Abstract
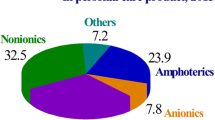
Lipase can catalyze varieties of reactions at the interface of aqueous and organic phase. Among various alternatives to modify catalytic performance of lipase, the addition of surfactants, particularly nonionic surfactants, has been widely studied. Low concentrations of nonionic surfactants augment lipase catalysis; on increasing surfactant concentration, often the catalytic performance decreases. Mole ratio of water to (nonionic) surfactant also has a profound effect on lipase activity. Catalytic abilities of some lipases are either enhanced or reduced in the presence of all nonionic surfactants of the same type, whereas for some other lipases, nonionic surfactants of the same type have mixed effect. Nonionic surfactant even changes substrate specificity of lipase. Water-in-ionic liquid microemulsion involving nonionic surfactant often performs better than other systems in improving catalytic ability of lipase. Tween and Triton surfactants often enhance enantiomeric separation catalyzed by lipase. Nonionic surfactants significantly affect activities of immobilized lipase, being present either as a component during immobilization or as a component in reaction medium. Lipases coated with nonionic surfactants act better than reverse micelles and microemulsions containing lipase. Thus, nonionic surfactants help lipase catalyzed processes in various media to enhance production of useful compounds like flavor ester, structured lipids, optically pure compounds, and noncrystalline polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipase, an enzyme of the class hydrolase, requires biphasic media comprising of a polar medium (to solubilize lipase) and a nonpolar medium (to solubilize substrates) in order to manifest catalytic action. Nonpolar medium like organic solvent inactivates lipase, either partially or completely. Hydrated surfactant aggregates like micelles, reverse micelle (RM), and microemulsions can solve this problem by increasing substrate solubilization and guarding lipase from a denaturing effect of organic solvent [1]. Factors like micelle formation, concentrations of free and micellar substrate, and their availability to lipase make molecular interactions complex. Conformational change of lipase from closed to open form leads to access of active site by substrate. As a large hydrophobic surface is exposed around active site in open conformation, only closed form is observed in water [2]. Surfactants preferentially interact with some of the binding sites of enzyme or form more powerful hydrophobic bonds than existing ones and thus change its structure and, simultaneously, catalytic ability [3]. High activity of lipase can also be achieved through stabilization of the hydrophobic surface by substrate or premicellar surfactant assemblies [4]. Surfactants can affect the interaction between protein (enzyme) and lipidic interface also [5].
As lipase acts at a hydrophobic/hydrophilic interface, catalytic activity of a specific lipase in micelle varies with a hydrophilic head group of respective surfactant [5]. Cationic and anionic surfactants have cationic and anionic head groups, respectively, which have denaturing effects on lipase due to electrostatic interactions. Nonionic surfactants with uncharged polar head groups cannot have any electrostatic interaction with enzyme. Naturally, these surfactants cannot deactivate enzyme like lipase through conformational changes, rather stabilize it through hydrogen bonding and hydrophobic interactions and work as a lipase activator through an increasing lipid–water interfacial area [6, 7]. Surfactant-induced structural change like helicity played a significant role in controlling the catalytic performance of lipase [8]. Nonionic surfactants, with 100 times smaller critical micellar concentration (CMC) than ionic surfactants containing similar hydrophilic groups, can solubilize more substrates by forming more micelles and thus can augment catalysis [3].
But, nonionic surfactants can inactivate enzymes at high concentrations (equal to or more than CMC) [9]. At extremely high concentrations of surfactant (a few times of CMC), most of the free substrate, being incorporated into micellar phase, was unavailable to lipase, leading to low lipase activity [10]. Nonionic surfactant types like Span (ester of sorbitol with fatty acid), Tween (contains additional polyethylene glycol segment over Span structure), and Triton (constitutes of octylphenoxy and ethoxy groups) have differences in their structure. Water-insoluble Span surfactants act as water-in-oil emulsifiers, but water-soluble Tween and Triton surfactants act as oil-in-water emulsifiers. Consequently, these surfactants affect the catalytic ability of lipase differently. The present work discusses impacts of various nonionic surfactants on catalytic abilities of lipases from different origins.
Effects of Nonionic Surfactants on Catalytic Ability of Free Lipase
Span, Tween, and Triton surfactants have widely varying effects on catalytic performances of different (free) lipases. Table 1 shows that sorbitan monooleate (Span 80) enhanced the catalytic activity of Candida rugosa lipase (CRL) but decreased the activity of porcine pancreas lipase (PPL) [11, 12]. Table 2 represents impacts of Tween surfactants on the catalytic performance of various free lipases [9, 10, 12,13,14,15,16,17,18,19,20]. Polyethylene glycol sorbitan monooleate (Tween 80) enhanced catalytic activities and enantioselectivities of some lipases [14,15,16] but reduced activities of some other lipases [12, 17,18,19]. Table 3 shows that Triton surfactants augmented activities of some lipases [9, 10, 15, 18, 21, 22] but reduced activities of some other lipases [12, 17, 23]. The results from Tables 1, 2, and 3 also indicate that activities of some lipases (PPL and Bacillus cereus C7) decreased in the presence of most of the nonionic surfactants.
Various groups of researchers compared impacts of Tween- and Triton-type surfactants on the same lipase. Their observations clearly signify that a particular nonionic surfactant augmented catalytic abilities of some lipases but reduced the same for some other lipases. Possibly, lipase–surfactant interaction varies with either type of lipase or that of nonionic surfactant. Polyethylene glycol sorbitan monopalmitate (Tween 40) and Tween 80 inhibited the activity of extracellular, alkalophilic lipase from Bacillus sp. LBN2, whereas octylphenoxy polyethoxyethanol (Triton X-100) and polyethylene glycol tert-octylphenyl ether (Triton X-114) augmented its activity [24]. Possibly, Triton-type surfactants enhanced the frequency of lipase–substrate contact and also helped lipase to remain in open form. Triton X-100 significantly enhanced the activity of organic solvent-stable Pseudomonas stutzeri LC2–8 lipase [25]. Triton X-100 was found to increase the activity of lipase from Chromohalobacter canadensis by a greater extent (112%) compared to Tween 80 (65%) and polyethylene glycol sorbitan monolaurate (Tween 20) (38%) [26]. Li et al. [27] noted that in case of Aureobasidium pullulans lipase, there was significant augmentation of activity on incorporation of Tween 20 (by 103.9%) and Tween 80 (by 159.6%). Triton X-100 increased it to a low extent (by 12%). Tween 80–activated Pseudomonas stutzeri PS59 lipase as conformation of active site became more flexible through lipase–surfactant hydrogen bond formation [28, 29].
Concentrations of nonionic surfactants played a significant role in controlling lipase activity. Studies summarized in Tables 2 and 3 show that almost all the nonionic surfactants increased the catalytic ability of lipase at low concentrations (0.0075 M or lower) but decreased catalysis when used at higher concentrations (0.01 M or higher). Nonionic surfactants, present at low concentrations, could co-adsorb with globular proteins (like enzymes). But, these surfactants displaced protein completely at high concentrations [30]. Tween 80 stimulated lipases at very low concentration (1/100 to 1/10,000 of CMC) [6]. Rise in concentration for all Span-type surfactants (except sorbitan monostearate (Span 60)) augmented the activity of Bacillus stearothermophilus MC7 lipase. Tween surfactants at low concentrations (less than CMC) also activated this lipase, possibly by enhancing substrate solubility [31]. High concentrations of nonionic surfactants of polyoxyethylene (1%, w/v) or alkylarylpolyoxyethanol (10%, v/v) type inhibited triolein hydrolysis by hog pancreas lipase remarkably [32]. Nonionic surfactants like alkyl maltosides and octyl glucosides inhibited Thermomyces lanuginosus lipase (TLL) at high concentrations but activated TLL by 10 times at low concentrations, and in both cases, concentration was below CMC [33]. Concentration of Triton X-100 was found to be statistically significant during sardine oil hydrolysis catalyzed by Cryptococcus sp. MTCC 5455 lipase [34]. But, there are exceptions also. Catalytic ability of CRL increased in the presence of both high [11, 16] and low [9] concentrations of nonionic surfactants. Low concentrations of polyoxyethylene- (1%, w/v) or alkylarylpolyoxyethanol-type nonionic surfactant had no impact on hog pancreas lipase activity. In gum arabic (GA)–stabilized oil-in-water emulsion, a low concentration (< 0.1%) of Tween 80 did not affect free fatty acid (FFA) production from lipid, but FFA production was enhanced as concentration exceeded 0.1% [35]. At certain high concentrations (0.7% and higher), Tween 80 remained attached with lipase and thus displaced GA from the interface. This obstructed interfacial lipase adsorption and decreased its catalytic activity [36].
