Abstract
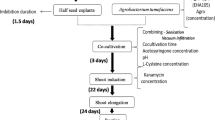
Soybean is a recalcitrant crop to Agrobacterium-mediated genetic transformation. Development of highly efficient, reproducible, and genotype-independent transformation protocol is highly desirable for soybean genetic improvement. Hence, an improved Agrobacterium-mediated genetic transformation protocol has been developed for cultivar PK 416 by evaluating various parameters including Agrobacterium tumefaciens strains (LBA4404, EHA101, and EHA105 harboring pCAMBIA1304 plasmid), sonication duration, vacuum infiltration pressure, and vacuum duration using cotyledonary node explants of soybean prepared from 7-day-old seedlings. The transformed plants were successfully developed through direct organogenesis system. Transgene expression was assessed by GUS histochemical and gfp visual assays, and integration was analyzed by PCR and Southern blot hybridization. Among the different combinations and durations evaluated, a maximum transformation efficiency of 18.6 % was achieved when the cotyledonary node explants of cv. PK 416 were sonicated for 20 s and vacuum infiltered for 2 min at 250 mmHg in A. tumefaciens EHA105 suspension. The amenability of the standardized protocol was tested on four more soybean cultivars JS 90-41, Hara Soy, Co 1, and Co 2 in which all the cultivars responded favorably with transformation efficiency ranging from 13.3 to 16.6 %. The transformation protocol developed in the present study would be useful to transform diverse soybean cultivars with desirable traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean [Glycine max (L.) Merrill], an economically important oil seed crop, belongs to the family Fabaceae. Soybean seed contains 40 % protein and 20 % oil which made the crop as world's foremost provider of protein and oil. The world annual soybean production is 251.5 million metric tons. The USA is the leading country in soybean production with an annual output of 82.3 million metric tons followed by Brazil, Argentina, China, and India [1]. Apart from protein and oil, soybean also contain significant amount of pharmacologically important compounds such as isoflavones, phytic acids, omega 3-fatty acids, and vitamin E.
Soybean is susceptible to several biotic and abiotic factors including salinity, drought, high temperature, bacterial, viral, and fungal pathogens. These biotic and abiotic factors cause considerable damage in soybean and reduce the crop productivity. Great deals of efforts were made in the form of classical breeding to develop superior soybean cultivars against the aforementioned problems. However, classical breeding is difficult due to the fact that soybean is a self-pollinating crop and the genetic variation between different varieties of soybean is narrow [2]. Recent developments in plant genetic engineering made possible to isolate and transfer desirable traits into economically important crop like soybean. Agrobacterium-mediated genetic transformation and particle bombardment are commonly used to transform different cultivars of soybean, and the transformed plants were recovered by direct organogenesis, indirect organogenesis, or somatic embryogenesis. Several reports are available on Agrobacterium-mediated genetic transformation and subsequent transformed soybean plants recovery by direct organogenesis using cotyledonary node explants [3–17]. Extensive research has been carried out in soybean cotyledonary node transformation to improve the transformation percentage which includes addition of acetosyringone during infection [5], addition of thiol compounds during co-cultivation [10, 11], increasing the infection sites by using multi-needle [14], and addition of surfactants into Agrobacterium suspension [15]. Even though these reports showed significant improvement in soybean cotyledonary node transformation, there is a necessity to further improve transformation efficiency by refining the available transformation protocols to meet the increasing demand for genetically modified soybean.
