Abstract
Siderophores are low-molecular-weight ligands produced by bacteria, fungi and plants to solubilize and take up iron. Iron is essential for the growth and development of almost all living organisms and acts as a catalyst in some of the most fundamental enzymatic processes, including oxygen metabolism, electron transfer and deoxyribonucleic acid (DNA)/ribonucleic acid (RNA) synthesis. However, despite its abundance on earth, iron is biologically unavailable in most environments as it aggregates into insoluble oxy-hydroxide polymers. Hence, to acquire this iron from natural ecosystems, bacteria have evolved multiple parallel pathways, the most important being siderophore production.
This study was aimed at studying the incidence of siderophore-producing bacteria in the two coastal ecosystems of Goa: sand dunes and mangroves. The two isolates TMR2.13 and NAR38.1 selected for the study were identified as Pseudomonas aeruginosa and Bacillus amyloliquefaciens, respectively. Sodium benzoate was found to have a remarkable effect on siderophore production in P. aeruginosa TMR2.13 and increased iron demand of the organism as compared to the metabolism of simple substrates such as glucose. Studies were further carried out with B. amyloliquefaciens NAR38.1 to understand the effect of the presence of both biotic and abiotic metals in the growth medium. The presence of metals showed a varied effect on growth and siderophore production with increase in production of siderophores in the presence of zinc, cobalt, manganese, lead and aluminium which can be exploited in phytoremediation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
2.1.1 Need for Iron
Iron is essential for the growth and development of almost all living organisms. It acts as a catalyst in some of the most fundamental enzymatic processes, including oxygen metabolism, electron transfer and deoxyribonucleic acid (DNA)/ribonucleic acid (RNA) synthesis (Guan et al. 2001) . However, despite its abundance on earth and the micromolar concentrations required for microbial growth, iron is biologically unavailable in most environments. In aerobic inorganic environments, iron is present in the oxidized ferric form Fe+3, which aggregates into insoluble oxy-hydroxide polymers (Wandersman and Delepelaire 2004) .
The most obvious effect of iron deficiency on microbial cells is the decrease in quantity of cellular biomass. Iron deficiency has been reported to induce changes in activities of certain enzymes. For example, iron deficiency has been reported to result in 24-fold stimulation of NADase activity and is known to decrease the concentrations of cytochromes, peroxidase and catalase. Such a decrease in cytochrome concentration has been reported in Torula utilis; decrease in peroxidase, catalase and oxidase activity has been reported in Candida guilliermondii. Nicotinamide adenine dinucleotide (NADH) dehydrogenase is a membrane-bound enzyme with iron-sulfur centres which brings about NADH oxidation in the terminal electron transport systems of aerobes. Its enzyme activity and polypeptide chain composition have been found to be affected by iron limitation. Aconitase activity has also been found to diminish due to iron deficiency as opposed to aldolase whose activity is found to increase. Diminution in alcohol dehydrogenase activity has been observed in C. guilliermondii with decrease in iron concentration in the growth medium. Iron exerts an effect on enzymes involved in the biosynthesis and degradation of iron-chelating agents, siderophores (Light and Clegg 1974) .
Besides affecting enzyme activity, iron deficiency results in morphological changes in microorganisms. In Mycobacterium smegmatis, an increase in cell length has been observed as compared with the cells grown in iron-sufficient medium. This could be due to inhibition of DNA synthesis without proportional inhibition of cell growth or due to a direct effect on cell division or cell separation (Light and Clegg 1974) . Hence, to acquire this iron from natural ecosystems, bacteria have evolved multiple parallel pathways, the most important of these is, siderophore production.
2.1.2 Siderophores
Siderophores are low-molecular-weight ligands (20–2000 Da) produced by bacteria, fungi and plants to solubilize and take up iron (Chu et al. 2010; Hider and Kong 2010) . Siderophores chelate iron with an affinity constant of almost 1030M− 1. More than 500 different siderophores mostly from bacteria have been described. The iron ligation groups have been tentatively classified into three main chemical types: hydroxamate, catecholate and hydroxycarboxylates; however other varieties of siderophore structures have been resolved which include oxazoline, thiazoline, hydroxypyridinone, α- and β-hydroxy acids and α–keto acid components. The most important property of siderophores is their denticity (number of iron coordinating atoms per molecule) which ranges from bidentate to hexadentate (Raymond and Dertz 2004) . The peptide backbone of siderophores is usually made of several nonprotein amino acid analogs including both modified and D-amino acids. Some bacteria produce one type of siderophore while many produce multiple types of siderophores-permitting microorganisms to grow in different environments.