The interaction between nonionic surfactant and lipase depends on pH also. Mesa et al. [37] observed that on increasing pH, the effect of Triton X-100 on TLL diminished. At pH 5, Triton X-100 had a hyperactivating effect on TLL, and this diminished after 48 h. At pH 7, it had nonhyperactivating effect but stabilized TLL even after 48 h. At pH 9, it had no positive effect on TLL performance. Concentration of a polyethylene glycol dodecyl ether-type nonionic surfactant (Thesit) had a significant effect on Bacillus subtilis lipase A, the smallest known lipase. This impact was also controlled by pH, through control of hydrophobic surface area [38].
Some studies revealed another stark fact about effects of surfactants on activities of lipases. Catalytic activity of lipase from one origin was either increased or decreased by all nonionic surfactants of the same type, whereas lipase from another origin was affected in widely different ways by different nonionic surfactants of the same type. Fusarium oxysporum lipase was inhibited by all Tween surfactants but was activated by all Triton surfactants [18]. In olive oil hydrolysis, Rhizopus delemar lipase (RDL) was completely inhibited by all Tween surfactants (Tween 20, Tween 40, Tween 60, Tween 80). All Span surfactants (Span 20, Span 40, Span 60, Span 80) also decreased its activity [39]. Geotrichum candidum lipase and Candida cylindracea lipase were inhibited by all surfactants. In case of Chromobacterium viscosum lipase (CVL), sorbitan monolaurate (Span 20) increased its catalytic performance highly, Span 80 a little, and sorbitan monopalmitate (Span 40) had no effect, whereas Span 60 decreased it. Pseudomonas sp. lipase acted as a completely 1,3-specific lipase in the presence of Tween surfactants. Span 40 was the best for this lipase, Tween 20 enhanced its activity little, but Tween 40, polyethylene glycol sorbitan monostearate (Tween 60), and Tween 80 inhibited it [39].
Water played an important role in controlling the catalytic ability of lipase, particularly in the presence of polyethylene glycol monododecyl ether-type nonionic surfactants. In palm oil transesterification with stearic acid using Rhizopus sp. lipase, triethylene glycol monododecyl ether was the best performing nonionic surfactant. In hydrocarbon phase, lipase molecules remained dispersed by hydrated nonionic surfactant and had better access of reactants than in the case involving anionic surfactant like sodium bis(2-ethylhexyl) sulfosuccinate (AOT) [40]. Mole ratio of water to (nonionic) surfactant, i.e., ω0, was a critical parameter behind hydration of nonionic surfactant. Tetraethylene glycol monododecyl ether (C12EO4) was applied in RDL-catalyzed esterification of hexanol with oleic acid in decane. Extent of esterification was low at low ω0 (< 5), attained a plateau for intermediate ω0, and on increasing ω0 further, increased again [41]. Catalytic performance of Pseudomonas cepacia lipase (PCL) was optimum at ω0 of 8 during esterification of octanol with lauric acid in C12EO4/isooctane microemulsion, as water necessary for hydration of every ethylene oxide group of surfactant was available at that ω0 [42]. Above this ω0, shapeless aggregates changed to spherical RMs. Lipase exhibited maximum activity at this structural transition. On increasing ω0, additional water barrier formed between lipase and micellar interphase. This decreased substrate diffusion and, simultaneously, rate of esterification [43]. Naoe et al. [44] carried out esterification of oleic acid with octyl alcohol catalyzed by RDL in RM of sugar ester DK-F-110. Reaction rate was dependent on ω0 and DK-F-110 concentration. DK-F-110 RM, with higher turnover number, helped in better catalysis compared to AOT RM. The maximum rate was obtained at a ω0 of 2.5, pH 6, and 40 °C. The extremely low ω0 pinpointed that sugar ester RM created a proper hydrophilic environment for catalysis by lipase during esterification molecules. Uehara et al. [45] examined the catalytic ability of RDL during hydrolysis of triolein in a w/o microemulsion consisting of DK-ester-F-110, isooctane, and butanol (a co-solvent). The maximum initial rate was enhanced about 2-fold on addition of butanol. Solubilized water content also had impact on the initial rate of hydrolysis. Sufficient hydrophobicity of water pool and fluidity of microinterface were also necessary for adequate hydrolysis.
A nonionic surfactant named Lutensol AT50 helped PCL to catalyze complete polymerization of pentadecalactone in hexadecane; leading to formation of biodegradable polymer nanoparticles. PCL also successfully catalyzed co-polymerization of pentadecalactone and dodecalactone at low temperature to synthesize noncrystalline polyesters in mini-emulsion of Lutensol AT50 [46].
Catalysis by Free Lipase in Nonionic Surfactant–Formulated Water-in-Ionic Liquid Microemulsions
Nonionic surfactant–formulated water-in-ionic liquid (w/IL) microemulsions can affect the catalytic activity of free lipase substantially. A ternary system with a hydrophobic ionic liquid (IL) named 1-butyl-3-methylimidazolium hexafluorophosphate ((BMIM)(PF6)), nonionic surfactant (Tween 20 or Triton X-100), and aqueous phase (buffer of pH 7.5) retained the catalytic ability of CRL, CVL, and TLL during esterification of lauric acid with 1-propanol [47]. Variation of catalytic abilities of lipases with ω0 passed through a maximum. At higher temperature (50 °C), the operational stability of free lipases in this w/IL microemulsion was significantly higher than that of other microheterogeneous systems. Circular dichroism and Fourier transform infrared spectroscopy found that lipase in this microemulsion retained original structure or sometimes attained more robust structure [47]. Lima et al. [48] applied Tween 20 and (BMIM)(PF6) to prepare w/IL microemulsion-based organogels (MBGs). Activities of Candida antarctica lipase (CAL) and CVL immobilized in these MBGs were up to 4.4-fold higher than water in oil-based MBGs during esterification of lauric acid with butanol. Lipases in w/IL MBGs retained activity for a long time even at 70 °C. The rates and conversions were significantly higher in Tween 20–based w/IL microemulsions than those in AOT-based RM, water-saturated (BMIM)(PF6), and surfactant-free microemulsion-like ternary systems [48]. Commercial CRLs could not effectively catalyze esterification of phytosterols with fatty acid in a conventional reaction system but efficiently catalyzed the same in a w/IL microemulsion containing nonionic surfactant like (BMIM)(PF6)/Tween 20/H2O. These studies clearly show that nonionic surfactant (in particular, Tween 20)–based w/IL microemulsion can act as an effective medium to augment catalysis by free lipase [49].
Nonionic Surfactant Aided Oil Removal by Free Lipase
Triton X-100 enhanced Penicillium chrysogenum lipase activity by 50%, while SDS suppressed it during bioremediation of waste cooking oil [50]. Triton X-100 (1%) also increased Ralstonia pickettii lipase activity by 30% at pH 7 and 37 °C during removal of oil in laundry. Oil removal increased with an increase in temperature [51]. Lipase from a thermoalkalophilic Pseudomonas species was significantly active in the presence of polyethylene glycol sorbitan trioleate (Tween 85), Span 80, and Span 20 during olive oil removal from cotton fabric. As this lipase was stable in high pH and temperatures in the presence of surfactant, it could act as an additive in detergent formulations [52]. A lauryl glucoside-type nonionic surfactant (Glucopone 600 (G600)) was better than ionic surfactants in retaining stability and interfacial adsorption of genetically engineered variants of TLL during fatty soil cleaning [53]. G600 also retained the highest stability of T1 lipase during dishwashing. G600 lowered thermal denaturation and thus helped T1 lipase to express better activity, whereas Tween 80 slightly inhibited this lipase [54]. To the contrary, cleaning of soil involving tributyrin or triolein by TLL was diminished in the presence of commercial nonionic surfactants. Possibly, the lipase–substrate interaction decreased due to surfactant adsorption at the soil surface [55].
