Abstract
Metronidazole (MTNZ) is an antiprotozoa drug, could be labeled with the 99mTc. MTZL could be used as an ideal vehicle to deliver radioactive decay energy of 99mTc to the sites of tumor, thus facilitate tumor imaging. The process of labeling was done using tin chloride as reducing agent. The optimum conditions required to label 25 μg MTZL were 100 μg stannous chloride, 30 min reaction time, room temperature at pH 7–9 using 0.5 M phosphate buffer. The radiochemical purity of the labeled compound, at the above conditions, was determined using paper chromatography. The yield was about 93%. About 2.5 × l06 of Ehrlich Ascites Carcinoma (EAC) was injected intrapritoneally (i.p) to produce ascites and intramuscularly (i.m) in the right thigh to produce solid tumor in female mice. Biodistribution studies were carried out by injecting solution of 99mTc-MTZL in normal and tumor bearing mice. The uptake in ascites was over 5% of the injected dose per gram tissue body weight, at 4 h post injection and above 4% in solid tumor. These data revealed localization of the tracer in the tumor tissues with high percentage sufficient to use 99 mTc MTZL as promising tool for diagnosis of tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It was reported that the earlier diagnosis of tumor the more the % success in treatment of cancer [1]. The goal is to deliver labeled chemotherapeutic drugs or radioisotopes to the specific tumor site with decreased toxicity to other proliferating tissues as well as neighboring tissues [1]. Many drugs were labeled with radioisotopes to fulfill this purpose. These drugs may be Antimetabolites, antibiotics, antiinflamatories, antibodies or other drugs [2–4]. Many radioisotopes are widely used in cellular radiation studies like 99mTc, 131I and 123I [5–7].
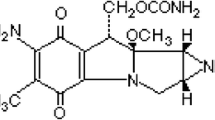
Imidazole derivatives are widely used in medicine [8]. Metronidazole (MTZL) is a 5-nitroimidazole derivative with activity against anaerobic protozoa and anaerobic bacteria. It also has a radiosensitizing effect on hypoxic tumor cells. MTZL act by interfere with DNA by a metabolite in which the nitro group has been reduced [9, 10].
Previous work was done for labeling of many drugs with 99mTc for imaging study like MDP (methylene diphosphonates for bone imaging, DTPA (dithiopentaacetic acid for kidney scanning) [11, 12].
This study was conducted to label metronidazole as a vehicle to carry 99mTc to a tumor cells. This was achieved by injecting EAC to mice either intraperitoneally to induce ascites or intramuscularly in the right thigh to produce solid tumor. Biodistribution study of 99mTc-MTZL in normal mice was investigated. In addition in vitro stability of 99mTc-MTZL and its in vivo biodistribution in EAC mice was also investigated.
Materials and methods
Drugs and chemicals
-
1
Metronidazole was supplied as a gift from El-Kahera for Drugs Chemical Co. USA.
-
2
99mTc was obtained as saline eluent of an expired Mo column.
-
3
Tin chloride was purchased from Sigma Chemical Company, USA.
-
4
All other chemical reagents were of analytical grade (AR), obtained from reputed manufacturers.
-
5
Ehrlich ascites carcinoma (EAC) was kindly supplied from National Cancer Institute, Cairo, Egypt.
Animals
Female Swiss Albino mice weighing 20–25 gm were purchased from the Institute of Eye Research Cairo, Egypt. The animals were kept at constant environmental and nutritional conditions throughout the experimental period and kept at room temperature (22 ± 2 °C) with a 12 h on/off light schedule. Female mice were used in this study due to their susceptibility to Ehrlich ascites carcinoma more than male mice [13]. Animals were kept with free access to food and water all over the experiment.
Labeling procedure and requirement
99mTc-metronidazole was prepared by the following procedures [14]. One milligram MTZL was dissolved in 3 mL purged distilled water with stirring. Tin chloride was added to MTZL solution in evacuated vial with Hamilton syringe and approximately 200–400 MBq 99mTc at room temperature. After a specified interval of time, chromatographic analysis was developed using paper chromatography ascending techniques [15]. The yield of the reaction and the radiochemical purity were determined by paper chromatography using acetone as mobile phase to distinguish between free at the top and both complex and reduced colloids near the point of spotting. On the other hand, 4 N NaoH as a mobile phase differentiate between reduced colloids which persist near the point of spotting and both complex and free, which move towards the front of chromatogram.
Factors affecting % labeling yield
This experiment was conducted to study the different factors that affect labeling yield such as: (1) tin content, (2) substrate content, (3) reaction temperature, (4) pH of the reaction and (5) reaction time.