2.1.3 Marine Siderophores
Marine bacteria are known to require micromolar concentration of iron. However, iron concentration in the surface waters of the oceans is only 0.01–2 nM (Sandy and Butler 2009) . Therefore, many of the marine bacteria belonging to alpha and gamma proteobacteria produce siderophores. Marine siderophores can be structurally categorized into two major groups: (1) Siderophores that are produced as suites of amphiphiles and differ in the chain length of a fatty acid appendage (Homann et al. 2009; Ito and Butler 2005; Martin et al. 2006; Martinez et al. 2003) and (2) siderophores that are produced with an α-hydroxy carboxylic acid moiety, which is photoreactive when coordinated with Fe+3 (Barbeau 2006; Barbeau et al. 2001, 2002, 2003; Kupper et al. 2006; Hickford et al. 2004) . The aquachelins, marinobactins, ochrobactins and synechobactins coordinate Fe+3 by both oxygen atoms of each hydroxamate group and both oxygen atoms of the α-hydroxy carboxylate group. The amphibactins coordinate Fe+3 with the three hydroxamate groups. The alterobactins and pseudoalterobactins coordinate Fe+3 via the two β-hydroxy aspartate moieties and one catecholate group, whereas petrobactin and petrobactin sulfonate coordinate Fe+3 with the two catecholates and the α-hydroxy acid portion of the citrate backbone.
The most characteristic and distinguishing feature of marine siderophores is their photoreactivity (Barbeau et al. 2001) . Siderophores that contain α-hydroxycarboxylate moiety when in complex with Fe+3 undergo oxidation and Fe+3 is reduced to Fe+2. Fatty acid-containing marine siderophores have the important property of amphiphilicity, the degree of which varies in marine siderophores. Such variations in amphiphilicity arise due to the differences in the head-group composition relative to the fatty acid chain length. Amphibactins and ochrobactins are highly hydrophobic and are extracted from the bacterial pellet (Martin et al. 2006; Martinez et al. 2003) whereas aquachelins are isolated from aqueous supernatant which indicates their hydrophilic nature. Synechobactins are isolated from the supernatant as well as the pellet (Ito and Butler 2005) . Hydrophobic amphibactins have smaller peptide portion consisting of four amino acids while fatty acid chains are longer. Marinobactins and aquachelins have longer peptide chain consisting of 6–7 amino acids and a shorter fatty acid chain. The ochrobactins are also quite hydrophobic as a result of two fatty acid appendages, and a small head group, whereas the synechobactins, with a similarly small head group, are less hydrophobic than the ochrobactins as they have only one fatty acid (Vraspir and Butler 2008) .
Other marine organisms that have been studied for siderophore production are: Marinobacter hydrocarbonoclasticus (petrobactin), M. aquaeolei (petrobactin sulfonate(s)), Aeromonas hydrophila (amonabactins) and fish pathogens such as Vibrio anguillarum, which produces vanchrobactin and anguibactin. Petrobactin produced by M. aquaeolei is different from the petrobactin produced by B. anthracis in that it is a mono or di sulphonated derivative. Sulphonation of the catechol group has also been observed in pseudoalterobactin which is structurally related to alterobactin (Sandy and Butler 2009) .
2.2 Factors Affecting Siderophore Production
2.2.1 Iron Concentration
Iron concentration is the most important factor which regulates siderophore production, since they are produced under iron-limiting conditions (Budzikiewicz 1993; Vasil and Ochsner 1999; Visca et al. 1993) . Iron availability depends upon the environment in which the microorganism is growing. In an intensively aerated liquid medium, iron exists mainly in the form of Fe+3 which is extremely insoluble at neutral pH. Thus, the increase of oxygen pressure in the culture broth can reduce iron availability (Kim et al. 2003) . With laboratory culture media, the amount of siderophores produced by iron-sufficient growth can be as little as 0.1 % of that produced under iron-deficient conditions. It is therefore necessary to remove the traces of iron from the culture medium for a successful production of siderophores (Messenger and Ratledge 1985) .
The influence of iron on siderophore production has been studied by many authors (Braud et al. 2006; Ochsner et al. 2002) . Studies carried out with different Pseudomonas strains confirm that siderophore production is associated with iron concentration in the medium. Siderophore production is suppressed at Fe+3 concentration of 100 μM in Pseudomonas fluorescens (VTE94558), P. fluorescens (VTT/ ELT 116) and Pseudomonas chlororaphis (VTT-E-94557) (Laine et al. 1996) . Iron concentrations of about 10 μM are considered high enough and generally result in excellent cell-mass accumulation with only modest yields of siderophores (Neilands 1984) ; even so, P. fluorescens 94 produces siderophores at an iron concentration of 50 μM (Manninen and Mattila-Sandholm 1994) . On the other hand, siderophore production in Pseudomonas syringae B30ID is gradually repressed at Fe+3 concentrations from 1 to 10 μM (Bultreys and Gheysen 2000) . Villegas et al. (2002) have observed that cell growth of Pseudomonas aeruginosa PSS reaches a maximum at 10 μM Fe+3, however, the biosynthesis of siderophores is lowered at this concentration, since cell growth and siderophore production are inversely proportional under such conditions. P. aeruginosa NCCB 2452 and ATCC 15692 produce the siderophores pyoverdine and pyochelin. The concentration of these was found to increase in iron-limited culture, although the level of pyochelin was ten times lower than that of pyoverdine (Kim et al. 2003) .