Sonication and vacuum infiltration are the two important transformation parameters that significantly improve the transformation efficiency in many crops. Sonication helps in creating micro-wounds by cavitation across the explants, and vacuum infiltration efficiently infiltrates the Agrobacterium cells into the meristematic region of the explants. Sonication and vacuum infiltration have been successfully employed to improve the transformation efficiency of several economically important crops such as radish [18], citrus [19], cowpea [20], banana [21], lentil [22], and sugarcane [23]. Still, there is no study in soybean cotyledonary nodes emphasizing the role and application of vacuum infiltration as available in other crops. In addition, to date, there has been no report on soybean transformation describing the combined usage of sonication and vacuum infiltration methods for efficient gene delivery into the target cells. Hence, the present study was undertaken with an objective of developing efficient cotyledonary node genetic transformation for Indian soybean cv. PK 416 by employing sonication and vacuum infiltration. Further, the standardized protocol was applied to various Indian soybean cultivars to evaluate the influence of genotype on cotyledonary node transformation.
Materials and Methods
Plant Material
The seeds of five soybean cultivars (PK 416, JS 90-41, Hara Soy, Co 1, and Co 2) were collected from the National Research Center for Soybean (NRCS), Indore, Madhya Pradesh, India, and Tamil Nadu Agricultural University (TNAU), Coimbatore, Tamil Nadu, India. The seeds were multiplied during the appropriate season in the departmental research garden, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India. The standardization was carried out using the soybean cultivar PK 416 due to its better response during initial stage of experiments.
In vitro Seed Germination and Explants Preparation
The seeds of soybean cv. PK 416 were chlorinated for 16 h in a tightly sealed desiccator (Tarsons, Kolkata, India) containing chlorine gas produced by mixing 3.5 ml of 12 N HCl and 100 ml of 5.25 % sodium hypochlorite [24]. The surface-sterilized seeds were inoculated with the hilum proximal to the MS basal medium [25] (pH 5.8) solidified with 0.2 % phytagel (Sigma, St. Louis, USA) and incubated for 3 days under complete darkness at 25 ± 2 °C and later incubated for 4 days under a 16-h photoperiod with light supplied by cool white fluorescent lamps (Philips, New Delhi, India) at an intensity of 50 μmol m−2 s−1. The cotyledonary node explants (~8 mm in size) were prepared from 7-day-old seedlings by removing cotyledons, primary shoot, and hypocotyl.
MIC of Hygromycin B
To determine the sensitivity concentration of Hygromycin B (Sigma, St. Louis, USA) on shoot regeneration of cotyledonary node explant, the explants were cultured on shoot induction medium [SIM: Murashige and Skoogs (MS) salts, MSIII iron, B5 vitamins [26], 87.65 mM sucrose, 3 mM 2-(N-morpholino) ethanesulfonic acid (MES), 2.22 μM N 6-benzylaminopurine (BA), and 0.2 % phytagel (pH 5.8)] for 10 days without hygromycin B. After 10 days of initial culture, the explants were sub-cultured three times (at 10 days interval) into fresh SIM containing different concentrations (2–10 mg l−1) of hygromycin B. After 40 days of culture on SIM, the explants with surviving shoots were transferred to shoot elongation medium [SEM: MS salts, MSIII iron, B5 vitamins, 87.65 mM sucrose, 3 mM MES, 1.45 μM gibberellic acid (GA3), and 0.2 % phytagel (pH 5.8)] containing respective concentration of hygromycin B and incubated for 30 days with sub-culture at every 10 days interval. The minimum inhibitory concentration (MIC) of hygromycin B was determined at the end of shoot elongation period. A control was maintained of explants in respective regeneration medium devoid of hygromycin B. The sensitivity concentration of hygromycin B was also determined at rooting stage to reduce the escapes. Individual elongated shoots of cotyledonary node explants were excised and transferred to rooting medium [RM: MS salts, MSIII iron, B5 vitamins, 87.65 mM sucrose, 3 mM MES, 4.93 μM indole-3-butyric acid (IBA), and 0.2 % phytagel (pH 5.8)] containing various concentrations (2–10 mg l−1) of hygromycin B along with control without hygromycin B. The MIC was determined after 30 days of culture. All the cultures were incubated at 25 ± 2 °C under a 16-h photoperiod at an intensity of 50 μmol m−2 s−1.