In the process of labeling, trials and errors were performed for each factor under investigations till obtains the optimum value. The experiment was repeated with all factors kept at optimum changing except the factor under study, till the optimal conditions achieved. [16].
Paper chromatography
Paper chromatography was achieved using two mobile phases acetone and 4 N NaoH with ascending technique [17];
In vitro stability
This experiment was conducted to determine the stability of 99mTc-MTZL after labeling and the impact of time on that compound. The yield was measured at different time intervals (1, 2, 4, 6 and 12 h) after labeling [18].
Induction of tumor in mice
The parent tumor line (Ehrlich Ascites Carcinoma) was withdrawn from 7 days old downer female Swiss albino mice and diluted with sterile physiological saline solution to give 12.5 × 106 cells/ml. About 0.2 ml solution was then injected in mice intraperitoneally to produce ascites, or intramuscularly in the right thigh to produce solid tumor. The animals were maintained till the tumor development was apparent for about 10–15 days [19].
In vivo biodistribution
In normal mice
In vivo biodistribution studies were performed using four groups each comprise six mice. Each animal was injected in the tail vein with 0.2 ml solution containing 50–100 KBq of 99mTc-MTZL. The mice were kept in metabolic cages for the required time. Each group was subjected to scarification by cervical dislocation at the recommended time (15 min, 1 h, 6 h or 12 h) after injection. Organs or tissues of interest were removed, washed with saline, weighted and counted. Correction was made for background radiation and physical decay during the experiment [20]. The weights of blood, bone and muscles were assumed to be 7%, 10% and 40% of the total body weight, respectively [21].
In tumor bearing mice
Biodistribution of 99mTc-MTZL was carried out in two groups of animals each group consists of 24 mice, one ascites bearing group and the other solid tumor bearing mice. Each animal was injected in the tail vein with 0.2 ml solution containing 50–100 KBq of 99mTc-MTZL 2 weeks post inoculation. Each group subdivided to four subgroups of six mice each. Animals in each group were kept in metabolic cages for scarification at its required time, after 15 min, 1 h, 6 h or 12 h post injection of the labeled drug. Sacrification of mice was done by cervical dislocation and the organs or tissues of interest were isolated, weighted and counted for its uptake of radioactivity. Ascites fluid was drained and counted as a whole. The counting tubes, including a standard equivalent to 1% of the injected dose, were assayed in a well type NaI (TI) gamma counter and the results were calculated as percentages of injected dose (I.D) per gram tissue. The final results were expressed as mean ± one standard error [22].
Statistical analysis
The results are expressed as means ± SEM for the indicated number of different experiments. The statistical significance of differences was assessed by unpaired Student’s t-test p < 0.05.
Results and discussion
Paper chromatography
The analysis of chromatographic data revealed the high percentage labeling yield of 99mTc-MTZL. Free 99mTc was obtained from paper acetone chromatogram. Colloid was obtained from 4 N NaoH chromatogram. Complex 99mTc-MTZL was obtained by subtracting colloid from activity obtained near the spotting in acetone chromatogram.
Factors affecting labeling yield
Tin content
Results obtained in this study showed the high yield obtained for 99mTc-MTZL sing tin chloride as reducing agent (Table 1). It was observed that the radiochemical yield significantly increased by increasing the amount of tin from 5 μg to 100 μg (optimum content) at which maximum labeling yield was obtained. By increasing the amount of tin to 200 μg, the yield showed significant decrease in % complex 99mTc-MTZL. A significant reduction in the labeling yield was noted by decreasing the concentration of tin below 100 μg may be explained as at low concentrations of tin, not all 99mTc-was reduced. While by increase the tin content to 200 μg colloid may be increased and hence, % complex decreased [22].
Effect of substrate content
The influence of MTZL content as a substrate on the labeling yield using tin chloride was shown in Table 2. The increase of the concentration of MTZL was accompanied by a significant increase in the labeling yield, where it reached above 90% at 25 μg of MTZL. Increasing the amount of MTZL above 25 μg produced no significant increase in the labeling yield. Increasing the concentration of starting material is usually increases the total incorporation of 99mTc-MTZL since there is a minimum limit to the volume used [23]. A total of 25 μg of MTZL was required to obtain maximum labeling yield, below this concentration significant decrease in the yield. On the other hand, using higher concentration did not significantly affect labeling yield.