2.2.2 Nature of Nitrogen and Carbon Source
Nitrogen is one of the major components of important cellular elements including proteins, nucleic acid and cell wall. Amino acids are particularly good sources of nitrogen, generally inducing an increased growth rate. Glutamic acid as the sole source of carbon and nitrogen has been reported to improve the production of siderophores (Casida 1992) . Villegas et al. (2002) have studied siderophore production by P. aeruginosa PSS in a glutamic minimum medium with glutamic acid as the sole carbon source and siderophore production as high as 140 μM has been obtained. Different nitrogen sources have been reported for siderophore production including the supplements of other amino acids (Albesa et al. 1985) . Bultreys and Gheysen (2000) have reported high siderophore production in strains of P. syringae with all the 20 amino acids when used as the sole source of both carbon and nitrogen.
Another amino acid commonly present in such media is asparagine, which is used as carbon and nitrogen source by almost all fluorescent Pseudomonas (Palleroni 1984) . Solid and liquid culture media containing asparagine are reported to be highly effective for the induced production of siderophores by strains of P. syringae (Bultreys and Gheysen 2000) . This amino acid is usually combined with sucrose in the medium known as sucrose-asparagine (SA) for the production of siderophores in Pseudomonas (Laine et al. 1996; Morris et al. 1992) .
Siderophore production can also be achieved with several organic substrates (Meyer and Abdallah 1978) . Glycerol used as the carbon source in different media (Nowak-Thompson and Gould 1994) , for example, the King’s medium B (King et al. 1954) , stimulates pyoverdine production. Duffy and Defago (1999) have reported increased pyochelin production in P. fluorescens CHAO with glucose but not with glycerol. However, salicylic acid production was found to increase significantly with glycerol. Use of sodium succinate has also been reported for increase in siderophore production (Boruah and Kumar 2002; Meyer and Abdallah 1978; Sharma and Johri 2003) .
2.2.3 Metal Ions
Metals other than iron also stimulate or inhibit siderophore production in a number of bacteria, even in the presence of high iron concentrations . For example, Duhme et al. (1998) have studied the effect of molybdenum on siderophore production by Azotobacter vinelandii and found that at concentrations upto 100 mM of molybdenum, azotochelin production is activated, whereas at higher metal concentrations the synthesis of the siderophore is completely repressed. Reports also show that high concentrations of aluminium increase the production of schizokinen and N-deoxyschizokinen (two hydroxamate siderophores) in iron-limited cultures, but not in iron-rich cultures of Bacillus megaterium (Hu and Boyer 1996) . Pyoverdine production is induced by 10 mM aluminium, copper, gallium, manganese and nickel when grown in the iron-limited succinate medium (Braud et al. 2009a) . Teitzel et al. (2006) have made an interesting observation wherein under iron-limited conditions, exposure to 10 mM copper upregulates genes involved in the synthesis of pyoverdine and downregulates those involved in the synthesis of pyochelin. Increase in pyoverdine even in the presence of iron (100 mM) has been reported by Braud et al. (2010) when 10 and 100 mM copper and nickel (290 and 380 %, respectively) are added in growth medium. In all these experiments, metals were added at the beginning of the cultures. Most of these studies have been carried out with only a single or in some cases two metal concentrations and not for a large range of concentrations . It is probable that as iron concentration regulates siderophore production, it can be both activated and inhibited by many of these metals, depending on their concentrations. Therefore, studies involving varied concentrations of each metal are necessary to establish their exact roles in the regulation of siderophore production.
It is not clear how metals other than iron stimulate siderophore production. One possible explanation is that the free siderophore concentration in the medium is reduced in the presence of other metals as a result of complex formation. Such a decrease in the siderophore concentration may be sufficient to activate further secretion of additional siderophore into the medium. Induction of siderophore production by heavy metals indicates their role in bacterial heavy metal tolerance. Toxic metals enter the periplasm of Gram-negative bacteria mostly by diffusion across the porins (Li et al. 1997; Lutkenhaus 1977; Pugsley and Schnaitman 1978) . The binding of metals to siderophores in the extracellular medium reduces the free metal concentration, affecting diffusion (the molecular mass of the resulting siderophore–metal complex is too great for diffusion via porins) and therefore their toxicity. Growth assays have also shown that P. aeruginosa strains capable of producing pyoverdine and pyochelin are more resistant to metal toxicity than a siderophore nonproducing strain (Braud et al. 2010) .