Agrobacterium Strain and Binary Vector
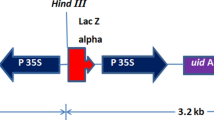
Three Agrobacterium tumefaciens strains such as LBA4404, EHA101, and EHA105 harboring the binary vector pCAMBIA1304 (Fig. 1) were used in the present investigation. A. tumefaciens LBA4404 is an octopine strain with Ach5 chromosomal background carrying pAL4404 as virulence plasmid [27]. EHA101 contains a disarmed version of the agropine-type super virulent Ti plasmid pTiBo542 [28]. EHA105 is a l,l-succinamopine strain with a C58 chromosomal background and contains pEHA105 as virulence helper plasmid [28, 29].
Linear map of the plasmid vector pCAMBIA1304 present within the Agrobacterium tumefaciens strains EHA105, EHA101, and LBA4404 that was used for the transformation experiments. The T-DNA region of pCAMBIA1304 showing the assembly of hpt II gene expression cassette (CaMV 35S P: hpt II: 35S poly A) and gfp-gus fusion gene expression cassette (CaMV 35S P: gfp-gus: nos poly A). CaMV 35S P cauliflower mosaic virus 35S promoter, hpt II hygromycin phosphotransferase II, 35S poly A cauliflower mosaic virus 35S poly A terminator, gfp-gus green fluorescent protein–β glucuronidase fusion gene, nos poly A nopaline synthase poly A terminator
The T-DNA region of the binary vector contains cauliflower mosaic virus 35S (CaMV 35S) promoter-driven hygromycin phosphotransferase II (hpt II) gene and green fluorescent protein-β-glucuronidase (gfp-gus) fusion gene as plant selection and reporter markers, respectively (Fig. 1). The backbone of the vector carries neomycin phosphotransferase II (npt II) gene for bacterial selection. The Agrobacterium strains were maintained on solid AB agar medium supplemented with 10 mg l−1 rifampicin (Sigma, St. Louis, USA) and 50 mg l−1 of kanamycin (Sigma, St. Louis, USA).
Agrobacterium Infection and Co-cultivation of Explants
A single colony from each of the three A. tumefaciens strains was inoculated into 35 ml LB broth containing the aforesaid antibiotics and incubated on an orbital shaker at 28 °C for 16 h at 180 rpm. The bacterial cells were harvested by centrifugation at 6000 rpm for 8 min and suspended in liquid infection medium (LIM) comprising half strength MS salts, MSIII iron, B5 vitamins, 87.65 mM sucrose, 20 mM MES, and 2.22 μM BA (pH 5.4). The OD600 of the bacterial suspensions was adjusted to 0.8 prior to infection and filter sterilized 200 μM acetosyringone (Sigma, St. Louis, USA) was added to the suspension and incubated for 1 h at 28 °C on an orbital shaker (180 rpm).
The cotyledonary node explants (Fig. 2a) were prepared as described earlier, pricked gently, and randomly at the axillary and apical meristematic regions using a sterile hypodermic needle (27G1/1) (Dispovan, New Delhi, India). The wounded explants were inoculated in to the Agrobacterium suspensions and incubated at room temperature with occasional gentle agitation. After 30 min, the cotyledonary node explants were separated from the Agrobacterium suspension; air-dried on sterile Whatman no. 1 filter paper to blot off the excess Agrobacterium, and co-cultivated horizontally for 5 days on co-cultivation medium [CCM: MS salts, MSIII iron, B5 vitamins, 87.65 mM sucrose, 20 mM MES, 200 μM acetosyringone, 3.3 mM l-cysteine, 1.0 mM sodium thiosulfate (STS), 1.0 mM dithiothreitol (DTT), and 0.2 % phytagel (pH 5.4)]. During co-cultivation period, the cultures were incubated at 25 ± 2 °C under total darkness.