Enantioselective Separation Catalyzed by Free Lipase in the Presence of Nonionic Surfactants
Nonionic surfactants had significant impacts on lipase-catalyzed enantioselective separations. Tween 60, Tween 80, and nonyl phenol polyoxyethylene ether (OP-10) stimulated the catalytic ability of crude and purified CRL in enantioselective hydrolysis of 2-chloroethyl ester of ketoprofen. Nearly optically pure (S)-ketoprofen (S-2-(3-benzoylphenyl) propionic acid) with high enantiomeric excess was obtained. Tween 80 (2%, w/v) augmented enantiomeric ratio by 5 and 12 times for crude and purified CRL, respectively. OP-10 was even better than Tween 80. At higher concentrations of OP-10, the hydrophobic part of free surfactant moved away from the lipase surface or might form a bilayer around a lipase molecule, leading to lower catalysis by lipase [56]. RDL was the best lipase in increasing enantioselectivity during enantioselective esterification of racemic glycidol with lauric acid in cyclohexane in the presence of dioleyl-N-d-glucono-l-glutamate, a nonionic surfactant [57]. In hydrolysis of 2-phenylpropane-1,3-diol diacetate ester using crude PPL, all alkyl (thio)glucoside- and maltoside-type nonionic surfactants reduced conversion but octyl thioglucopyranoside and n-dodecyl glucoside augmented enantiomeric excess. All Span surfactants decreased conversion, but Span 80 led to higher enantiomeric excess. Triton X-100 and Triton X-114 reduced conversion and enantiomeric excess. All polyoxyethylene-type nonionic surfactants decreased conversion, but octyl glycol and octadecyl glycol increased enantiomeric excess [58]. In the transesterification of (RS)-1-phenylethanol with vinyl acetate, Aspergillus oryzae lipase, modified by Tween 40, showed high catalytic ability and enantioselectivity for the kinetic resolution of (RS)-1-phenylethanol in the presence of an organic co-solvent of tetrahydrofuran and a phosphate buffer of pH 7.0. (R)-1-phenylethyl acetate was obtained at an enantiomeric excess of more than 99% [59]. These outcomes also made it clear that nonionic surfactant–lipase interaction varies with a type of surfactant. Low concentration of surfactant was generally more effective in enhancing enantiomeric excess.
Effects of Nonionic Surfactants on the Catalytic Performance of Immobilized Lipase
Nonionic surfactants significantly affected activities of immobilized lipase, either being present as a component during immobilization or as a component in reaction medium. Zeta potential analysis showed that the electrostatic interaction between the enzyme and the support for immobilization is critical for high loading density of enzyme through adsorption and covalent bonding [60]. Nonionic surfactants most possibly alter this interaction. Inappropriate immobilization lacks proper orientation for which there can be burial of lid, ultimately leading to a loss of activity. But, surfactants can form an interface which keeps the lid of lipase open. Interfacially activated lipase can be immobilized in such open state, ultimately augmenting the activity [61, 62].
Aspergillus niger lipase (ANL), immobilized on silk fiber, manifested poor catalytic activity during sunflower oil hydrolysis in biphasic oil–water medium, but Triton X-100 augmented its activity significantly [63]. Tween 20, Tween 80, and Triton X-100 augmented the catalytic performance of ANL, immobilized on magnetic nanoparticle, during production of glycerol carbonate [64]. A very small concentration of Triton X-100 (below CMC) greatly enhanced activities of Pseudomonas fluorescens lipase (PFL) and CAL immobilized on glyoxyl agarose and cyanogen bromide–activated sepharose. Activity of PFL–glyoxyl agarose increased in the presence of Triton X-100. Increment in the concentration of nonionic surfactant actually diminished lipase activity, even when it was immobilized [21]. The surfactant partly helped to keep lipase in open form, but partial inhibition might also take place due to the competition between substrate and surfactant molecules for the adsorption site of lipase [21]. The presence of Tween 80 during immobilization of CAL B on carboxy-functionalized single-walled carbon nanotubes enhanced the catalytic performance of the lipase during production of biodiesel [65].
CRL, immobilized through adsorption as nanoconjugates on acid-functionalized multi-walled carbon nanotubes, acted as a catalyst for producing methyl oleate ester [66]. Immobilized CRL, in the presence of Triton X-100 and Tween 80, led to the increased extent of esterification when compared with free CRL in the presence of the same surfactants. These nonionic surfactants altered surface charge density at the interfacial region through the hydrophobic modification of CRL and thus assisted immobilized CRL to concentrate at the interface. The hydrophobic surfaces of carbon nanotubes possibly interacted with an amphiphilic alpha-helix peptide covering the active site of CRL; consequently, the stability of open conformation of CRL increased and thus its catalytic ability augmented further [66]. But, Tween 80 and Triton X-100 could not enhance conversion during pentyl valerate synthesis, catalyzed by CRL immobilized in MBGs. Low ω0 and weak, fragile MBGs were mainly responsible for the low conversion [67]. Zhang et al. [68] used nonionic surfactants during immobilized CRL (in MBGs)–catalyzed transesterification of 2-phenylethanol with vinyl acetate. Surfactants, working as the interface between buffer and organic phase, acted as the first level of protection to lipase. Surprisingly, the Triton X-100–based MBG was less catalytically active than the AOT-based MBG. Dave and Madamwar [69] observed that nature of co-surfactants and oil-affected CRL-catalyzed esterification of ethyl alcohol with butyric acid in quaternary (Triton X-100/water/1-hexanol/n-hexane) water-in-oil MBG. Esterification increased with increasing ω0 at constant Z, i.e., (moles co-surfactant)/(moles surfactant). At ω0 of 30 and Z of 8, 100% esterification was achieved. For several recycles, MBGs were stable and lipase retained its activity. Thermal stability increased five times on immobilization. Tween 80 enhanced the ability of immobilized CRL to produce S-naproxen from enantioselective hydrolysis of racemic naproxen methyl ester [70]. CRL, immobilized on glyoxyl agarose, manifested better catalytic ability in the presence of Triton X-100 [71]. Cross-linked and entrapped CRL manifested eight times higher activity than soluble CRL during esterification of conjugated linoleic acid and ethanol in nonaqueous medium. The MSU-H-type mesoporous silica support was modified by a nonionic surfactant of the triblock co-polymer Pluronic P123 before immobilization. Covalent and noncovalent interactions like electrostatic repulsion and hydrophobic interaction were present between CRL and support, activated by the surfactant [72]. Rhizomucor miehei lipase, adsorbed on chitosan beads and subsequently cross-linked with glutaraldehyde, showed excellent catalytic ability and thermal stability even at a higher temperature (60 °C) during synthesis of flavor esters in the presence of Triton X-100 [73]. TLL-magnetic cross-linked enzyme aggregates (mCLEAs) retained activity for a long time on repeated use for more than 4 weeks [74]. The addition of Tween 80 (0.0006 M) to the TLL-mCLEA system enhanced the yield of biodiesel by 1.5 times, and stability and reusability were also high. Surfactant-activated TLL-mCLEA manifested high activity, improved stability, and high reusability. Yang and Zhang [75] observed that Burkholderia cepacia lipase (BCL), when pretreated with 0.0001 M Triton X-100 and subsequently immobilized in mCLEAs with hydroxyapatite-coated magnetic nanoparticles, experienced a maximum increase in activity (~ 15%), whereas small activity enhancement (~ 5%) was achieved with 0.0002 M Tween 80. Nonionic surfactant during pretreatment possibly fixed BCL with its lid open and thus enhanced its activity, ultimately facilitating biodiesel production in solvent without further addition of water. Catalytic performance of immobilized (cross-linked) and nonionic surfactant (Tween 80 or Triton X-100)–pretreated Rhizopus oryzae lipase (ROL) during esterification was better than that of free lipase and nonpretreated, immobilized lipase. In terms of pretreating ability, Tween 80 was better than Triton X-100 [61]. Liu et al. [62] synthesized a novel immobilized lipase from Yarrowia lipolytica lipase LIP2, covalently immobilized on functionalized Fe3O4 magnetic nanoparticles (MNPs) in RM. The addition of Span 20 lifted catalytic activity by 12 times. At high ω0, excess water led to loose lipase structure and ultimately diminished its catalytic ability [76]. Nonionic colloidal liquid aphron (CLA) formulated by ANL with 1% Tween 80/mineral oil and 1% Tween 20 increased its catalytic ability by 7 times. Changes in the Michaelis–Menten constant (Km) of lipase supported the fact that immobilization led to large conformational changes [77]. Sánchez-Otero et al. [78] immobilized Geobacillus thermoleovorans CCR11 lipase on porous polypropylene, a hydrophobic