Abstract
In this study, it was the first report that Bacillus sp. CCZU11-1 was used for the biotransformation of 1,3-propanediol cyclic sulfate (1,3-PDS) and its derivatives. The catalytic performance of Bacillus sp. sulfatase in the biotransformation of 1,3-PDS was significantly improved by biocatalyst permeabilization and immobilization. Using cell permeabilization, the hydrolytic activity of the whole-cell biocatalyst was increased by 3.5-fold after 1.5 h of pretreatment with 10 % (v/v) toluene at 30 °C and pH 7.0. Biotransformation of 20 mM 1,3-PDS for 24 h, 1,3-propanediol (1,3-PD) could be obtained in the yield of 97.4 % under the optimized reaction condition. Additionally, the immobilized biocatalysts, permeabilized cells entrapped in calcium alginate, and cross-linked enzyme aggregates were further employed to biotansform 1,3-PDS. Moreover, the total operational time of the immobilized biocatalysts could reach above 240 h with high conversion rate (>90 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Short-chain diols (e.g., ethylene glycol (EG), 1,2-propanediol (1,2-PD), and 1,3-propanediol (1,3-PD)) are one kind of important platform chemicals that serve as the basic starting materials for producing chemical intermediates, building blocks, and polymers [1–5]. The chemical synthesis of short-chain diols from petroleum materials has been employed for decades [6]. However, the chemical methods for the synthesis of diols often require expensive catalysts, dependence on non-renewable materials, high temperature and/or high pressure, and release of toxic intermediates, resulted in complex processes and low diol yields [7]. Compared to the conventional chemical methods, biocatalytic synthesis methods can offer highly selective reactions, energy-effective operations, and environmentally benign processes, and thus of great interest [2, 5, 8]. Various biocatalysts (e.g., exposide hydrolase, esterase, reductase, and oxidase) have been effectively used for synthesizing diols [5, 8–11].
It is well known that sulfatases can catalyze the hydrolytic cleavage of sulfate esters by liberating inorganic sulfate and the corresponding alcohol [12–15]. In recent years, Acidianus infernus DSM 3191, Bacillus sphaericus FCC 098, Comamonas sp. DSM 115091, Cupriavidus necator DSM 5536, Gulosibacter molinativorax DSM 13485, Metallosphaera sedula DSM 5348, Nocardia nova DSM 43843, Paracoccus sp. DSM 6392, Pseudomonas sp. DSM 6978, Rhizobiaceae sp. FCC 175, Rhodococcus ruber DSM 44541, Synechococcus sp. PCC 7942, Sulfolobus solfataricus DSM 1617, Sulfurisphaera ohwakuensis DSM 12421, and Xanothbacter autotrophicus DSM 431 have been used for the biotransformation of alkylsulfate esters [12–16]. Utilization of whole cells instead of isolated enzymes as biocatalysts for the biotransformation of alkylsulfate esters is a relatively simple performance that does not require the procedure of complex protein separation or the addition of exogenous cofactors [12, 15]. However, less attention has been given to the use of whole-cell catalytic system for catalyzing cyclic sulfates into diols. Cyclic sulfates are a class of versatile synthons for the synthesis of important intermediates [17, 18] and are also employed as functional additives [19, 20]. Significantly, it is a feasible alternative to biosynthesize diols from the corresponding cyclic sulfates by sulfatase.
In this study, it was the first report that the whole cells of Bacillus sp. CCZU11-1 [21, 22] were used for the biotransformation of 1,3-propanediol cyclic sulfate (1,3-PDS) and its derivatives into diols. Moreover, permeabilization and immobilization technologies were used for the enhancement of biocatalytic efficiency. Additionally, the potential application of sulfatase from Bacillus sp. CCZU11-1 was successfully demonstrated in the biotransformation or biodegradation of cyclic sulfates or sulfites.
Materials and Methods
Materials
1,2-Propanediol cyclic sulfate (1,2-PDS; 1a) and ethylene sulfate (ES; 3a) were obtained from Quzhou Ruierfeng Chemical Co., Ltd. (Zhejiang, China). 1,3-Propanediol cyclic sulfate (1,3-PDS; 2a) was purchased from Sigma-Aldrich Co. Ltd. (USA). Glycol sulfide (GS; 4a) was obtained from Aladdin Chemical Reagent Co. Ltd. (Shanghai, China). Dimethyl sulfate (DS; 5a) was purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All other chemicals were obtained from commercial sources and were of analytical grade.