Effect of pH
In order to reach the suitable pH value for maximum radiochemical yield, labeling of MTZL with 99mTc was carried out at different pH ranging from 2 to 12. The test was performed using 25 μg of MTZL, 100 μ1 of 0.5 M phosphate buffer of pH7 at 30-min reaction time. The experiment was repeated using 100 μl of each buffer at different pH values. As shown in Table 3, pH 7 is the optimum pH at which the maximum yield was obtained (94.8%). Also, it was observed that at pH 2 or 4, the yield was 8.4%, and 65%, respectively, while at pH values 9 and 11, the yield was 56.0%, 89.5%, respectively. There was significant difference between all pH values of the reaction mediums. The observation of this study that the optimum pH is 7, using phosphate buffers is constant with other previous work [22].
Effect of reaction time
Table 4 shows the relationship between the reaction time and the yield of 99mTc-MTZL. Radiochemical yield was significantly increased from 56.9% to 94.8% with increasing reaction time from 1 to 15 min. Extending the reaction time to 60 min, produced no significant change of the radiochemical yield. Extending the reaction time more than 1 h was associated with decrease in labeling yield. The efficiency of reducing agent may be affected by time and thus yield decreased [24].
In vitro stability of 99mTc-MTZL
In the present experiment, a significant decrease in the stability of 99mTc-MTZL from 94.8% to 91% at 12 h post labeling was observed. Further significant reduction was observed at 24 h post labeling, as the yield was 88%. The labeling yield was about 85% at 48 h post labeling (Tables 5).
Biodistribution of 99mTc-MTZL
In normal mice
Biodistribution study of 99mTc-MTZL in normal mice showed that 99mTc-MTZL was distributed rapidly in blood, stomach, heart and kidney at 15 min post injection. After 1 h, 99mTc-MTZL uptake was significantly decreased in organs like blood, heart, liver and intestine. However, 99mTc-MTZL uptake was significantly increased in bone, muscle, and thyroid after 1 h. At 12 and 24 h post injection, the majority of tissues showed significant decrease in 99mTc-MTZL uptake. Thyroid gland showed significant increase in 99mTc-MTZL uptake at 12 h post injection (Table 6).
In ascites bearing mice
The results of this experiment showed that the sites of greatest uptake of 99mTc-MTZL after 15 min post injection were the blood, heart and lung (16.5, 8 and 7.5), respectively. Table 7 shows that the concentration of 99mTc-MTZL was the lowest in thyroid, muscle and spleen at 15 min post injection. The uptake of 99mTc-MTZL in ascitic fluid was rapidly take place as each ml of ascitic fluid received 3.3% of total activity. The uptake of ascitic fluid was significantly increased after 1 and 12 h to reach 5.2% and 6.5% per 1 ml, respectively. No significant change in the uptake of 99mTc-MTZL at 24 h post injection was observed when compared to its previous value. The data also showed that some organs exhibit significant increase of uptake at 1 h post injection like stomach, ascitic fluid, bone and thyroid. On the other hand, significant decrease in 99mTc-MTZL uptake was observed in blood, heart, kidney and lung at the same time. At 12 h post injection, the majority of organs showed significant decrease in uptake of 99mTc-MTZL. Significant increase was only observed in ascitic fluid and thyroid at 12 h post injection. Similarly, at 24 h post injection, the majority of organs showed additional significant decrease in 99mTc-MTZL uptake. The results of biodistribution study of 99mTc-MTZL in ascites bearing animal revealed that ascites was one of the most site of uptake of 99mTc-MTZL and this was clear at l h and lasted to 24 h post injection. 99mTc-MTZL uptake in ascites was about 25% of the injected dose at 12 h post injection before reflecting the uptake per gram tissue. The uptake of each ml of ascites was 5.2, 6.5 and 6.3 at 1, 12 and 24 h, respectively. It was also observed that ascites was the site of highest uptake considering the average volume of ascites (8.2 ± 0.7). This result suggests the use 99mTc-MTZL in imaging of tumor. The high uptake of 99mTc-MTZL in kidney may reflect the excretion of the drug via urine [23].
In solid tumor bearing mice
Biodistribution of 99mTc-MTZL in solid tumor bearing mice was found to be greatest in blood, heart and stomach (22.8, 12 and 11.1, respectively) at 15 min post injection and lowest in left leg, bone and thyroid (0.8, 1.2 and 2, respectively) (Table 8). The biodistribution of 99mTc-MTZL in the right thigh (inoculated) was greater than that of left one. The uptake of 99mTc-MTZL in right thigh was significantly increased with time at 1 h and 12 h post injection, as it was 5.5 and 7% per g, respectively.