support, by adsorption. Immobilization with Triton X-100 augmented the reusability and activity of lipase, possibly due to breaking of protein (enzyme, i.e., lipase) aggregates and/or stabilization of an open form of lipase [21]. But, Pencreac’h et al. [79] noticed that PCL, immobilized on polypropylene, lost its activity totally after 10 min of exposure to 0.4% Triton X-100. This difference clearly points out that the hydrophobic interaction between the Geotrichum thermoleovorans lipase and the polypropylene support was strong. Another study observed a similar change in lipase activity [80]. The presence of Triton X-100 during immobilization of TLL on polypropylene doubled activity retention even after 30 h at 50 °C. Lipase immobilized without Triton X-100 showed better thermal stability, probably due to the presence of oligomers and other lipase aggregates [81]. The difference in catalytic abilities of various immobilized lipases in the presence of the same surfactant (Triton X-100) emphasized that the type of lipase controlled the lipase–surfactant interaction even in its immobilized state. Triton X-100 somewhat modified dependence of thermal stability on immobilization, as increasing temperature decreased activity further. Zhao et al. [82] applied a nonionic surfactant for interfacial activation of ROL, after which it was immobilized on nanoparticle of Fe3O4, previously modified by 3-aminopropyltriethoxysilane (APTES) and glutaraldehyde. Sucrose esters-11 worked better than other nonionic surfactants like Tween 20, Tween 60, Tween 80, Triton X-100, and sucrose esters-15 as activator. Immobilized ROL had hydrolytic activities 9.16 times and 31.6 times that of free lipase with p-nitrophenyl butyrate and p-nitrophenyl palmitate, respectively, as substrates. Its specific esterification activity was also 1.5 times that of free lipase. It had higher thermostability than free lipase. On increasing concentration of sucrose esters-11, lid of lipase opened more and the activity increased. Zhao et al. [83] immobilized ROL, interfacially activated by Tween 80, modified by APTES and glutaraldehyde, on nanoparticle of Fe3O4. This immobilized ROL had specific hydrolytic and esterification activities 16.6 and 2.6 times that of free ROL.
Catalysis by Nonionic Surfactant–Coated Lipases
Stability and simultaneous catalytic activity of lipase remain quite low in organic solvents due to partial deactivation. Coating by surfactant prevents this deactivation and enhances the solubility of lipase in organic solvents [84]. Nonionic surfactants might shift equilibrium of lipase towards open conformation by coating the hydrophobic area surrounding its active site [85]. High reaction rate and no necessity to control water content in organic media are advantages of surfactant-coated lipase (SCL) system [84]. SCL acted better than AOT-isooctane RM, two-phase isooctane–water system, and isooctane–powder lipase system. The activity of SCL, comprising of nonionic surfactants, depended strongly on the structure of the hydrophobic part of surfactant. SCL from nonionic surfactant having two hydrocarbon chains like glutamic acid dioleyl ester ribitol amide (2C18∆9GE) performed better than SCL from nonionic surfactant having one hydrocarbon chain (1C18∆9GE) [86]. SCL having more hydrophobic groups was more soluble in organic solvent, and this probably led to such observation [85].
In general, the molecular structure of the hydrophilic part of surfactant has great effect on the activity of SCL [85]. Naturally, SCLs having different nonionic surfactants will manifest a wide variation in their catalytic abilities. Table 4 describes the performance of lipases coated with 2C18Δ9GE, the most extensively used coating surfactant. CRL coated with 2C18Δ9GE showed a remarkable increase in catalytic ability during methyl ester formation from Chinese tallow kernel oil [87], olive oil hydrolysis [88, 89], and esterification [90]. Lipases (ANL, Pseudomonas sp., Rhizopus sp., and Mucor javanicus) showed significant activity enhancement when coated with 2C18Δ9GE during resolution of racemic menthol [91] and esterification of lauric acid with benzyl alcohol [92].
Table 5 [93,94,95,96,97,98,99] describes the performance of lipase coated with Span-type surfactants. Span 60 was the preferred coating surfactant in most of the studies. It augmented catalytic abilities of lipases like CRL and Rhizopus japonicus lipase. In a stunning observation, Pseudomonas sp. lipase coated with Span 60 showed much higher activity in ethanol than in water [97]. The same SCL efficiently catalyzed esterification, whereas crude lipase in the presence and in the absence of surfactant showed no activity [98].
Catalysis by Nonionic Surfactant–Coated Lipases Immobilized on Specific Supports
CRL, coated with surfactant and immobilized on GA-coated magnetic nanoparticles of ferrosoferric oxide, acted as a biocatalyst for ethyl isovalerate (a flavor ester) synthesis [85]. Lipase coated with 1 mM Triton X-100 resulted in much better esterification compared to lipase coated with cationic (cetyltrimethylammonium bromide (CTAB)) and anionic (AOT) surfactants. Magnetic support material kept lipase activity constant by retaining exact amount of water. The surface of GA might interact with the surface of lipase coated with nonionic surfactant, leading to enhanced rate of esterification. Catalytic performance of the SCL was constant even after the seventh recycle [85]. CRL coated with Tween 80 and immobilized in microemulsion of Triton X-100–based organogels outperformed the same SCL, immobilized in CTAB- and AOT-based organogels and uncoated lipase [100].
CRL, coated with surfactant and immobilized on silica, catalyzed ethyl butyrate (a flavor ester) production also [101]. Triton X-100 was better than Tween 80 as a coating surfactant. But, in another study [102], Tween 80 was found as the best surfactant in coating CRL, which was subsequently immobilized in sol–gel supports for transesterification of sucrose with acetic acid to produce sucrose-6-acetate. At elevated temperatures, SCL immobilized in sol–gel was more catalytically active than free CRL, CRL immobilized in sol–gel, and SCL without immobilization. Coating followed by immobilization protected lipase effectively against thermal denaturation. Conformational limitation on the enzyme movement, due to electrostatic interaction and enzyme–support hydrogen bonding, and low restriction in substrate diffusion at high temperature could be possible reasons behind such improvements.
Catalysis by Nonionic Surfactant–Coated Lipases in Organic Solvents
SCLs prepared with nonionic surfactants often showed sensitivity to organic solvents. BCL, coated with propylene glycol monostearate (a nonionic surfactant), catalyzed production of ascorbyl palmitate in the presence of molecular sieves [103]. The SCL was completely inactive in methanol, hexane, acetone, chloroform, and isopropanol but was active in tert-butanol. Unlike most of the organic solvents, tert-butanol did not strip essential water molecule bound to lipase, rather the microaqueous environment around it augmented stability. Temperature had a profound effect on the catalytic activity of SCL in organic solvents. At optimum temperature (50 °C), SCL was 8 times more active compared to free lipase [103]. Nonionic surfactant–coated RDL showed high stability and activity in hydrophobic organic solvents, but no activity in halogenated solvents [104]. SCL from RDL and Pseudomonas fragi 22-39B lipase (PFRL) catalyzed diacylglycerol and triacylglycerol syntheses from monoacylglycerols and aliphatic acids in homogeneous and dry benzene assisted by molecular sieves. SCL from PFL was more thermostable than free lipase and catalyzed ester exchange reactions in organic solvents containing a very low amount of water (250 ppm), but no reaction occurred in dry organic solvents [77]. PFRL, coated with synthetic nonionic surfactant didodecyl N-d-glucono-l-glutamate, worked better than SCLs from Pseudomonas sp., Penicillium roqueforti, Pseudomonas niger, and RDL for hydrolysis of lipophilic esters in an aqueous-organic system. The extent of reaction increased with increasing aqueous phase volume but was independent of its pH. So, hydrolysis definitely occurred in organic phase with water molecules coming from aqueous phase. SCL from PFRL was the best for esterification of triglyceride and enantioselective esterification of 1-phenylethanol also [105]. PFRL, coated with a dialkyl nonionic surfactant, esterified racemic alcohols with excess aliphatic acids enantioselectively in various relatively dry organic solvents (40–80 ppm water) in the presence of molecular sieves [106]. Rate increased with increasing alkyl chain length, but high enantioselectivity was obtained with hexanoic and dodecanoic acids. PCL, coated with 2C18Δ9GE, enhanced conversion of pentadecalactone during polymerization by 10 times. This SCL led to a higher molecular weight of polymer and was a better catalyst for ring-opening polymerization due to its higher solubility in organic solvent, compared to uncoated lipase [107].