Microorganism and Growth Condition
Bacillus sp. CCZU11-1 with sulfatase activity was preserved in our laboratory [22]. Cell cultivation was performed in a 250-mL flask containing 50 mL of growth medium (10 g/L glucose, 10 g/L peptone, 4.4 g/L KH2PO4, 1.3 g/L Na2HPO4·12H2O, 0.1 g/L MgSO4, 1 g/L NaCl, 1 mM 1,3-PDS, pH 7.0) on an orbital shaker incubator at 180 rpm and 30 °C. After 48 h, whole-cell biocatalysts were harvested by centrifugation (10,000×g) for 5 min at 4 °C, washed, and lyophilized by vacuum-freezing process.
Sulfatase Assays
One unit of activity is defined as the amount of dry cell weight (DCW, g) required to catalyze the formation of 1 μmol diol per minute at 30 °C and pH 7.0. All experiments were performed in triplicate.
Treatment of Lyophilized Cells by Sonication
The lyophilized cells were resuspended in phosphate buffer (100 mM, pH 7.0) to a final cell concentration of 0.018 g DCW/mL [0.1 g (wet weight)/mL]. Cell walls of Bacillus sp. CCZU11-1 were disrupted with the ultrasonic cell disruptor (Ningbo Scientz Biotechnology, JY9Z-II, China) in a cold ice-water bath for 30 times at 400 W (working 3 s and intervals 7 s as one cycle [23]). After the treated samples were found to be disrupted entirely with microscope, the cell debris was removed by centrifugation (20,000×g) for 30 min at 4 °C, and this supernatant obtained was designated as cell-free extract (CFE). The biotransformation, consisting of 20 mM 1,3-PDS and 10 mL CFE, was performed at 30 °C, pH 7.0 and 180 rpm.
Pretreatment of the Whole Cell Biocatalysts for Permeabilization
The lyophilized cells were resuspended in phosphate buffers (100 mM, pH 7.0) to final concentration 0.18 g DCW/10 mL; permeabilization reagent (chloroform, CTAB, DMSO, EDTA, ethanol, n-hexane, toluene, or Triton X-100) was added to final concentration from 2.5 to 15 % (v/v), and then stirred gently on a rotary shaker (180 rpm) at different temperature (20–40 °C) for different time (0.5–3 h). After this, the cells were recentrifuged (10,000×g, 5 min) at 4 °C, washed twice with the same buffer, and analyzed for its sulfatase activity.
Immobilization of Biocatalysts
In order to recycle and reuse the biocatalysts, immobilization of permeabilized cells is an alternative [24]. The permeabilized cells of Bacillus sp. were immobilized with calcium alginate according to the previous report [23]. The immobilization of crude proteins in CFE with glutaraldehyde as cross-linked enzyme aggregates (CLEAs) was performed as follows: after precipitation of the crude proteins with 60 % (w/v) ammonium sulfate saturation, the crude protein was obtained by centrifugation (15,000×g) for 30 min at 4 °C, and then, it was redissolved in phosphate buffer (100 mM, pH 7.0) to form the solution containing 1 mg/mL proteins. Furthermore, the given amount of glutaraldehyde (20 mM) was used for the preparation of CLEAs for 30 min at 15 °C. After that, the suspension was centrifuged (15,000×g, 4 °C) for 30 min, and the CLEAs were then redissolved in the same buffer for the following biotransformation.
Reaction Condition with Permeabilized Cells
The biotransformation reaction with permeabilized cells in the aqueous media was generally performed as follows: certain amount of permeabilized cells (0.04–0.16 g/mL, wet weight) were resuspended in 10 mL KPB buffer (100 mM) containing 20 mM substrate and metal ion additive (Al3+, Ca2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, or Zn2+) (0.1 mM) at certain reaction temperature (20–40 °C), reaction pH (6.0–8.0), and cell dosage (0.0072–0.0288 g DCW/mL) in a shaker (180 rpm). Samples (80 μL each) from reaction media were withdrawn at regular intervals. The bioreaction was stopped by adding 20 μL HCl (1 M) to an 80-μL sample, followed by centrifugation (10,000×g, 5 min). The supernatant (50 μL) was used for the assay.
