative Screening of Siderophore Production
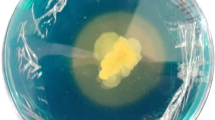
5 out of 7 isolates found positive for the siderophore production with formation of varying intensity of orange zones (13, 31, 16, 8 and 22 mm in DR1, DR2, DR4, DR6 and DR7 respectively) (Fig. 1). Among them the isolate DR2 showed highest siderophore zone (31 mm) on CAS agar plate. Similarly, Jabborova et al. [18] reported maximum siderophore zone by salinity tolerant B. subtilis 1 (15.8 mm), impacting on plant growth of wheat under saline soil.
Quantitative Assay for Siderophore Production
Among these 5 isolates, DR2 showed maximum siderophore production i.e. 69.81 SU% as compared to DR1 (27.51 SU%), DR4 (31.95 SU%), DR6 (15.67 SU%) and DR7 (53.49 SU%) (Fig. 1). Similarly, Kumar et al. [6] estimated 60.06% siderophore production by Bacillus sp. VITVK5 and 61.79% by Enterobacter sp. VITVK6 respectively. In another study, similar results were reported in B. subtilis CTS-G24 which produced 59 and 64% siderophore units in Nutrient and Succinate media, respectively [19]. Bagmare and Ismail [20] also reported Pseudomonas fluorescens produced maximum amount of siderophore (75%), followed by Azospirillum lipoferum (67%), Bacillus subtilis (62%), Pseudomonas striata (58%) and Bacillus megaterium (44%), which is identical to the outcome of our experiment. On the basis of optimum siderophore producing abilities, the isolate DR2 was selected for further optimization and pot experiment studies.
Phenotypic and Genotypic Characterization of Isolates
Among them, the promising isolate, namely DR2 (the highest Siderophore producing strain) appeared as Gram positive, rod having motility and the colony with creamy white, round, irregular margin. The isolate was also found positive for oxidase, citrate, ammonia, Voges-Proskauer reaction and starch hydrolysis. Based on their morphological, biochemical and phylogenetic analysis the isolate DR2 was identified as Bacillus subtilis DR2 (accession no. KP455653) (Fig. 2). Genus Bacillus is the most commonly reported PGPB with its well documented abilities of PGP properties like siderophore production, IAA production, nitrogen fixation, ACC deaminase synthesis and P-solubilization by numerous researchers [21, 22].
Characterization of Siderophores
Several workers across the globe reported several plant growth promoting rhizobacteria having ability to produce catecholates and hydroxamate type, siderophore [23, 24]. In our investigation, B. subtilis DR2 showed a strong positive reaction with formation of red color in the Arnow’s test and negative reaction in tetrazonium salt infers the presence of catechol-type siderophore (Fig. 3). Our results are also supported with the findings of Sinha et al. [25]. B. subtilis strain CAS15 produced the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine mind the gap here trimeric ester bacillibactin [26]. In similar way, Ferreira et al. [23] detected catechol type siderophores in B. subtilis and Rhizobium radiobacter, while hydroxamate-type siderophores in Pantoea allii and B. megaterium.
Optimization of Physicochemical Parameters for Siderophore Production
Optimization of culture media. Optimization of nutrient media prior to physicochemical parameters is essential for ensuring maximum microbial growth and hence, maximum siderophore production. For this three different media viz., nutrient broth, JNFb– and succinate medium were individually inoculated with the test strainB. subtilis DR2. Among the three different nutrient media tested, highest siderophore production was observed in succinate medium (71.51 SU%) without the addition of iron, while its productivity may be repressed due to presence of iron traces in nutrient broth (67.23 SU%) and JNFb- (59.31 SU%) broth media (Fig. 4). Similar study has been reported by Patil et al. [19], where maximum siderophore production (64 SU%) was found in succinate media.
Optimization of incubation time. There is variation in siderophore production at various time intervals and was found to be optimal after 72 h incubation (72.48 SU%). The isolate B. subtilis DR2 showed a gradual increase in siderophore production till 72 h, after which the production declined from 50.38 to 32.36% at 96 to 120 h, respectively of incubation as noted in our study (Fig. 5). Several workers have reported the siderophore production by Bacillus spp., under the range of 24 to 120 h of incubation [10, 23]. In accordance with this, optimum siderophore production at 72 h of incubation has been reported in Bacillus spp. IFM22 [27].