The presence of siderophores is apparently highly beneficial for bacteria in an environment contaminated with toxic metals and bacteria able to produce siderophores are more resistant to heavy metals than those not producing siderophores. Though siderophores provide an extracellular protection for bacteria by sequestering heavy metals outside the bacteria and avoiding their diffusion through porins into the bacteria, however, it is possible that siderophore production in response to heavy metal exposure can also have detrimental effects. If the siderophore uptake receptor does not distinguish between the metal–siderophore and the ferri–siderophore complexes, siderophores may provide a secondary mechanism for the uptake of toxic metals. Therefore, the ferri–siderophore pathways must have high metal specificity to transport or accumulate only the appropriate metal(s).
2.3 Occurrence of Siderophore-Producing Bacteria in Coastal Ecosystems of Goa
During our study on siderophore-producing bacteria from coastal ecosystems of Goa, such bacteria were isolated from two distinct ecosystems, one, a low-nutrient sand dune ecosystem and the other a nutrient-rich mangrove ecosystem. Sand dunes represent large amounts of shifting sand barren to plants. Sand dunes are largely categorized into two types. The first kinds are extremely dry interior deserts such as Sahara in Africa or Rajasthan in India. Coastal sand dunes occur along the coasts of the Atlantic, Pacific, North America and Australia. In Asia, coastal sand dunes occur in Japan, India and several other countries (Boorman 1977; Carter 1998; Desai and Untawale 2002) . Vegetation plays an important role in determining size, shape and stability of the dunes. Dead plants and humus from sand dune vegetation adds humus to the sand. Furthermore, microorganisms growing in these sand dunes help reduce the nutrient stress.
Mangroves are highly reproductive ecosystems which host a wide range of coastal and off-shore marine organisms. Mangroves provide a unique ecological environment for diverse bacterial communities (Ramanathan et al. 2008) . Tidal variations, salinity and intense human activities such as transportation through ships, barges and accidental spills result in diverse microflora in the marine coastal ecosystem (Rawte et al. 2002) . Bacteria largely influence nutrient cycling in mangroves and thus contribute to soil and vegetation patterns (Hossain et al. 2012) . Environments wherein microorganisms grow are highly variable with respect to temperature, pH, redox potential, osmotic pressure, water activity, salinity and general and essential nutrients. Previous studies have shown the presence of a large number of bacteria in these two ecosystems (Godinho and Bhosle 2002) .
During our study, 13 siderophore positive isolates from sand dunes and mangrove which showed the optimum siderophore production were subjected to routine biochemical tests and 16S rDNA sequencing for tentative identification. Of the thirteen isolates selected, ten belonged to the Bacillus spp, two were Streptomyces spp. and one was identified as P. aeruginosa (Gaonkar et al. 2012) .
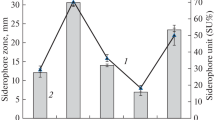
Amongst the many siderophore-producing organisms, the two isolates TMR2.13 (P. aeruginosa) (Fig. 2.1a, b, c) and NAR38.1 (Bacillus amyloliquefaciens) (Fig. 2.2a, b, c) were selected to study various aspects of siderophore production. Functional group tests proved that the siderophores produced by TMR2.13 and NAR38.1 were of carboxamate and catecholate type, respectively.
2.3.1 Effect of Iron on Siderophore Production
Iron is one of the most important micronutrients required by bacteria for their metabolism as a cofactor for a large number of enzymes and iron-containing proteins (Harrington and Crumbliss 2009) . However, its availability in the environment may not be sufficient to support microbial growth. Studies with NAR38.1 showed the production of siderophore up to 1 µM of Fe+2 and up to 30 µM of Fe+3, suggesting that higher levels of Fe+3 are required to suppress siderophore production as compared to the Fe+2. This could possibly be because of the well known fact that Fe3+ form is not the highly soluble form of iron as opposed to Fe2+ ion which has a much better solubility (Chu et al. 2010) . However, the effect of iron with P. aeruginosa TMR2.13 showed a significant siderophore production up to 54 µM which was suppressed at 108 µM irrespective of it being divalent or trivalent. The fact that pyoverdine binds to both the forms of Fe, as has been reported earlier (Xiao and Kisaalita 1998), results in the suppression of siderophore production with the same concentration of Fe+2 and Fe+3 .
2.3.2 Effect of Sodium Benzoate on Siderophore Production
Pseudomonas is known to be versatile in its ability to metabolize various organic compounds. A number of natural and anthropogenic compounds are known to be degraded, transformed or cometabolized by organisms belonging to this genus (Zeyaullah et al. 2009) . Many of the reactions involve the enzyme oxygenase which is the key enzyme involved in the opening of the aromatic ring structure.