Reaction Condition with Immobilized Biocatalysts
The biotransformation reaction with immobilized biocatalysts was performed as follows: 5.0 g immobilized cells (containing 0.18 g DCW, 1.28 U/g CDW cells) with calcium alginate or 3.0 mg CLEAs (76.8 U/g CLEAs) were resuspended in the 10 mL Tris–HCl buffer (100 mM, pH 7.0) containing 20 mM substrate and 0.1 mM Fe2+ at 30 °C and 180 rpm. Samples (80 μL each) from reaction media were taken at regular intervals for the assay. For the reusability investigation, the performances were carried out according to the previous method [23].
Analytical Methods
In the bioconversion experiments, various diols, 1,3-PD and its derivatives, were assayed by GC according to the reported method [25].
Results and Discussion
Biotransformation with Lyophilized Cells and CFE
In the biotransformation of 1,3-PDS by the lyophilized cells (unpermeabilized cells) of Bacillus sp. CCZU11-1, 1,3-PD was obtained in a low yield of 87.0 % after 24 h (Fig. 1a). To test if the permeability barrier affect the biotransformation of 1,3-PDS, CFE obtained after the sonication was further employed to transform 1,3-PDS (Fig. 1b). Yield of 98.1 % was obtained after 24 h. It could be concluded that, in whole-cell biocatalysis, permeability barrier affect the biotransformation of 1,3-PDS.
Biotransformation with Permeabilized Cells
Optimization of Permeabilization Condition for Whole Cells
To reduce permeability barrier, modification of whole cells is an alternative approach for increasing whole-cell catalyzed enzymatic reactions [23, 24]. It was reported that permeabilization could enhance the permeability of cell membranes [26]. It is well-known that surfactants and organic solvents have been both proved to be able to break the permeability barriers successfully and to accelerate biocatalytic reactions [23, 26]. As presented in Fig. 2, chloroform, DMSO, ethanol, n-hexane, toluene, and Triton X-100 could be used as permeabilization reagent for improving the catalytic activity. In contrast, CTAB and EDTA drastically decreased the sulfatase activity. Among these reagents, toluene was found to be the most effective enhancer (Fig. 2), and the catalytic activity of the whole-cell biocatalyst was increased by 1.7-fold after the permeabilization with 5 % (v/v) of toluene.
Effects of different permeabilization reagent on the sulfatase activity. Permeabilization reagent (chloroform, CTAB, DMSO, EDTA, ethanol, n-hexane, toluene, or Triton X-100) was added to final concentration 5 % (v/v) and then stirred gently on a rotary shaker (180 rpm) at 30 °C for 1.5 h. All experiments were performed in triplicate
Furthermore, the effects of toluene concentration on the cell permeabilized process were investigated (Fig. 3a), where the toluene concentration ranged from 2.5 to 15 % (v/v). Highest sulfatase activity was obtained when 10 % (w/v) toluene was used for permeabilizing the whole cells. Less sulfatase activity at low concentrations of toluene (≤7.5 %, v/v) might be due to the insufficient amount of reagent for effective permeabilization. The decrease in sulfatase activity at higher concentration of toluene (≥12.5 %, v/v) might be attributed to the leakage of the sulfatases from the whole cells or cell lysis. In addition, the permeabilization time had significant effects on catalytic activity [23]. Thirty minutes of pretreatment time might be insufficient for effective permeabilization, and the sulfatase activity exhibited a maximum after being permeabilized with 10 % (v/v) toluene for 1.5 h (Fig. 3b). Moreover, different permeabilization temperatures were tested to investigate these effects on sulfatase activity (Fig. 3c), and low permeabilized temperature (<30 °C) was propitious to cell permeability. The optimum result was achieved at 30 °C. When the pretreatment temperature was over 30 °C, the catalytic activity declined. At 40 °C, the sulfatase activity decreased greatly. Thus, the optimum toluene concentration, permeabilization time, and permeabilization temperature were 10 % (v/v), 1.5 h, and 30 °C, respectively. Compared to the unpermeabilized cells, the catalytic activity of the whole-cell biocatalyst was increased by 3.5-fold after the permeabilization under the optimized condition.