Summary
Formation of micelle by surfactants, concentrations of free and micellar substrate, and their availability to lipase made the interactions among lipases, substrates, and surfactants quite complex. The hydrophobic as well as hydrophilic part of surfactant played a distinct role in such interactions. Low concentration of nonionic surfactants mostly stabilized lipase through hydrophobic interactions and enhanced substrate solubilization. This ultimately augmented the catalytic ability of lipase. But, at higher concentration, nonionic surfactants often decreased rate of reaction by solubilizing substrate in micelles of excess surfactant, thus reducing effective substrate concentration in interfacial region (the working zone for lipase). Lipase displacement from the interface at high surfactant concentration also led to reduced catalysis. In GA-stabilized emulsions, this trend was somewhat opposite. The pH of reaction medium has the potential to alter the interaction between nonionic surfactant and lipase. The presence of Tween surfactants changed positional specificity of Pseudomonas sp. lipase from nonspecific to completely 1,3-specific. Alteration of the electrostatic interaction between lipase and immobilization support by nonionic surfactant increased the activity of some immobilized lipases, where the surfactant was used either as a component in reaction medium or as a component in immobilization support. PPL showed enhanced activity when immobilized on ordered mesoporous silica, prepared using a nonionic surfactant. Tween 80, Triton X-100, and Span 60 were responsible in augmenting enantiomeric separation catalyzed by CRL, Pseudomonas sp. lipase, etc. Among various lipases, only CRL always showed higher catalytic ability in the presence of Span, Tween, and Triton surfactants. So, this lipase can be a good choice in future studies. Denaturation of lipase in organic solvents could be resisted by coating it with nonionic surfactant like 2C18Δ9GE and Span surfactants. So, nonionic surfactant–coated lipase can be considered as a better catalyst in comparison with free lipase in organic solvents. Lipase coated with nonionic surfactant and then immobilized on supports like magnetic nanoparticles, microemulsion-based organogels, and silica also acted as better catalysts compared to free lipase.
Abbreviations
- ANL:
-
Aspergillus niger lipase
- AOT:
-
Sodium bis(2-ethylhexyl) sulfosuccinate
- BCL:
-
Burkholderia cepacia lipase
- (BMIM)(PF6):
-
1-Butyl-3-methylimidazolium hexafluorophosphate
- 2C18∆9GE:
-
Glutamic acid dioleyl ester ribitol amide
- C12EO4 :
-
Tetraethylene glycol monododecyl ether
- CAL:
-
Candida antarctica lipase
- CLA:
-
Colloidal liquid aphron
- CMC:
-
Critical micellar concentration
- CRL:
-
Candida rugosa lipase (formerly, Candida cylindracea lipase)
- CTAB:
-
Cetyltrimethylammonium bromide
- CVL:
-
Chromobacterium viscosum lipase
- FFA:
-
Free fatty acid
- GA:
-
Gum arabic
- IL:
-
Ionic liquid
- K m :
-
Michaelis–Menten constant
- MBG:
-
Microemulsion-based organogel
- mCLEA:
-
Magnetic cross-linked enzyme aggregate
- OP-10:
-
Nonyl phenol polyoxyethylene ether
- PCL:
-
Pseudomonas cepacia lipase
- PFL:
-
Pseudomonas fluorescens lipase
- PFRL:
-
Pseudomonas fragi 22-39B lipase
- PPL:
-
Porcine pancreas lipase
- RDL:
-
Rhizopus delemar lipase
- RM:
-
Reverse micelle
- ROL:
-
Rhizopus oryzae lipase
- SCL:
-
Surfactant-coated lipase
- SDS:
-
Sodium dodecyl sulfate
- Span 20:
-
Sorbitan monolaurate
- Span 40:
-
Sorbitan monopalmitate
- Span 60:
-
Sorbitan monostearate
- Span 80:
-
Sorbitan monooleate
- TLL:
-
Thermomyces lanuginosus lipase
- Tween 20:
-
Polyethylene glycol sorbitan monolaurate
- Tween 40:
-
Polyethylene glycol sorbitan monopalmitate
- Tween 60:
-
Polyethylene glycol sorbitan monostearate
- Tween 80:
-
Polyethylene glycol sorbitan monooleate
- Triton X-100:
-
Octylphenoxy polyethoxyethanol
- Triton X-114:
-
Polyethylene glycol tert-octylphenyl ether
- V max :
-
Maximum rate of reaction
- ω 0 :
-
(Moles water)/(moles surfactant)
- w/IL:
-
Water-in-ionic liquid
- Z :
-
(Moles co-surfactant)/(moles surfactant)
References
Li, Y., Li, G., & Ma, C. (2000). Enzymology in surfactant association systems. J Dispers Sci Technol, 21(4), 409–432.
Belle, V., Fournel, A., Woudstra, M., Ranaldi, S., Prieri, F., & Thome, V. (2007). Probing the opening of the pancreatic lipase lid using site-directed spin labeling and EPR spectroscopy. Biochemistry, 46(8), 2205–2214.
Fendler, J. H., & Fendler, E. J. (1975). Catalysis in Micellar and macromolecular systems. New York: Academic.
Jutila, A. K., Patkar, S. A., Vind, J., Svendsoen, A., & Kinnumen, P. K. (2000). Detergent induced conformational changes of Humicola lanuginosa lipase studied by fluorescence spectroscopy. Biophys J, 78(3), 1634–1642.
Antipova, A. S., Semenova, M. G., Belyakova, L. E., & Il’in, M. M. (2001). On relationships between molecular structure, interaction and surface behavior in mixture: small-molecule surfactant+protein. Colloids Surf B: Biointerfaces, 21(1–3), 217–230.
Delorme, V., Dhouib, R., Canaan, S., Fotiadu, F., Carrière, F., & Cavalier, J.-F. (2011). Effects of surfactants on lipase structure, activity, and inhibition. Pharm Res, 28(8), 1831–1842.
Fernández-Lorente, G., Palomo, J. M., Mateo, C., Munilla, R., Ortiz, C., Cabrera, Z., Guisán, J. M., & Fernández-Lafuente, R. (2006). Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules, 7(9), 2610–2615.
Alam, P., Rabbani, G., Badr, G., Badr, B. M., & Khan, R. H. (2015). The surfactant-induced conformational and activity alterations in Rhizopus niveus lipase. Cell Biochem Biophys, 71(2), 1199–1206.
Helistö, P., & Korpela, T. (1998). Effects of detergents on activity of microbial lipases as measured by the nitrophenyl alkanoate esters method. Enzym Microb Technol, 23(1–2), 113–117.
Lai, D. T., & O’Connor, C. J. (2000). Synergistic effects of surfactants on kid pregastric lipase catalyzed hydrolysis reactions. Langmuir, 16(1), 115–121.
Goswami, D., Basu, R. K., & De, S. (2010). Surfactant enhanced ricinoleic acid production using Candida rugosa lipase. Bioresour Technol, 101(1), 6–13.
Goswami, D., De, S., & Basu, J. K. (2012). Effects of process variables and additives on mustard oil hydrolysis by porcine pancreas lipase. Braz J Chem Eng, 29(3), 449–460.