Optimization of temperature. Like incubation, microorganisms are also profoundly affected by temperature of their habitat as it influences their growth and metabolite secretion. The siderophore production varied with variation in temperatures and thus 35°C under shaking condition of 120 rpm (74.54 SU%) was observed for maximum production (Fig. 6). At low temperature, as the growth rate is slow hence, the siderophore production is also low. However, as the temperature increases up to optimal limit, the growth rate enhances, leading to production of more biomass, consequently higher amount of siderophore production takes place. Optimum production of siderophores have also been reported over a wide range of temperatures i.e., 25–45°C by Bacillus VITVK5, Bacillus VITVK6 and Enterobacter spp. [6].
Optimization of pH. pH plays a vital role in the solubility of iron in production media and thereby, siderophore production. In our study the maximum siderophore production (75.80%) was found at pH 8.0 (Fig. 7). A change in pH of culture medium affects both microbial growth and bioavailability of iron [28]. In the present work effect of pH clearly reflected that pH close to ambient condition (8) supported maximum growth and siderophore production. As per the report of Agro services international (Florida based soil fertility and plant nutrition laboratory) at a high pH value, insolubility of iron increases, which is in accordance with our finding [6]. At pH 8, iron becomes more insoluble in the soil solution and it might have stimulated the production of siderophore. In a similar experiment siderophore production increased at pH 7.5 and ranged between 18 to 30%, while at pH of 8.5, siderophore production was reported at peak, ranging from 30 to 60% by five PGPR (Bacillus cereus, Pseudoalteromonas tetraodonis, Micrococcus aloeverae, Psychrobacter pocilloporae, and Pseudomonas weihenstephanensis) [25].
Optimization of carbon source. Carbon source provides energy for growth and various metabolic activities of microorganism. Supplementation of growth media with various carbon sources increases the growth capacity of bacteria and therefore enhances siderophore production. Siderophore production of B. subtilis is influenced by the nature of the carbon source. The current study looked at the effect of various carbon sources on siderophore production, including sucrose, glucose, fructose, mannitol, and lactose. It was found that sucrose has the most profound effect, acting as the best inducer of B. subtilis DR2, because it produces the optimum siderophore (78.06 SU%) when compared to other carbon sources (Fig. 8). Other workers have also reported sucrose, as best inducer of siderophore production in Bacillus spp. VITVK5 as 83.17 SU% [6].
Optimization of nitrogen source. Nitrogen, as one of the most important nutritional factor, serves as the building block material of organisms, so is used as the basal component of medium. Hence, various organic and inorganic compounds were tested in media as a source of nitrogen for siderophore production. During the evaluation of different suitable nitrogen sources in culture media, sodium nitrate was appeared to be the best suited for siderophore production as 80.60 SU% by B. subtilis DR2. The other nitrogen sources, such as ammonium sulphate (60.32 SU%), potassium nitrate (43.86 SU%) and urea (21.67 SU%) gave lesser amount of siderophore production (Fig. 9). Similar results were reported in the Bacillus sp. VITVK5 (61.94%) and Enterobacter spp. VITVK6 (61.32%), where sodium nitrate was used as nitrogen source [6].
Pot studies. Pot experiments are cost efficient, easy to conduct and broadly applicable. Such pot culture is a simple and fast method to demonstrate that inoculation of rhizobacteria can increase the biomass and yield of any test plant. In our pot study, the results revealed that inoculation of Coriandrum sativum seeds with bacterial strain B. subtilis DR2 had a positive stimulatory effect on all the growth parameters, as compared to the control (Table 1). In the tested plant material % enhancements in shoot length, root length, and biomass were recorded as 47.65, 51.85, and 70.94, respectively (Fig. 10). Our finding is similar to Mishra et al. [29], who recorded that treatment with B. megaterium ISB28 produced the highest shoot length (16.07 cm) being at par with Bacillus aerophilus Cor-15 (15.20 cm), B. subtilis NRCSS-I (15.76 cm) and B. subtilis NRCSS-II (15.36 cm) with respect to control (12.20 cm) and root length varied from 12.05, 10.69, 11.25 and 11.13 cm with respect to control 8.64 cm respectively in Coriandrum sativum plant. The enhancement in growth parameters after inoculation with B. subtilis DR2 may be, due to total nutrient uptake, different PGP activities, such as siderophore production, IAA production, phosphate solubilization, nitrogen fixation, etc. [21]. These results corroborate with the previous finding, which reported that B. cereus ALT1 diminished Cd stress, strengthened antioxidant system and boosted growth in Soybean [30]. Recently, Lastochkina et al. [31] reported that a siderophore producing B. subtilis enhanced the growth of wheat. Such sort of extensive research work becomes necessary as it reflects that replacement of chemical fertilizers with PGPR as inoculants is a much effective and sustainable approach for plant growth promotion.