Effects of toluene concentration (a), permeabilization time (b), and permeabilization temperature (c) on the sulfatase activity. Toluene was added to final concentration from 2.5 to 15 % (v/v) and then stirred gently on a rotary shaker (180 rpm) at different temperatures (20–40 °C) for different times (0.5–3 h). All experiments were performed in triplicate
Biotransformation with Toluene-Permeabilized Cells
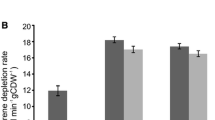
To improve the catalytic efficiency, it was necessary to optimize reaction conditions (e.g., reaction temperature, reaction pH, metal ion additive, and cell dosage) [27–29]. It was found that the sulfatase activity clearly increased with a rise in reaction temperature, reaching a maximum at 30 °C (Fig. 4a). At temperatures over 30 °C, the sulfatase activity decreased considerably, possibly due to the thermal deactivation of sulfatase in the cells during the biotransformation. As shown in Fig. 4b, the highest sulfatase activity was found at pH 7.0. Moreover, metal ions (Al3+, Ca2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, and Zn2+) (0.1 mM) were added into the reaction media, respectively. It was found that Fe2+ (0.1 mM) was the optimum metal ion additive (data not shown). It was reported that Fe2+ was also the appropriate metal ion additive for the sulfatase from Rhodococcus ruber DSM44541 [13]. Finally, the cell dosage was tested on the biocatalytic activity (Fig. 4c), the sulfatase activity was considerably increased with cell dosage increased, reaching a maximum at 0.018 g DCW/mL. When the cell dosage was over the value, the sulfatase activity had no significant increase.
Based on the above results, the optimum reaction conditions were obtained as follows: reaction temperature 30 °C, reaction pH 7.0, Fe2+ 0.1 mM, and cell dosage 0.018 g DCW/mL. Under these conditions, time courses for the biotransformation of 20 mM 1,3-PDS were further investigated (Fig. 5). After 24 h, 1,3-PD was obtained in the high yield (97.4 %). Prolonged the reaction time for another 6 h, the concentrations of 1,3-PDS and 1,3-PD did not change significantly.
To investigate substrate spectrum of toluene-permeabilized cells, various substrates (20 mM) were tested (Table 1). Clearly, the sulfatase from Bacillus sp. showed broad substrate specificity in the hydrolysis of 1,3-PDS (2a) and its derivatives. It showed the highest catalytic activity toward 1,2-PDS (1a), and (S)-1,2-PD could be obtained in the yield of 42.3 % with e.e. value of >99 %. 1,2-PD is a major commodity chemical intermediate currently derived from propylene [3]. Using DS (5a) as substrate, the least catalytic activity was obtained. ES (3a) and GS (4a) could be transformed into the corresponding diols with the yields of 71.6–86.7 %. In this study, it was the first report that short chain diols (e.g., EG 1,2-PD and 1,3-PD) could be synthesized from the corresponding cyclic sulfates by whole cells of Bacillus sp. CCZU11-1. These diols were important platform chemicals for the production of chemical intermediates and polymers [1–4]. As compared to other reported biocatalysts (e.g., exposide hydrolase, esterase, reductase, and oxidase) [8–11], sulfatase has unique biocatalytic properties for catalyzing these sulfates (1a–4a) to diols. Moreover, 1,3-PDS and ES are the additives in propylene carbonate (PC)-based electrolyte for lithium-ion batteries [19, 20]. Direct discharge of these sulfates to the environment may cause serious environmental pollution. Clearly, biotransformation and biodegradation of cyclic sulfates by sulfatase from Bacillus sp. CCZU11-1 is of great interest owing to the desirability of conducting such conversions under mild conditions.
Biotransformation with Immobilized Biocatalysts
In order to recycle and reuse the biocatalysts, immobilized whole-cell biocatalysts are preferred as in this form they could be recycled for reuse, thereby avoiding the need to purify intracellular enzymes and reducing process costs [22, 29–31]. Entrapment seemed to be a better choice for cell immobilization [29, 32]. In this study, toluene-permeabilized cells were immobilized with calcium alginate. Furthermore, the reusability of immobilized whole-cell biocatalysts was investigated. Each batch biotransformation was performed for 24 h. After each batch reaction was over, the immobilized biocatalysts were recovered and then reused for another batch reaction. As shown in Fig. 6, the immobilized cells had good stability and reusability. After the tenth batch, the conversion rate was over 90 %. However, free toluene-permeabilized cells could be used only four times with the conversion rate over 50 %. Significantly, an efficient catalyst recycling was obtained.