Transformation and regeneration of plantlets from cotyledonary node explants of soybean cv. PK 416 infected and co-cultivated with A. tumefaciens strain EHA105 harboring pCAMBIA1304. a Cotyledonary node explants prepared from 7-day-old in vitro seedlings; b induced shoot buds in SIM containing 200 mg l−1 cefotaxime and 50 mg l−1 vancomycin after 10 days of initial culture; c–e selection of regenerated shoots in SIM supplemented with 200 mg l−1 cefotaxime, 50 mg l−1 vancomycin, and 10 mg l−1 hygromycin B (c after 10 days, d after 20 days, and e after 30 days of selection); f elongated shoots in SEM amended with 100 mg l−1 cefotaxime, 25 mg l−1 vancomycin, and 10 mg l−1 hygromycin B after 30 days of culture; g rooted shoot on RM containing 4 mg l−1 hygromycin B after 30 days of culture; h putatively transformed soybean plantlets in growth chamber; i fertile putatively transformed soybean plant grown in greenhouse
Influence of Sonication on Transformation Efficiency
For sonication treatments, the cotyledonary node explants were inoculated into 35 ml Agrobacterium suspension of EHA105 and sonicated for different time durations (0, 5, 10, 20, 30, 40, or 50 s) using water bath sonicator (model 1510 Branson, Branson Ultrasonics, Kanagawa, Japan). After sonication, the explants were transferred into fresh Agrobacterium suspension and incubated at room temperature for 30 min with occasional gentle agitation, air-dried in sterile Whatman no. 1 filter paper, and co-cultivated in CCM for 5 days in dark at 25 ± 2 °C.
Influence of Sonication Combined with Vacuum Infiltration on Transformation Efficiency
In another set of experiment, the cotyledonary node explants sonicated for 20 s were transferred into fresh Agrobacterium suspension of EHA105 and vacuum infiltered at different vacuum pressures (0, 100, 250, 500, or 750) for different time durations (0, 1, 2, or 3 min) using a desiccator (Tarsons, Kolkata, India) connected to a vacuum pump (Indian high vacuum pumps, Bangalore, India). The infiltered explants were incubated in fresh Agrobacterium suspension for 30 min and co-cultivated as described earlier.
Selection and Regeneration of Transformed Plants
After co-cultivation, the explants were washed thrice with sterile double-distilled water and then with sterile liquid shoot induction medium containing 200 mg l−1 cefotaxime (Duchefa, Haarlem, Netherlands) and 50 mg l−1 vancomycin (Duchefa, Haarlem, Netherlands) to remove the Agrobacterium. After washing, the explants were blot dried on sterile Whatman no. 1 filter paper. The cotyledonary node explants were then inoculated into SIM containing 200 mg l−1 cefotaxime and 50 mg l−1 vancomycin without hygromycin B to stimulate shoot induction for the first 10 days, and thereafter, the explants were inoculated into SIM amended with the aforesaid antibiotics along with 10 mg l−1 hygromycin B and sub-cultured trice at 10 days interval to develop multiple shoots. The cotyledonary node explants with multiple shoots were then transferred into SEM containing 100 mg l−1 cefotaxime, 25 mg l−1 vancomycin, and 10 mg l−1 hygromycin B for shoot elongation. The explants with shoots were sub-cultured in SEM at every 10 days interval. After 30 days of culture, the elongated shoots were separated from cotyledonary node explants and inoculated into RM supplemented with 4 mg l−1 hygromycin B and incubated for 30 days. All the cultures were incubated at 25 ± 2 °C under a 16-h photoperiod (50 μmol m−2 s−1) provided by cool white fluorescent lamps (Philips, Delhi, India).