CONCLUSIONS
Iron is a vital element required by all living organisms for their numerous cellular activities. Under iron-deficit condition, PGPR produce low molecular weight siderophores to chelate iron (Fe3+) molecules from the environment for their survival and support for overall crop improvement. The isolate B. subtillis DR2 was found to be quite effective in promoting seed germination and seedling growth of Coriandrum sativum, in terms of enhanced root, shoot length and biomass production. Therefore, it is suggested that the use of this promising strain as a potent biofertilizer can be beneficial for coriander cultivation and other crops also. Its application in pot experiment favours integration of biological management for plant improvement. The reproducibility of the result needs to be further standardized, so that the bacterium could be recommended as biofertilizers.
REFERENCES
Kumar, A., Soni, R., Kanwar, S.S., and Pabbi, S., Stenotrophomonas: a versatile diazotrophic bacteria from the rhizospheric soils of Western Himalayas and development of its liquid biofertilizer formulation, Vegetos, 2019, vol. 32, pp. 103–109.
Freschet, G.T., Roumet, C., Comas, L.H., Weemstra, M., Bengough, A.G., Rewald, B., Bardgett, R.D., De Deyn, G.B., Johnson, D., Klimesova, J., and Lukac, M., Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs, New Phytol., 2021, vol. 232, no. 3, pp. 1123–1158. https://doi.org/10.1111/nph.17072
Nazir, N., Kamili, A.N., and Shah, D., Mechanism of plant growth promoting rhizobacteria (PGPR) in enhancing plant growth – A review, Int. J. Eng. Res. Manage. Technol., 2018, vol. 8, pp. 709–721.
Bholay, A.D., Jadhav, P.U., Borkhataria, B.V., and Dhalkari, M.V., Fluorescent Pseudomonas as plant growth promoting rhizobacteria and their siderophoregenesis, J. Pharm. Biol. Sci., 2012, vol. 1, pp. 27–32.
Kumar, P., Thakur, S., Dhingra, G.K., Singh, A., Pal, M.K., Harshvardhan, K., Dubey, R.C., and Maheshwari, D.K., Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant, Biocatal. Agric. Biotechnol., 2018, vol. 15, pp. 264–269.
Kumar, V., Menon, S., Agarwal, H., and Gopalakrishnan, D., Characterization and optimization of bacterium isolated from soil samples for the production of siderophores, Resour.-Effic. Technol., 2017, vol. 3, pp. 434–439.
Colombowala, A. and Aruna, K., Studies on optimization of siderophore production by Pseudomonas aeruginosa azar 11 isolated from aquatic soil and its antibacterial activity, Int. J. Pharm. Biol. Sci., 2018, vol. 8, pp. 714–731.
Ferreira, M.J., Silva, H., and Cunha, A., Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review, Pedosphere, 2019a, vol. 29, no. 4, pp. 409–420. https://doi.org/10.1016/S1002-0160(19)60810-6
Nithyapriya, S., Lalitha, S., Sayyed, R.Z., Reddy, M.S., Dailin, D.J., El Enshasy, H.A., Luh Suriani, N., and Herlambang, S., Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame, Sustainability, 2021, vol. 13, p. 5394. https://doi.org/10.3390/su13105394
Kumar, P., Dubey, R.C., and Maheshwari, D.K., Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens, Microbiol. Res., 2012, vol. 167, pp. 493–499. https://doi.org/10.1016/j.micres.2012.05.002
Hashem, A., Tabassum, B, and Abd_Allah, E.F., Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress, Saudi J. Biol. Sci., 2019, vol. 26, pp. 1291–1297.
Kashyap, B.K., Solanki, M.K., Pandey, A.K., Prabha, S., Kumar, P., and Kumari, B., Bacillus as plant growth promoting rhizobacteria (PGPR): a promising green agriculture technology, Plant Health Under Biotic Stress, Ansari, R., and Mahmood I., Eds., Springer-Verlag, 2019, pp. 219–236.
Muralidharan, B., Jagannathan, A., and Ramasamy, S., Investigation of antimicrobial activity and chemical constituents of Eragrostis cynosuroides by GC-MS, Res. J. Phar. Tech., 2016, vol. 9, pp. 267–271.
Wilson, L., Spices and flavoring crops: fruits and seeds, in Encyclopedia of Food and Health, Academic Press, 2016, pp. 73–83, https://doi.org/10.1016/B978-0-12-384947-2.00647-4
Schwyn, B. and Neilands, J.B., Universal chemical assay for the detection and determination of siderophores, Anal. Biochem., 1987. vol. 160, pp. 47–56.