Biotransformation of 1,3-PDS by immobilized cells with calcium alginate and free lyophilized cells. The bioreaction, consisting of 20 mM substrate, Fe2+ (0.1 mM), and 5.0 g immobilized cells (containing 0.18 g DCW) or 0.18 g DCW free lyophilized cells (1.28 U/g DCW) in 10 mL Tris–HCl buffer (100 mM, pH 7.0), was carried out for 24 h at 30 °C and 180 rpm. After each cycle, the beads were washed with physiological saline (0.85 % NaCl, w/v) and transferred into a fresh Tris–HCl buffer (100 mM, pH 7.0) for the biotransformation
Additionally, a repeated batch reaction with the glutaraldehyde cross-linked biocatalysts was also carried out. After each run of reaction, the CLEAs were recovered, washed three times with phosphate buffer (100 mM, pH 7.0), and used for the next round of biotransformation. As shown in Fig. 7, the CLEAs were also very stable. After the 12th batch, the conversion rate was over 90 %. As shown in Figs. 6 and 7, the CLEAs had higher catalytic activity and better reusability than the immobilized cells with calcium alginate. Notably, the preparation of CLEAs can be conducted without using harsh chemical such as toluene. However, the immobilized cells were easily recovered than the CLEAs.
Biotransformation of 1,3-PDS with the glutaraldehyde cross-linked biocatalysts. The bioreaction, consisting of 20 mM substrate, Fe2+ (0.1 mM), and 3.0 mg CLEAs (76.8 U/g CLEAs) in 10 mL Tris–HCl buffer (100 mM, pH 7.0), was carried out for 24 h at 30 °C and 180 rpm. After each cycle, the biocatalysts were obtained by centrifugation (15,000×g) for 30 min at 4 °C. Then, they were transferred into a fresh Tris–HCl buffer (100 mM, pH 7.0) for the biotransformation
Based on the above data, it was found that the permeabilized cells entrapped in calcium alginate and CLEAs could be effectively used to tansform 1,3-PD. These two kind of biocatalysts have potential application in the biotransformation of 1,3-PDS and its derivatives.
Conclusion
In this case, it was the first report that Bacillus sp. CCZU11-1 was employed to biotransform 1,3-PDS and its derivatives. After the optimization, the appropriate cell permeabilization conditions were obtained: toluene concentration 10 % (v/v), permeabilization time 1.5 h, and permeabilization temperature 30 °C. Moreover, the optimum reaction temperature, reaction pH, and cells dosage were 30 °C, 7.0, Fe2+ 0.1 mM, and 0.018 g DCW/mL, respectively. 1,3-PD could be obtained from 20 mM 1,3-PDS sulfate in a high yield of 97.4 % by toluene-permeabilized cells after 24 h. Finally, the immobilized biocatalysts, permeabilized cells entrapped in calcium alginate and CLEAs, were used to tansform 1,3-PDS, respectively. Furthermore, the total operational time of the immobilized biocatalysts could reach over 240 h with high conversion rate (>90 %). Significantly, Bacillus sp. CCZU11-1 has high potential in the biotransformation or biodegradation of cyclic sulfates or sulfites.
References
Afschar, A., Vaz Rossell, C., Jonas, R., Quesada, C. A., & Schaller, K. (1993). Journal of Biotechnology, 27, 317–329.
Ainala, S. K., Ashok, S., Ko, Y., & Park, S. (2013). Applied Microbiology and Biotechnology, 97, 5001–5011.
Altaras, N. E., & Cameron, D. C. (1999). Applied and Environmental Microbiology, 65, 1180–1185.
Altaras, N. E., & Cameron, D. C. (2000). Biotechnology Progress, 16(6), 940–946.
Liu, X., Pan, Z. J., & Xu, J. H. (2011). Progress in Chemistry (In Chinese), 23, 903–913.
Haas, T., Jaeger, B., Weber, R., Mitchell, S., & King, C. (2005). Applied Catalysis A: General, 280, 83–88.
Deckwer, W. D. (1995). Fems Microbiology Review, 16, 143–149.
He, Y. C., Ma, C. L., Zhang, X., Li, L., Xu, J. H., & Wu, M. X. (2013). Applied Microbiology and Biotechnology, 97, 7185–7194.
Chang, L., Ouyang, L. M., Xu, Y., Pan, J., & Xu, J. H. (2010). Journal of Molecular Catalysis B: Enzymatic, 66, 95–100.
Hu, Q. S., Xu, Y., & Nie, Y. (2010). Bioresource Technology, 101, 8461–8463.
Reetz, M. T., Bocola, M., Wang, L. W., Sanchis, J., Cronin, A., Arand, M., Zou, J. Y., Archelas, A., Bottalla, A. L., Naworyta, A., & Mowbray, S. L. (2009). Journal of the American Chemical Society, 131, 7334–7343.