Sorour, N., Karboune, S., Saint-Louis, R., & Kermash, S. (2012). Lipase-catalyzed synthesis of structured phenolic lipids in solvent-free system using flaxseed oil and selected phenolic acids as substrates. J Biotechnol, 158(3), 128–136.
Syed, M. N., Iqbal, S., Bano, S., Khan, A. B., Ali-ul-Qader, S., & Azhar, A. (2010). Purification and characterization of 60 kD lipase linked with chaperonin from Pseudomonas aeruginosa BN-1. Afr J Biotechnol, 9(45), 7724–7732.
Mateos Diaz, J. C., Cordova, J., Baratti, J., Carriere, F., & Abousalham, A. (2007). Effect of nonionic surfactants on Rhizopus homothallicus lipase activity: a comparative kinetic study. Mol Biotechnol, 35(3), 205–214.
Wu, H.-Y., Xu, J.-H., & Liu, Y.-Y. (2001). A practical enzymatic method for preparation of (s)-ketoprofen with a crude Candida rugosa lipase. Synth Commun, 31(22), 3491–3496.
Dutta, S., & Ray, L. (2009). Production and characterization of an alkaline thermostable crude lipase from an isolated strain of Bacillus cereus C7. Appl Biochem Biotechnol, 159(1), 142–154.
dos Prazeres, J. N., Cruz, J. A. B., & Pastore, G. M. (2006). Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz J Microbiol, 37(4), 505–509.
Polizelli, P. P., Tiera, M. J., & Bonilla-Rodriguez, G. O. (2008). Effect of surfactants and polyethylene glycol on the activity and stability of a lipase from oilseeds of Pachira aquatica. J Am Oil Chem Soc, 85(8), 749–753.
Salameh, M. A., & Wiegel, J. (2010). Effects of detergents on activity, thermostability and aggregation of two alkalithermophilic lipases from Thermosyntropha lipolytica. The Open Biochemistry Journal, 4, 22–28.
Fernández-Lorente, G., Palomo, J. M., Cabrera, Z., Fernandez-Lafuente, R., & Guisán, J. M. (2007). Improved catalytic properties of immobilized lipases by the presence of very low concentrations of detergents in the reaction medium. Biotechnol Bioeng, 97(2), 242–250.
Qian, L. L., Chen, S. X., & Shi, B. Z. (2007). Preparation of enantiopure (R)-flurbiprofen catalyzed by a newly isolated Bacillus cereus C71. Biocatalysis and Biotransformation, 25(1), 29–34.
Fendri, A., Frikha, F., Mosbah, H., Miled, N., Zouari, N., Bacha, A. B., Sayari, A., Mejdoub, H., & Gargouri, Y. (2006). Biochemical characterization, cloning, and molecular modelling of chicken pancreatic lipase. Arch Biochem Biophys, 451(2), 149–159.
Bora, L., & Bora, M. (2012). Optimization of extracellular thermophilic highly alkaline lipase from thermophilic Bacillus sp. isolated from hotspring of Arunachal Pradesh, India. Braz J Microbiol, 43(1), 30–42.
Cao, Y., Zhuang, Y., Yao, C., Wu, B., & He, B. (2012). Purification and characterization of an organic solvent-stable lipase from Pseudomonas stutzeri LC2-8 and its application for efficient resolution of (R, S)-1-phenylethanol. Biochem Eng J, 64, 55–60.
Ai, L., Huang, Y., & Wang, C. (2018). Purification and characterization of halophilic lipase of Chromohalobacter sp. from ancient salt well. J Basic Microbiol, 58(8), 647–657.
Li, Y., Liu, T.-J., Zhao, M.-J., Zhang, H., & Feng, F.-Q. (2019). Screening, purification, and characterization of an extracellular lipase from Aureobasidium pullulans isolated from stuffed buns steamers. Journal of Zhejiang University-Science B (Biomedicine & Biotechnology), 20(4), 332–342.
Zadymova, N. M., Yampol’skaya, G. P., & Filatova, L. Y. (2006). Interaction of bovine serum albumin with nonionic surfactant Tween 80 in aqueous solutions: complexation and association. Colloid Journal, 68(2), 162–172.
Li, X.-L., Zhang, W.-H., Wang, Y.-D., Dai, Y.-J., Zhang, H.-T., Wang, Y., Wang, H.-K., & Lu, F.-P. (2014). A high-detergent-performance, cold-adapted lipase from Pseudomonas stutzeri PS59 suitable for detergent formulation. J Mol Catal B Enzym, 102, 16–24.
Wilde, P. J., Husband, F. A., Mackie, A. R., Ridout, M. J., & Morris, V. J. (2002). Protein surfactant interactions at interfaces, their influence on interfacial structure, and the stability of foams and emulsions. Abstr Pap Am Chem Soc, 223, U453.
Guncheva, M., Zhiryakova, D., Radchenkova, N., & Kambourova, M. (2007). Effect of nonionic detergents on the activity of a thermostable lipase from Bacillus stearothermophilus MC7. J Mol Catal B Enzym, 49(1–4), 88–91.
Wills, E. D. (1955). The effect of surface-active agents on pancreatic lipase. Biochem J, 60(4), 529–534.
Mogensen, J. E., Sehgal, P., & Otzen, D. E. (2005). Activation, inhibition, and destabilization of Thermomyces lanuginosus lipase by detergents. Biochemistry, 44(5), 1719–1730.
Aarthy, M., Saravanan, P., Ayyadurai, N., Gowthaman, M. K., & Kamini, N. R. (2016). A two step process for production of omega 3-polyunsaturated fatty acid concentrates from sardine oil using Cryptococcus sp. MTCC 5455 lipase. J Mol Catal B Enzym, 125, 25–33.
Yao, X., Nie, K., Chen, Y., Jiang, F., Kuang, Y., Yan, H., Fang, Y., Yang, H., Nishinari, K., & Phillips, G. O. (2018). The influence of non-ionic surfactant on lipid digestion of gum arabic stabilized oil-in-water emulsion. Food Hydrocoll, 74, 78–86.
Li, Y., & Mcclements, D. J. (2011). Inhibition of lipase-catalyzed hydrolysis of emulsified triglyceride oils by low-molecular weight surfactants under simulated gastrointestinal conditions. Eur J Pharm Biopharm, 79(2), 423–431.
Mesa, M., Pereañez, J. A., Preciado, L. M., & Bernal, C. (2018). How the Triton X-100 modulates the activity/stability of the Thermomyces lanuginose lipase: insights from experimental and molecular docking approaches. International Journal of Biological Macromolecules, 120(Pt B), 2410–2417.
Kuebler, D., Bergmann, A., Weger, L., Ingenbosch, K. N., & Hoffmann-Jacobsen, K. (2017). Kinetics of detergent induced activation and inhibition of a minimal lipase. J Phys Chem B, 121(6), 1248–1257.
Matori, M., Asahara, T., & Ota, Y. (1991). Reaction conditions influencing positional specificity index (psi) of microbial lipases. J Ferment Bioeng, 72(6), 413–415.
Holmberg, K., & Osterberg, E. (1987). Enzymatic transesterification of a triglyceride in microemulsions. In J. C. Eriksson, B. Lindman, & P. Stenius (Eds.), Progress in colloid and polymer science (vol. 74) (pp. 98–102). Berlin: Springer Verlag.
Kolisis, F. N., Valis, T. P., & Xenakis, A. (2006). Lipase-catalyzed esterification of fatty acids in nonionic microemulsions. Ann N Y Acad Sci, 613(1), 674–680.
Stamatis, H., Xenakis, A., Dimitriadis, E., & Kolisis, F. N. (1995). Catalytic behavior of Pseudomonas cepacia lipase in w/o microemulsions. Biotechnol Bioeng, 45(1), 33–41.
Valis, T. P., Xenakis, A., & Kolisis, F. N. (1992). Comparative studies of lipase from Rhizopus delemar in various microemulsion systems. Biocatalysis, 6(4), 267–279.
Naoe, K., Ohsa, T., Kawagoe, M., & Imai, M. (2001). Esterification by Rhizopus delemar lipase in organic solvent using sugar ester reverse micelles. Biochem Eng J, 9(1), 67–72.