Liver showed significant increase in % 99mTc-MTZL uptake at 15 min, 1 h and 12 h post injection, when compared to ascetic bearing animals. In addition, 99mTc-MTZL uptake in the stomach of solid tumor mice was significantly increased at 15 min, 1 h and 24 h post injection when compared to ascetic bearing mice.
In the present study, the increase in % of 99mTc-MTZL in the blood of solid tumor bearing mice may be due to the large volume of ascetic fluid that are formed in ascetic bearing animals [25]. Significant increase in 99mTc-MTZL uptake in bone of ascites bearing mice may be due to high vascularities to ascetic fluid that may lead to destruction of blood cells. This may activate bone marrow and increase uptake of 99mTc-MTZL in the bone [24].
Conclusion
Incorporation of 99mTc-MTZL to a tumor site was achieved by labeling of MTZL with 99mTc. The appropriate conditions for labeling of 99mTc-MTZL (94% yield) were l00 μg tin as reducing agent, 25 μg MTZL as substrate, at pH 7, at room temperature and 15–30 min reaction time. The great incorporation of 99mTc-MTZL in tumor sites (asites or solid tumor) also facilitates tumor imaging. 99mTc-MTZL was found to be highly localized in tumor sites which considered an ideal victor to carry iodine-125 to the nucleus of tumor cells [25]. Also, increasing the dose (radioactivity) of 99mTc-MTX produced significant increase in the % non-viable cells revealed radiotoxicity of 99mTc-MTX on tumor cells. In conclusion, this study demonstrates a hopeful approach for cancer imaging.
References
Roelvid TA, Horenblas S, Moonen LM, Te Velde A, Meinhardt W, Bartilink H (1997) Ned Tijdschr Geneskd 3:171
Boyd RE (1997) Appl Radiat Isot 48:1027
Molinski V (1982) Int J Appl Radiat Isot 33:811
Davison A (1983) The coordination chemistry of technetium, Cortina International. Verona, Italy, pp 3–14
Robbins PJ (1984) Chromatography of technetium-99m radiopharmaceuticals a practical guide. Society of Nuclear Medicine, New York, p 51
Motaleb MA (2001) Ph.D. Thesis, Faculty of Science, Ain-Shams University, p 25
Boyd RE (1986) J Nucl Spectrum Aust 2(1):18
El-Kolaly MT, Talaat H, Botros N (1996) Proceedings of the sixth conference of nuclear sciences and applications, vol 3. Egypt, p 124
Delgado JN, Remers WA (eds) (1992) Wilson and Gisvold’s textbook of organic and pharmaceutical chemistry, 9th edn., JB Lippincott Company, Philadelphia, p 313
Martindale W (2001) In: The extra pharmacopoeia, 29th edn., Pharmaceutical Press, London, p 658
Svoboda K, Lezama J, Melichor F (1985) J Radioanal Nucl Chem Lett 96:405
El-Kolaly MT (1993) J Radioanal Nucl Chem Lett 170:293
Johannsen B, Spies H (1991) In: Workshop on generator and cyclotron produced radiopharmaceuticals, Riyad, Saudi Arabia, 13–31 October 1991
Abd El-Ghany E (1998) M.Sc. Thesis, Faculty of Pharmacy, Cairo University, p 71
Meares CF, Goodwin DA (1984) J Pro Chem 3(2):215
Subramanian G, Mcafee JG, Blair RJ (1975) J Nucl Med 16:744
Mock B, English D (1987) J Nucl Med 28:1471
Subramanian G, McAfee J (1971) Radiology 98:192
El-Asrag H, El-Kolaly M, El-Sayed AA, Abd El-Bary A (1995) J Radioanal Nucl Chem 196(1):45
Korde A, Venkatesh M (1998) In: International symposium on modem trends in radiopharmaceuticals for diagnosis and therapy, vol 30, IAEA, Lisbon, Portugal, 3 April 1998, SM-355/13
Dupertuis Y, Buchegger F, Pichard C (2003) Cancer Biother Radiopharm 18(1):7
Guzman FM, Archundia LVD, Cortés SH (2008) J Braz Chem Soc 19(3):380
Proulx A, Ballinger J, Galenchyn Y (1989) Int J Rad Appl Instrum A 40:95
Ballinger RJ (2001) Semin Nucl Med 31(4):321
Kenji Y, Hideo K, Kazuki F (1999) J Nucl Med 40:854
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibrahim, I.T. Preparation of 99mTc-metronidazole as a model for tumor imaging. J Radioanal Nucl Chem 281, 669–674 (2009). https://doi.org/10.1007/s10967-009-0040-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0040-8