Gadler, P., & Faber, K. (2006). Trends in Biotechnology, 25, 83–88.
Pogorevc, M., Strauss, U. T., Riermeier, T., & Faber, K. (2002). Tetrahedron Asymmetry, 13, 1443–1447.
Schober, M., Toesch, M., Knaus, T., Strohmeier, G. A., van Loo, B., Fuchs, M., Hollfelder, F., Macheroux, P., & Faber, K. (2013). Angewandte Chemie International Edition, 52, 3277–3279.
Toesch, M., Schober, M., & Faber, K. (2014). Applied Microbiology and Biotechnology, 98, 1485–1496.
Wallner, S. R., Nestl, B. M., & Faber, K. (2005). Organic and Biomolecular Chemistry, 3, 2652–2656.
Megia-Fernandez, A., Ortega-Muñoz, M., Hernandez-Mateo, F., & Santoyo-Gonzalez, F. (2012). Advanced Synthesis & Catalysis, 354, 1797–1803.
Steinmann, J. G., Phillips, J. H., Sanders, W. J., & Kiessling, L. L. (2001). Organic Letters, 3, 3557–3559.
Li, X. C., Yin, Z. L., Li, X. H., & Wang, C. (2013). Ionics. doi:10.1007/s11581-013-1036-5.
Yao, W. H., Zhang, Z. R., Gao, J., Li, J., Xu, J., Wang, Z. C., & Yang, Y. (2009). Energy and Environmental Science, 2, 1102–1108.
Zhang, D. P., Tao, Z. C., Liu, F., Wang, L. Q., Cai, Z. Q., Qing, Q., & He, Y. C. (2014). 6th Chinese Fermentation Engineering Congress, 4, 116.
He, Y. C., Tao, Z. C., Zhang, D. P., Yang, Z. X., Gao, S., & Ma, C. L. (2014). Biotechnology Letters. doi:10.1007/s10529-014-1670-7.
He, Y. C., Zhang, Z. J., Xu, J. H., & Liu, Y. Y. (2010). Journal of Industrial Microbiology and Biotechnology, 37, 741–750.
Park, Y. M., Choi, E. S., & Rhee, S. K. (1994). Biotechnology Letters, 16, 345–348.
Zheng, Y., Zhang, H. Y., Zhao, L., Wei, L. J., Ma, X. Y., & Wei, D. Z. (2008). Journal of Chemical Technology and Biotechnology, 83, 1409–1412.
Kumar, A., & Pundle, A. (2009). Journal of Molecular Catalysis B: Enzymatic, 57, 67–71.
He, Y. C., Pan, X. H., Xu, X. F., & Wang, L. Q. (2014). Applied Biochemistry and Biotechnology, 172, 3223–3233.
He, Y. C., Yang, Z. X., Zhang, D. P., Tao, Z. C., Chen, C., Chen, Y. T., Guo, F., Xu, J. H., Huang, L., Chen, R. J., & Ma, X. F. (2014). Applied Biochemistry and Biotechnology, 173, 2042–2053.
He, Y. C., Zhou, Q., Ma, C. L., Cai, Z. Q., Wang, L. Q., Zhao, X. Y., Chen, Q., Gao, D. Z., Zheng, M., Wang, X. D., & Sun, Q. (2012). Bioresource Technology, 115, 88–95.
Zhang, T., Li, W. L., Chen, X. X., Tang, H., Li, Q., Xing, J. M., & Liu, H. Z. (2011). World Journal of Microbiology and Biotechnology, 27, 299–305.
He, Y. C., Wu, Y. D., Pan, X. H., & Ma, C. L. (2014). Biotechnology Letters, 36, 341–347.
He, Y. C., Xu, J. H., Su, J. H., & Zhou, L. (2010). Applied Biochemistry and Biotechnology, 160, 1428–1440.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21102011), the Natural Science Foundation of Jiangsu Province (No. BK20141172), and the Open Project Program of the State Key Laboratory of Bioreactor Engineering.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, YC., Liu, F., Zhang, DP. et al. Biotransformation of 1,3-Propanediol Cyclic Sulfate and Its Derivatives to Diols by Toluene-Permeabilized Cells of Bacillus sp. CCZU11-1. Appl Biochem Biotechnol 175, 2647–2658 (2015). https://doi.org/10.1007/s12010-014-1457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1457-2