Uehara, A., Imai, M., & Suzuki, I. (2008). The most favorable condition for lipid hydrolysis by Rhizopus delemar lipase in combination with a sugar–ester and alcohol W/O microemulsion system. Colloids Surf A Physicochem Eng Asp, 324(1–3), 79–85.
Taden, A., Antonietti, M., & Landfester, K. (2003). Enzymatic polymerization towards biodegradable polyester nanoparticles. Macromol Rapid Commun, 24(8), 512–516.
Pavlidis, I. V., Gournis, D., Papadopoulos, G. K., & Stamatis, H. (2009). Lipases in water-in-ionic liquid microemulsions: structural and activity studies. J Mol Catal B Enzym, 60(1–2), 50–56.
Lima, V. M. G., Krieger, N., Mitchell, D. A., & Fontana, J. D. (2004). Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem Eng J, 18(1), 65–71.
Zeng, C., Qi, S., Li, Z., Luo, R., Yang, B., & Wang, Y. (2015). Enzymatic synthesis of phytosterol esters catalyzed by Candida rugosa lipase in water-in-(Bmim)PF6 microemulsion. Bioprocess Biosyst Eng, 38(5), 939–946.
Kumar, S., Mathur, A., Singh, V., Nandy, S., Khare, S. K., & Negi, S. (2012). Bioremediation of waste cooking oil using a novel lipase produced by Penicillium chrysogenum SNP5 grown in solid medium containing waste grease. Bioresour Technol, 120, 300–304.
Hemachander, C., & Puvanakrishnan, R. (2000). Lipase from Ralstonia pickettii as an additive in laundry detergent formulations. Process Biochem, 35(8), 809–814.
Khoo, M. L., & Ibrahim, C. O. (2009). Lipase from thermoalkalophilic Pseudomonas species as an additive in potential laundry detergent formulations. Malaysian Journal of Microbiology, 5(1), 1–5.
Jurado-Alameda, E., Román, M. G., Vaz, D. A., & Pérez, J. L. J. (2012). Fatty soil cleaning with ozone and lipases, a way to develop more environmentally friendly washing processes. Household and Personal Care Today, 7(4), 49–56.
Naganthran, A., Masomian, M., Rahman, R. N. Z. R. A., Ali, M. S. M., & Nooh, H. M. (2017). Improving the efficiency of new automatic dishwashing detergent formulation by addition of thermostable lipase, protease and amylase. Molecules, 22(9), 1577–1594.
Jurado, E., Bravo, V., Luzón, G., Fernández-Serrano, M., Garcia-Román, M., Vaz, D. A., & Vicaria, J. M. (2007). Hard-surface cleaning using lipases: enzyme-surfactant interactions and washing tests. J Surfactant Deterg, 10(1), 61–70.
Liu, Y. Y., Xu, J. H., & Hu, Y. (2000). Enhancing effect of Tween-80 on lipase performance in enantioselective hydrolysis of ketoprofen ester. J Mol Catal B Enzym, 10(5), 523–529.
Okazaki, S., Kamiya, N., Goto, M., & Nakashio, F. (1997). Enantioselective esterification of glycidol by surfactant-lipase complexes in organic media. Biotechnol Lett, 19(6), 541–543.
Bornemann, S., Crout, D. H. G., Dalton, H., & Hutchinson, D. W. (1994). The effects of surfactants on lipase-catalysed hydrolysis of esters: activities and stereoselectivity. Biocatalysis, 11(3), 191–221.
Yan, H. D., Guo, B. H., Wang, Z., & Qian, J. Q. (2019). Surfactant-modified Aspergillus oryzae lipase as a highly active and enantioselective catalyst for the kinetic resolution of (RS)-1-phenylethanol. 3 Biotech, 9(7), 265.
Min, K., Kim, J., Park, K., & Yoo, Y. J. (2012). Enzyme immobilization on carbon nanomaterials: loading density investigation and zeta potential analysis. J Mol Catal B Enzym, 83, 87–93.
Kartal, F. (2016). Enhanced esterification activity through interfacial activation and cross-linked immobilization mechanism of Rhizopus oryzae lipase in a nonaqueous medium. Biotechnol Prog, 32(4), 899–904.
Liu, T., Zhao, Y., Wang, X., Li, X., & Yan, Y. (2013). A novel oriented immobilized lipase on magnetic nanoparticles in reverse micelles system and its application in the enrichment of polyunsaturated fatty acids. Bioresour Technol, 132, 99–102.
Chatterjee, S., Barbora, L., Singh Cameotra, S., Mahanta, P., & Goswami, P. (2009). Silk-fiber immobilized lipase-catalyzed hydrolysis of emulsified sunflower oil. Appl Biochem Biotechnol, 157(3), 593–600.
Tudorache, M., Negoi, A., & Parvulescu, V. I. (2017). Enhancement of the valorization of renewable glycerol: the effects ofthe surfactant-enzyme interaction on the biocatalytic synthesis of glycerol carbonate. Catalysis Today, 297(Part 1), 71–76.
Bencze, L. C., Bartha-Vári, J. H., Katona, G., Toşa, M. I., Paizs, C., & Irimie, F.-D. (2016). Nanobioconjugates of Candida antarctica lipase B and single-walled carbon nanotubes in biodiesel production. Bioresour Technol, 200, 853–860.
Marzuki, N. H. C., Mahat, N. A., Huyop, F., Aboul-Enein, H. Y., & Wahab, R. A. (2015). Sustainable production of the emulsifier methyl oleate by Candida rugosa lipase nanoconjugates. Food Bioprod Process, 96, 211–220.
Raghavendra, T., Panchal, N., Divecha, J., Shah, A., & Madamwar, D. Biocatalytic synthesis of flavor ester “pentyl valerate” using Candida rugosa lipase immobilized in microemulsion based organogels: effect of parameters and reusability. Biomed Res Int, Volume 2014(Article ID 353845). https://doi.org/10.1155/2014/353845.
Zhang, W.-W., Wang, N., Zhou, Y.-J., He, T., & Yu, X.-Q. (2012). Enhancement of activity and stability of lipase by microemulsion-based organogels (MBGs) immobilization and application for synthesis of arylethyl acetate. J Mol Catal B Enzym, 78, 65–71.
Dave, R., & Madamwar, D. (2008). Candida rugosa lipase immobilized in Triton X-100 microemulsion based organogels (MBGs) for ester synthesis. Process Biochem, 43(1), 70–75.
Gilani, S. L., Najafpour, G. D., Heydarzadeh, H. D., & Moghadamnia, A. (2017). Enantioselective synthesis of (S)-naproxen using immobilized lipase on chitosan beads. Chirality, 29(6), 304–314.
Perna, R. F., Tiosso, P. C., Sgobi, L. M., Vieira, A. M. S., Vieira, M. F., Tardioli, P. W., Soares, C. M. F., & Zanin, G. M. (2017). Effects of Triton X-100 and PEG on the catalytic properties and thermal stability of lipase from Candida rugosa free and immobilized on glyoxyl-agarose. The Open Biochemistry Journal, 11, 66–76.
Yu, W. H., Fang, M., Tong, D. S., Shao, P., Xu, T. N., & Zhou, C. H. (2013). Immobilization of Candida rugosa lipase on hexagonal mesoporous silicas and selective esterification in nonaqueous medium. Biochem Eng J, 70, 97–105.
de Oliveira, U. M. F., Lima de Matos, L. J. B., de Souza, M. C. M., Pinheiro, B. B., dos Santos, J. C. S., & Gonçalves, L. R. B. (2018). Effect of the presence of surfactants and immobilization conditions on catalysts’ properties of Rhizomucor miehei lipase onto chitosan. Appl Biochem Biotechnol, 184(4), 1263–1285.
Zhang, W.-W., Yang, X.-L., Jia, J.-Q., Wang, N., Hu, C.-L., & Yu, X.-Q. (2015). Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J Mol Catal B Enzym, 115, 83–89.
Yang, H., & Zhang, W. (2019). Surfactant imprinting hyperactivated immobilized lipase as efficient biocatalyst for biodiesel production from waste cooking oil. Catalysts, 9(11), 914.