Oxygenases are enzymes which catalyse the incorporation of molecular oxygen into various substrates. The cofactors involved in oxygenase reactions are heme, nonheme iron, copper, flavin etc. However, the most frequent one is iron. Nearly 100 oxygenases are known till date, of which almost 50 % have iron built into their structure or require iron for their activity (Nozaki and Ishimura 1974) . When a benzene ring is cleaved by a dioxygenase reaction, hydroxylation of the benzene ring proceeds with the formation of catechol or phenol derivatives (Song et al. 2000) . Catechol derivatives are further cleaved by dioxygenases which are further divided into intradiol and extradiol. Intradiol is red in colour and contains trivalent iron while extradiol enzymes are colourless containing divalent iron (Nozaki and Ishimura 1974) . Hence, metabolism of aromatic compounds is expected to impose a specific iron requirement on cells due to the involvement of oxygenases. An attempt was therefore made in this study to understand the effect of the aromatic compound, sodium benzoate , on siderophore production in the organisms’ capability of utilizing sodium benzoate as the sole source of carbon.
Sodium benzoate was found to have a remarkable effect on siderophore production in P. aeruginosa TMR2.13. It was noted that while the production of siderophore was inhibited above 54 μM of added iron in Mineral salts medium (MSM) with glucose without affecting growth, in the presence of sodium benzoate, siderophore was produced even up to the presence of 108 μM of added iron (Gaonkar et al. 2012) .
A report on the effect of trace element requirement has shown that the iron demand in bacteria increases during the expression of alkane hydroxylase (Staijen and Witholt 1998) . A study on the effect of iron concentration on degradation of toluene by Pseudomonas strain reports a reduction in the efficiency of the culture when the iron concentration is low (Dinkla et al. 2001) . Observations from this study also suggest the requirement of a higher concentration of iron to sustain growth in a benzoate medium.
2.3.3 Effect of Metal Ions on Siderophore Production
Studies further carried out with B. amyloliquefaciens NAR38.1 to understand the effect of the presence of both biotic and abiotic metals in the growth medium, depicted following four responses (Gaonkar and Bhosle 2013) :
-
1.
In the presence of zinc, siderophore production increased, but no effect was seen on the growth while the presence of cobalt and manganese increased siderophore production with decrease in growth .
-
2.
In the presence of molybdenum and arsenic, siderophore production decreased, but there was no effect on growth.
-
3.
Decrease in siderophore production at lower concentrations but increase at higher concentration without affecting growth was manifested in the presence of lead and aluminium.
-
4.
Decrease in growth as well as siderophore production was brought about by the presence of cadmium and copper.
Such effects indicate that the potential of the organism to combat metal toxicity varies with the type of metal it is in contact with. Although bacteria are known to possess different mechanisms to alleviate the effects of toxic metals, the concentration of the metal plays an important role in the manifestation of such mechanisms. Production of siderophores in the presence of metals can be a useful trait for plant growth promoting organisms as the soils which are contaminated with metals are often iron deficient (Tank and Saraf 2009) . In fact, siderophore-producing bacteria have been considered important for inducing metal tolerance in plants and for promotion of metal accumulation in plants, especially in the phyto-extraction technology for remediation of metal-contaminated soils (Ahmed and Holmstrom 2014) . The effects of siderophore-producing bacteria on the uptake of metals by hyper-accumulator plants have been the focus of increased attention (Dimkpa et al. 2008; Braud et al. 2009b) . Braud et al. (2009b) have reported an increase in the bioavailability of Chromium and Lead in soils inoculated with P. aeruginosa. Bacterial siderophores can also provide iron to various plants, which helps in reducing the metal toxicity (Bar-Ness et al. 1991; Hussain and Joo 2014; Reid et al. 1986; Wang et al. 1993) . Such beneficial effects exhibited by siderophore-producing bacteria implicate that the inoculation with metal-resistant siderophore-producing bacteria may help in improving the process of phyto-extraction in metal-contaminated soils.
2.4 Conclusions and Future Prospects
This study has offered an insight into the response of siderophore-producing bacteria to the aromatic compound sodium benzoate and to metal ions. The present work can be extended to natural ecosystems exposed to the influx of such pollutants. Inoculation with metal-resistant siderophore-producing bacteria may help in improving the process of phyto-extraction in metal-contaminated soils.
Moreover, it would also be interesting to probe deeper into the results of the present studies and study effect of iron limitation on the enzymes involved in utilization of sodium benzoate in P. aeruginosa TMR2.13 and the role of siderophores in metal resistance in B. amyloliquefaciens NAR38.1.
References
Ahmed, E., & Holmstrom, S. J. M. (2014). Siderophores in environmental research: Roles and applications. Microbial Biotechnology, 7, 196–208.
Albesa, I., Barberes, L. I., Pajaro, M. C., & Craso, A. J. (1985). Pyoverdine production by Pseudomonas fluorescens in synthetic media with various sources of nitrogen. Journal of General Microbiology, 131, 3251–3254.
Barbeau, K. (2006). Photochemistry of organic iron (III) complexing ligands in oceanic systems. Photochemistry and Photobiology, 82, 1505–1506.