The survived well-rooted plantlets were separated from the culture tubes, washed thoroughly with sterile double-distilled water to remove the media particles from the roots, and transferred to plastic cups containing sterile sand, soil, and vermiculate (1:1:1 v/v/v). The plantlets were covered with polythene bags with minimum puncture and grown in growth chamber at 25 ± 2 °C with 85 % relative humidity (RH) for 2–3 weeks. The plantlets were irrigated once in 2 days. Upon growth, the plantlets were transferred to earthen pots containing sterile sand, soil, and vermiculate (1:1:1 v/v/v) and grown in greenhouse under controlled conditions.
GUS Histochemical Analysis
The gus gene expression was assessed in the putatively transformed cotyledonary node explants, regenerated multiple shoots, stem, leaves, flowers, floral parts, hand-cut sections of stem, and leaf (midrib) by following the method described by Jefferson et al. [30]. The putatively transformed materials and respective controls from wild-type (WT) plants were incubated for 12 h at 37 °C in GUS assay buffer [0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 10 mM EDTA, and 20 % methanol in 50 mM phosphate buffer (pH 7.0)] containing 2 mM X-Gluc (5-bromo-4-chloro-3-indolyl β-d-glucuronide) (SRL Pvt. Ltd., Mumbai, India). After incubation, the plant materials and tissue sections were subjected to dechlorophyllation by washing in acetone/methanol mixture (1:3 v/v) (Sigma, St. Louis, USA) and then visually observed for blue staining. The floral parts, sections of stem, and leaf midrib were observed under a stereozoom microscope (Nikon, Tokyo, Japan) and documented in Nikon color digital camera system DS-Fil-U2 (100–240 V) consisting of NIS elements software package (Nikon, Tokyo, Japan).
Visualization of gfp Gene Expression
The gfp gene expression in hand-cut tissue sections of stem and floral reproductive parts such as androecium, stamens, anthers, and pollen grains from putative transformants along with respective controls from WT plants were visualized under a MZFLIII stereomicroscope (Leica, Heerbrugg, Switzerland) equipped with a 100-W mercury lamp and a ‘GFP-2’ filter set (excitation 480 ± 40 nm; emission 510 nm). The hand-cut tissue sections of stem from putative transformants were subjected to dechlorophyllation by washing in acetone/methanol mixture (1:3 v/v) prior to documentation. The results were documented in a Nikon color digital camera system DS-Fil-U2 (100–240 V) mounted on the MZFLIII stereomicroscope.
Molecular Analysis of Putative Transformants
Five randomly selected GUS-GFP assay-positive putative transformants were analyzed for the hpt II gene integration by polymerase chain reaction (PCR) and Southern blot hybridization. Genomic DNA was isolated from the putative transformants and WT soybean plants by following the method described by Dellaporta et al. [31]. The primers, hpt II FP: 5-GATGTTGGCGACCTCGTATT-3 and hpt II RP: 5-GTGTCACGTTGCAAGACCTG-3, were used to amplify a 407-bp fragment of the hpt II gene. The PCR reaction consisted of 50 ng of genomic DNA or plasmid DNA, 0.2 mM of dNTPs (Genei, Bangalore, India), 1.0 U of Taq DNA polymerase (Sigma Genosys, TX, USA), 0.4 μM of each primer, and 2.5 μl of 10× Taq DNA polymerase buffer in a total of 25 μl reaction. The amplification was performed out in a PTC-100TM thermal cycler (MJ Research Inc., Waltham, MA, USA) programmed with an initial denaturation of DNA at 94 °C for 4 min, followed by 30 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. The reaction products were analyzed by electrophoresis on a 1 % (w/v) agarose gel and visualized by staining with ethidium bromide.