Liu, Y., Liu, T., Wang, X. F., Xu, L., & Yan, Y. J. (2011). Biodiesel synthesis catalyzed by Burkholderia cenocepacia lipase supported on macroporous resin NKA in solvent-free and isooctane systems. Energy Fuel, 25(3), 1206–1212.
Ward, K., Xi, J., & Stuckey, D. C. (2016). Immobilization of enzymes using non-ionic colloidal liquid aphrons (CLAs): activity kinetics, conformation, and energetics. Biotechnol Bioeng, 113(5), 970–978.
Sánchez-Otero, M. G., Valerio-Alfaro, G., García-Galindo, H. S., & Oliart-Ros, R. M. (2008). Immobilization in the presence of Triton X-100: modifications in activity and thermostability of Geobacillus thermoleovorans CCR11 lipase. J Ind Microbiol Biotechnol, 35(12), 1687–1693.
Pencreac’h, G., Leullier, M., & Baratti, J. C. (1997). Properties of free and immobilized lipase from Pseudomonas cepacia. Biotechnol Bioeng, 56(2), 181–189.
Castro-Ochoa, L. D., Rodríguez-Gómez, C., Valerio-Alfaro, G., & Oliart-Ros, R. M. (2005). Screening purification and characterization of the thermoalkalophilic lipase produced by Bacillus thermoleovorans CCR11. Enzym Microb Technol, 37(6), 648–654.
Palomo, J. M., Fuentes, M., Fernández-Lorente, G., Mateo, C., Guisán, J. M., & Fernández-Lafuente, R. (2003). General trend of lipase to self-assemble giving biomolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules, 4(1), 1–6.
Zhao, J.-F., Lin, J.-P., Yang, L.-R., & Wu, M.-B. (2019). Enhanced performance of Rhizopus oryzae lipase by reasonable immobilization on magnetic nanoparticles and its application in synthesis 1,3-diacyglycerol. Appl Biochem Biotechnol, 188(3), 677–689.
Zhao, J.-F., Wang, T., Lin, J.-P., Yang, L.-R., & Wu, M.-B. (2019). Preparation of high-purity 1,3-diacylglycerol using performance-enhanced lipase immobilized on nanosized magnetite particles. Biotechnol Bioprocess Eng, 24(2), 326–336.
Goto, M., Noda, S., Kamiya, N., & Nakashio, F. (1996). Enzymatic resolution of racemic ibuprofen by surfactant-coated lipases in organic media. Biotechnol Lett, 18(7), 839–844.
Mahmood, I., Ahmad, I., Chen, G., & Huizhou, L. (2013). A surfactant-coated lipase immobilized in magnetic nanoparticles for multicycle ethyl isovalerate enzymatic production. Biochem Eng J, 73, 72–79.
Goto, M., Kameyama, H., Goto, M., Miyata, M., & Nakashio, F. (1993). Design of surfactants suitable for surfactant-coated enzymes as catalysts in organic media. Journal of Chemical Engineering of Japan, 26(1), 109–111.
Gao, Y., Chen, W., Lei, H., Liu, Y., Lin, X., & Ruan, R. (2009). Optimization of transesterification conditions for the production of fatty acid methyl ester (FAME) from Chinese tallow kernel oil with surfactant-coated lipase. Biomass Bioenergy, 33(2), 277–282.
Song, B.-D., Ding, H., & Wang, S.-C. (2007). Hydrolysis of olive oil catalyzed by surfactant-coated Candida rugosa lipase in a hollow fiber membrane reactor. Biotechnol Bioprocess Eng, 12(2), 121–124.
Wu, J.-C., Ding, H., Song, B.-D., Hayashi, Y., Talukder, M. M. R., & Wang, S.-C. (2003). Hydrolytic reactions catalyzed by surfactant-coated Candida rugosa lipase in an organic-aqueous two-phase system. Process Biochem, 39(2), 233–238.
Song, B.-D., Xing, A.-H., Wu, J.-C., & Wang, S.-C. (2003). Stability of Candida rugosa lipase in isooctane. Chin J Chem Eng, 11(2), 217–219.
Kamiya, N., Goto, M., & Nakashio, F. (1995). Surfactant-coated lipase suitable for the enzymatic resolution of menthol as a biocatalyst in organic media. Biotechnol Prog, 11(3), 270–275.
Goto, M., Kamiya, N., Miyata, M., & Nakashio, F. (1994). Enzymatic esterification by surfactant-coated lipase in organic media. Biotechnol Prog, 10(3), 263–268.
Basheer, S., Mogi, K., & Nakajima, M. (1995). Surfactant-modified lipase for the catalysis of the interesterification of triglycerides and fatty acids. Biotechnol Bioeng, 45(3), 187–195.
Ko, W.-C., Wang, H.-J., Hwang, J.-S., & Hsieh, C.-W. (2006). Efficient hydrolysis of tuna oil by a surfactant-coated lipase in a two-phase system. J Agric Food Chem, 54(5), 1849–1853.
Babali, B., Aksoy, H. A., Tuter, M., & Ustun, G. (2001). Enzymatic esterification of (−)-menthol with lauric acid in isooctane by sorbitan monostearate-coated lipase from Candida rugosa. J Am Oil Chem Soc, 78(2), 173–175.
Basheer, S., Cogan, U., & Nakajima, M. (1998). Esterification kinetics of long-chain fatty acids and fatty alcohols with a surfactant-coated lipase in hexane. J Am Oil Chem Soc, 75(12), 1785–1790.
Isono, Y., Nabetani, H., & Nakajima, M. (1996). Preparation of lipase-surfactant complex for the catalysis of triglyceride hydrolysis in heterogeneous reaction systems. Bioprocess Eng, 15(3), 133–137.
Isono, Y., Nabetani, H., & Nakajima, M. (1995). Lipase-surfactant complex as catalyst of interesterification and esterification in organic media. J Ferment Bioeng, 80(2), 170–175.
Huang, S. Y., Chang, H. L., & Goto, M. (1998). Preparation of surfactant-coated lipase for the esterification of geraniol and acetic acid in organic solvents. Enzym Microb Technol, 22(7), 552–557.
Dandavate, V., & Madamwar, D. (2007). Novel approach for the synthesis of ethyl isovalerate using surfactant coated Candida rugosa lipase immobilized in microemulsion based organogels. Enzym Microb Technol, 41(3), 265–270.
Thakar, A., & Madamwar, D. (2005). Enhanced ethyl butyrate production by surfactant coated lipase immobilized on silica. Process Biochem, 40(10), 3263–3266.
Zhong, X., Qian, J., Guo, H., Hu, Y., & Liu, M. (2014). Biosynthesis of sucrose-6-acetate catalyzed by surfactant-coated Candida rugosa lipase immobilized on sol–gel supports. Bioprocess Biosyst Eng, 37(5), 813–818.
Hsieh, H. J., Nair, G. R., & Wu, W. T. (2006). Production of ascorbyl palmitate by surfactant-coated lipase in organic media. J Agric Food Chem, 54(16), 5777–5781.
Okahata, Y., & Ijiro, K. (1992). Preparation of a lipid-coated lipase and catalysis of glyceride ester syntheses in homogenous organic solvents. Bull Chem Soc Jpn, 65(9), 2411–2420.
Mori, T., Kishimoto, S., Ijiro, K., Kobayashi, A., & Okahata, Y. (2001). A lipid-coated lipase as an efficient hydrolytic catalyst in the two-phase aqueous-organic system. Biotechnol Bioeng, 76(2), 157–163.
Okahata, Y., Fujimoto, Y., & Ijiro, K. (1988). Lipase-lipid complex as a resolution catalyst of racemic alcohols in organic solvents. Tetrahedron Lett, 29(40), 5133–5134.
Noda, S., Kamiya, N., Goto, M., & Nakashio, F. (1997). Enzymatic polymerization catalyzed by surfactant-coated lipases in organic media. Biotechnol Lett, 19(4), 307–310.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goswami, D. Lipase Catalysis in Presence of Nonionic Surfactants. Appl Biochem Biotechnol 191, 744–762 (2020). https://doi.org/10.1007/s12010-019-03212-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03212-w