Barbeau, K., Rue, E. L., Bruland, K. W., & Butler, A. (2001). Photochemical cycling of iron in the surface ocean mediated by microbial iron (iii)-binding ligands. Nature, 413, 409–413.
Barbeau, K., Zhang, G., Live, D. H., & Butler, A. (2002). Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. Journal of the American Chemical Society, 124, 378–379.
Barbeau, K., Rue, E. L., Trick, C. G., Bruland, K. T., & Butler, A. (2003). Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnology and Oceanography, 48, 1069–1078.
Bar-Ness, E., Chen, Y., Hadar, Y., Marchner, H., & Romheld, V. (1991). Siderophores of Pseudomonas putida as an iron source for dicot and monocot plants. Plant and Soil, 130, 231–241.
Boorman, L. (1977). Sanddunes. In K. S. R. Barnes (Ed.), The coastline (pp. 161–197). New York: Wiley.
Boruah, H. P. D., & Kumar, B. S. D. (2002). Biological activity of secondary metabolites produced by strain of Pseudomonas fluorescens. Folia Microbiologica, 47, 359–363.
Braud, A., Jezequel, K., Leger, M. A., & Lebeau, T. (2006). Siderophore production by using free and immobilized cells of two pseudomonads cultivated in a medium enriched with Fe and/ or toxic metals (Cr, Hg, Pb). Biotechnology and Bioengineering, 94, 1080–1088.
Braud, A., Hoegy, F., Jezequel, K., Lebeau, T., & Schalk, I. J. (2009a). New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environmental Microbiology, 11, 1079–1091.
Braud, A., Jézéquel, K., Bazot, S., & Lebeau, T. (2009b). Enhanced phytoextraction of an agricultural Cr-, Hg- and Pb-contaminated soil by bioaugmentation with siderophore producing bacteria. Chemosphere, 74, 280–286.
Braud, A., Geoffroy, V., Hoegy, F., Mislin, G. L. A., & Schalk, I. J. (2010). The siderophores pyoverdine and pyochelin are involved inPseudomonas aeruginosa resistence against metals: Another biological function of these two siderophores. Environmental Microbiology Reports, 2, 419–425.
Budzikiewicz, H. (1993). Secondary metabolites from fluorescent pseudomonads. FEMS Microbiology Reviews, 104, 209–228.
Bultreys, A., & Gheysen, D. (2000). Production and comparison of peptide siderophores from strains of distantly related pathovars of Pseudomonas syringae and Pseudomonas viridiflava LMG2352. Applied Environmental Microbiology, 66, 325–331.
Carter, R. W. G. (1998). “Coastal dunes”. Coastal environments: An introduction to the physical, ecological and cultural ecosystems of coastlines. New York: Academic press.
Casida, L. E. Jr (1992). Competitive ability and survival in soil of Pseudomonas strain 679-2a dominant, nonobligate bacterial predator of bacteria. Applied Environmental Microbiology, 58, 32–37.
Chu, B. C., Garcia-Herrero, A., Johanson, T. H., Krewulak, K. D., Lau, C. K., Peacock, R. S., et al. (2010). Siderophore uptake in bacteria and the battle for iron with the host; a bird's eyeview. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine, 23, 601–611.
Desai, K. N., & Untawale, A. G. (2002). Sand dune vegetation of Goa; conservation and management. Botanical Society of Goa, Goa India.
Dimkpa, C. O., Svatoš, A., Dabrowska, P., Schmidt, A., Boland, W., & Kothe, E. (2008). Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomycesspp. Chemosphere, 74, 19–25.
Dinkla, I. J. T., Gabor, E. M., & Janssen, D. B. (2001). Effects of iron limitation on the degradation of Toluene by Pseudomonas Strains carrying the TOL(pWWO) plasmid. Applied Environmental Microbiology, 67, 3406–3412.
Duffy, B. K., & Defago, G. (1999). Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Applied Environmental Microbiology, 65, 2429–2438.
Duhme, R. C., Hider, M. J., Naldrett, M. J., & Pau, R. N. (1998). The stability of the molybdenum-azotochelin complex and its effect on siderophore production in Azotobacter vinelandii. Journal of Biological Inorganic Chemistry, 3, 520–526.
Gaonkar, T., & Bhosle, S. (2013). Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere, 93, 1835–1843.
Gaonkar, T., Nayak, P., Garg, S., & Bhosle, S. (2012). Siderophore-producing bacteria from a sand dune ecosystem and the effect of sodium benzoate on siderophore production by a potential isolate. The Scientific World Journal, 2012, 1–8.
Godinho, A., & Bhosle, S. (2002). Diazotrophic bacteria associated with coastal sand dune vegetation. National conference of coastal agriculture research. 6–7 April. ICAR Research Complex for Goa.
Guan, L. L., Kanoh, K., & Kamino, K. (2001). Effect of exogenous siderophores on iron uptake activity of marine bacteria under iron-limited conditions. Applied Environmental Microbiology, 67, 1710–1717.