Arnow, L.E., Colorimetric determination of the components of 3, 4-dihydroxy phenylalanine-tyrosine mixtures, J. Biol. Chem., 1937, vol. 118, pp. 531–37.
Kumar, P., Pahal, V., Gupta, A., Vadhan, R., Chandra, H., and Dubey, R.C., Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays, Sci. Rep., 2020, vol. 10, pp. 1–10.
Jabborova, D.P., Narimanov, A.A., Enakiev, Y.I., and Davranov, K.D., Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition, Bulg. J. Agric. Sci., 2020, vol. 26, pp. 744–747.
Patil, S., Bheemaraddi, M.C., Shivannavar, C.T., and Gaddad, S.M., Biocontrol activity of siderophore producing Bacillus subtilis CTS-G24 against wilt and dry root rot causing fungi in chickpea, IOSR J. Agric. Vet. Sci., 2014, vol. 7, p. 9.
Bagmare, R.R., Ismail, S., Ingole, A.J., and Bagmare, P.A., Siderophore production by plant growth promoting microorganisms, J. Pharm. Phytochem., 2019, vol. 8, pp. 1450–1452.
Yavarian, S., Jafari, P., Akbari, N., and Feizabadi, M.M., Selective screening and characterization of plant growth promoting bacteria for growth enhancement of tomato, Lycopersicon esculentum, Iran J. Microbiol., 2021, vol. 13, pp. 121–129.
Zhou, L., Song, C., Li, Z., and Kuipers, O.P., Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential, BMC Genomics, 2021, vol. 22, pp. 1–14.
Ferreira, C.M., Vilas-Boas, A., Sousa, C.A., Soares, H.M., and Soares, E.V., Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions, AMB Express, 2019b, vol. 9, pp. 1–12.
Ghazy, N., and El-Nahrawy, S., Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant, Arch. Microbiol., 2021, vol. 203, pp. 1195–1209.
Sinha, A.K., Parli Venkateswaran, B., Tripathy, S.C., Sarkar, A., and Prabhakaran, S., Effects of growth conditions on siderophore producing bacteria and siderophore production from Indian Ocean sector of Southern Ocean, J. Basic Microbiol., 2019, vol. 59, pp. 412–424.
Wang, X.Q., Zhao, D.L., Shen, L.L., Jing, C.L., and Zhang, C.S., Application and mechanisms of Bacillus subtilis in biological control of plant disease, in Role of Rhizospheric Microbes in Soil, Meena, V., Ed., Springer-Verlag, 2018, pp. 225–250.
Panda, S.H., Goli, J.K., Das, S., and Mohanty, N., Production, optimization and probiotic characterization of potential lactic acid bacteria producing siderophores, AIMS Microbiol., 2017, vol. 3, p. 88.
Gaonkar, T. and Bhosle, S., Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils, Chemosphere, 2013, vol. 93, pp. 1835–1843.
Mishra, B.K., Dubey, P.N., Aishwath, O.P., Kant, K.R., Sharma, Y.K., and Vishal, M.K., Effect of plant growth promoting rhizobacteria on coriander (Coriandrum sativum) growth and yield under semi-arid condition of India, Indian J. Agric. Sci., 2017, vol. 87, pp. 607–612.
Sahile, A.A., Khan, M.A., Hamayun, M., Imran, M., Kang, S.M., and Lee, I.J., Novel Bacillus cereus Strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress, Agronomy, 2021, vol. 11, p. 404.
Lastochkina, O., Garshina, D., and Pusenkova, L., Effect of endophytic Bacillus subtilis on drought stress tolerance of Triticum aestivum L. plants of Steppe Volga and Forest-Steppe West Siberian agroecological groups, 2nd International Conference Plants and Microbes: the Future of Biotechnology, Saratov, 2020, pp. 5–9.
ACKNOWLEDGMENTS
Authors are thankful to Department of Botany, Patna University, Patna, for providing the laboratory facilities to carry out the present investigation. The authors are also thankful to Xcelris, India for the molecular confirmation of our isolates by 16S rRNA gene sequencing.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AS designed the research; SK (First author) conducted the experiment; SK (1st), SK (2nd and 3rd), PK and AS analyzed data and wrote the paper. All authors have read and approved the final manuscript.
About this article
Cite this article
Kumari, S., Kumar, P., Kiran, S. et al. Optimization of Siderophore Production by Bacillus subtilis DR2 and Its Effect on Growth Promotion of Coriandrum sativum. Russ. Agricult. Sci. 48, 467–475 (2022). https://doi.org/10.3103/S1068367422060076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367422060076