Harrington, J. M., & Crumbliss, A. L. (2009). The redox hypothesis in siderophore-mediated iron uptake. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine, 22, 679–689.
Hickford, S. J. H., Kuepper, F. C., Zhang, G., Carrano, C. J., Blunt, J. W., & Butler, A. (2004). Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. Journal of Natural Products, 67, 1897–1899.
Hider, R. C., & Kong, X. (2010). Chemistry and biology of siderophores. Natural Product Reports, 27, 637–657.
Homann, V. V., Sandy, M., Tincu, J. A., Templeton, A. S., Tebo, B. M., & Butler, A. (2009). Loihichelins A-F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. Journal of Natural Products, 72, 884–888.
Hossain, H. Z., Aziz, C. B., & Saha, M. L. (2012). Relationships between soil physic chemical properties and total viable bacterial counts in Sunderban mangrove forests, Bangladesh. Dhaka Univ. Journal of Biological Sciences, 21, 169–175.
Hu, X., & Boyer, G. L. (1996). Siderophore-mediated aluminum uptake by Bacillus megaterium ATCC 19213. Applied and Environmental Microbiology, 62, 4044–4048.
Hussain, K. A., & Joo, J. H. (2014). Potential of siderophore production by bacteria isolated from heavy metal: Polluted and rhizhosphere soils. Current Microbiology, 6, 717–723.
Ito, Y., & Butler, A. (2005). Structure of synechobactins, new siderophores of the marine Cyanobacterium Synechococcus sp. PCC 7002. Limnology and Oceanography, 50, 1918–1923.
Kim, E. J., Sabra, W., & Zeng, A. P. (2003). Iron deficiency leads to inhibition of oxygen transfer and enhanced formation of virulence factors in cultures of Pseudomonas aeruginosa PAO1. Microbiology (Reading, England), 149, 2627–2634.
King, E. O., Ward, M. K., & Raney, D. E. (1954). Two simple media for the demostration of pyocyanin and fluorescin. Journal of Laboratory and Clinical Medicine, 44, 301–307.
Kupper, F. C., Carrano, C. J., Kuhn, J. U., & Butler, A. (2006). Photoreactivity of iron(III)-aerobactin: Photoproduct structure and iron(III) coordination. Inorganic Chemistry, 45, 6028–6033.
Laine, M. H., Karwoski, M. T., Raaska, L., & Mattila-Sandholm, T. (1996). Antimicrobial activity of Pseudomonas spp. against food poisoning bacteria and moulds. Letters in Applied Microbiology, 22, 214–218.
Li, X. Z., Nikaido, H., & Williams, K. E. (1997). Silver resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. Journal of Bacteriology, 179, 6127–6132.
Light, P. A., & Clegg, R. A. (1974). Metabolism in iron limited growth. In J. B. Neilands (Ed.), Microbial iron metabolism (1st ed., pp. 35–61). New York: Academic Press.
Lutkenhaus, J. F. (1977). Role of a major outer membrane protein in Escherichia coli. Journal of Bacteriology, 131, 631–637.
Manninen, E., & Mattila-Sandholm, T. (1994). Methods for the detection of Pseudomonas siderophores. Journal of Microbiological Methods, 19, 223–234.
Martin, J. D., Ito, Y., Homann, V. V., Haygood, M. G., & Butler, A. (2006). Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. Journal of Biological Inorganic Chemistry, 11, 633–641.
Martinez, J. S., Carter-Franklin, J. N., Mann, E. L., Martin, J. D., Haygood, M. G., & Butler, A. (2003). Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proceedings of the National Academy of Sciences of United States of America, 100, 3754–3759.
Messenger, A. J. M., & Ratledge, C. (1985). Siderophores. –In M. Moo-Youg (Ed.), Comprehensive biotechnology (Vol. 3, pp. 275–294). Pergamon Press, New York.
Meyer, J. M., & Abdallah, M. A. (1978). The fluorescent pigment of Pseudomonas fluorescens. Biosynthesis, purification and physicochemical properties. Journal of General Microbiology, 107, 319–328.
Morris, J., O’Sullivan, D. J., Koster, M., Leong, J., Weisbeek, P. J., & O’Gara, F. (1992). Characterization of fluorescent siderophore-mediated iron uptake in Pseudomonas sp.strain M114: Evidence for the existence of an additional ferric siderophore receptor. Applied and Environmental Microbiology, 58, 630–635.
Neilands, J. B. (1984). Siderophores of bacteria and fungi. Microbiological Sciences, 1, 9–14.
Nowak-Thompson, B., & Gould, S. J. (1994). A simple assay for fluorescent siderophores produced by Pseudomonas species and an efficient isolation of pseudobactin. BioMetals, 7, 20–24.
Nozaki, M., & Ishimura, Y. (1974). Oxygenases. In J. B. Neilands (Ed.), Microbial iron metabolism (1st ed., pp. 417–441). New York: Academic Press.
Ochsner, U. A., Wilderman, P. J., Vasil, A. I., & Vasil, M. L. (2002). Gene ChipR expression analysis of the iron starvation response in Pseudomonas aeruginosa: Identification of novel pyoverdine biosynthesis genes. Molecular Microbiology, 45, 1277–1287.
Palleroni, N. J. (1984). Family I Pseudomonadaceae. In N.R. Krieg, J.G. Holt (Eds.), Bergey’s manual of systematic bacteriology (Vol. 1, pp. 141–199). Baltimore: The Williams and Wilkins.
Pugsley, A. P., & Schnaitman, C. A. (1978). Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. Journal of Bacteriology, 133, 1181–1189.
Ramanathan, A. L., G, Singh., Majumdar, J., Samal, A. C., Chauhan, R., Ranjan, R. K., Rajkumar, K., & Santra, S. C. (2008). A study of microbial diversity and its interaction with nutrients in the sediments of Sunderban mangroves. Indian Journal of Marine Sciences, 37, 159–165.
Rawte, T., Padte, M., & Mavinkurve, S. (2002). Incidence of marine and mangrove bacteria accumulating polyhydroxyalkanoates on the mid-west coast of India. World Journal of Microbiology and Biotechnology, 18, 655–659.
Raymond, K. N., & Dertz, E. (2004). Biochemical and physical properties of siderophores. In J. H. Crosa, A. R. Mey & S. M. Payne (Eds.), Iron transport in bacteria (1st edn., pp. 3–17). Washington, DC: ASM press.
Reid, C. P., Szaniszlo, P. J., & Crowley, D. E. (1986). Siderophore involvement in plant iron nutrition. In T. R. Swinburne (Ed.), Iron siderophores and plant diseases (pp. 29–42). New York: Plenum Press.
Sandy, M., & Butler, A. (2009). Microbial iron acquisition: Marine and terrestrial siderophores. Chemical Reviews, 109, 4580–4595.
Sharma, A., & Johri, B. N. (2003). Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiological Research, 15, 243–248.
Song, J., Sung, J., Kim, Y. M., Zylstra, G. J., & Kim, E. (2000). Roles of the meta- and the ortho-Cleavage pathways for the efficient utilization of aromatic hydrocarbons by Sphingomonas yanoikuyae B1. The Journal of Microbiology, 38, 245–249.
Staijen, I. E., & Witholt, B. (1998). Synthesis of alkane hydroxylase of Pseudomonas oleovorans increases the iron requirement of alk + bacterial strains. Biotechnology Bioengineering, 57, 228–237.
Tank, N., & Saraf, M. (2009). Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. Journal of Basic Microbiology, 49, 195–204.
Teitzel, G. M., Geddie, A., De Long, S. K., Kirisits, M. J., Whiteley, M., & Parsek, M. R. (2006). Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. Journal of Bacteriology, 188, 7242–7256.
Vasil, M. L., & Ochsner, U. A. (1999). The response of Pseudomonas aeruginosa to iron: Genetics, biochemistry and virulence. Molecular Microbiology, 34, 399–413.
Villegas, M., Villa, P., & Frías, A. (2002). Evaluation of the siderophores production by Pseudomonas aeruginosa PSS. Revista Latinoamericana de Microbiologia, 44, 112–117.
Visca, P., Ciervo, A., Sanfilippo, V., & Orsi, N. (1993). Iron-regulated salicylate synthesis by Pseudomonas spp. Journal of General Microbiology, 139, 1995–2001.
Vraspir, J. M., & Butler, A. (2008). Chemistry of marine ligands and siderophores. Annual Review of Marine Science, 1, 43–63.
Wandersman, C., & Delepelaire, P. (2004). Bacterial iron sources: From siderophores to hemophores. Annual Review of Microbiology, 58, 611–647.
Wang, Y., Brown, H. N., Crowley, D., & Szaniszlo, P. J. (1993). Evidence for direct utilization of a siderophore, ferroxamine B, in axenically grown cucumber. Plant Cell Environment, 16, 579–585.
Xiao, R., & Kisaalita, W. S. (1998). Fluorescent pseudomonad pyoverdines bind and oxidize ferrous ion. Applied and Environmental Microbiology, 64, 1472–1476.
Zeyaullah, M., Abdelkafe, A. S., Zabya, W. B., & Ali, A. (2009). Biodegradation of catechols by micro-organisms-a short review. African Journal of Biotechnology, 8, 2916–2922.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gaonkar, T. (2015). Eubacterial Siderophores and Factors Modulating Their Production. In: Borkar, S. (eds) Bioprospects of Coastal Eubacteria. Springer, Cham. https://doi.org/10.1007/978-3-319-12910-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-12910-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12909-9
Online ISBN: 978-3-319-12910-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)