The PCR-positive plants were subjected to Southern blot hybridization to confirm the hpt II gene integration and to determine copy number. Ten micrograms of genomic DNA from PCR-positive plants and WT soybean plants and 5 μg of plasmid pCAMBIA1304 were digested by EcoRI which has single restriction site within the T-DNA region of the pCAMBIA1304 plasmid. The digested genomic DNA and plasmid DNA were size-fractionated on a 1 % (w/v) agarose gel and subsequently transferred to a Hybond N+ membrane (GE Healthcare Limited, Buckinghamshire, England). The membrane was hybridized with a probe prepared by labeling the PCR purified product of hpt II gene using AlkPhos Direct Labeling kit (GE Healthcare Limited, Buckinghamshire, England). The hybridized membrane was washed at 55 °C as per the manufacturer’s instructions (GE Healthcare Limited, Buckinghamshire, England), subjected to chemiluminescent development using CDP-Star substrate, and then exposed to X-ray film (Kodak Biomax Light 1).
Influence of Genotype on Transformation Efficiency
The amenability of the standardized sonication and vacuum infiltration assisted Agrobacterium-mediated genetic transformation protocol developed in the present study using soybean cv. PK 416 was adopted to check the genotypic effect of another four Indian soybean cultivars such as JS 90-41, Hara Soy, Co 1, and Co 2.
Statistical Analysis
For all the experiments, each treatment contained three replicates with at least 100 explants per replicate. Data were statistically analyzed using analysis of variance (ANOVA) using SPSS version 11.09 (IBM corporation). Data are presented as means ± standard error. The mean separations were carried out using Duncan’s multiple range test (DMRT), and significance was determined at 5 % level.
Results and Discussion
Plant Material
In the present investigation, cotyledonary nodes (Fig. 2a) prepared from 7-day-old seedlings were selected as target explants for Agrobacterium-mediated genetic transformation and regeneration of transformed plants. The cotyledonary node explants offered one of the better methods for regeneration of fertile soybean plants due to a short seed-to-seed generation time and needs no requirement for the maintenance of parental donor plants or long-term cultures [4]. In addition, cotyledonary node explants have better regeneration potential when compared to other explants. Hinchee et al. [3] reported that the cotyledonary node method is frequently used in soybean transformation system, which is based on Agrobacterium-mediated T-DNA delivery into regenerable cells in the axillary meristems of the cotyledonary node. In the last two decades, the cotyledonary node explants have been most commonly and successfully used to develop transformed plants in soybean [3, 5–7, 10–12, 15, 17].
MIC of Hygromycin B
Chimerism is a serious problem in transformed plant production. Hence, identifying the truly transformed tissues from non-transformed or partially transformed tissues is a major challenge in Agrobacterium-mediated genetic transformation. This problem could be overcome by using the selection agent in the regeneration medium. It is a well-known fact that the selection agent allows only transformed tissues to regenerate while it inhibits the development of non-transformed tissues. However, it is very crucial to identify the minimum concentration of the selection agent that will kill all the non-transformed cells and allow only the transformed cells to survive and finally regenerate into a complete transformed plant. Hygromycin B is a potent antibiotic that inhibits polypeptide elongation in protein synthesis and considered as an effective selection agent in soybean genetic transformation [32, 11, 15, 33].
In the present investigation, different concentration of Hygromycin B was used during different developmental stages to select the transformed tissues. The percentage of response to shoot induction, shoot elongation, and rooting gradually reduced with the increasing concentration of hygromycin B. Among the various concentrations of hygromycin B evaluated, 10 mg l−1 completely inhibited the shoot regeneration from the cotyledonary node explants and 4 mg l−1 completely arrested the root development from the elongated shoots. Hence, 10 mg l−1 hygromycin B was assigned as MIC during shoot induction and shoot elongation, and 4 mg l−1 hygromycin B was assigned as MIC at the stage of rooting. Olhoft et al. [11] supplemented SIM with 5 mg l−1 hygromycin B and SEM with 10 mg l−1 hygromycin B for selection of transformed soybean shoots. Liu et al. [15] initially supplemented SIM with 3 mg l−1 hygromycin B for 10 days and next 10 days with 5 mg l−1 hygromycin B to select the transformed shoots and carried out shoot elongation in SEM containing 8 mg l−1 hygromycin B. However, in both reports, the elongated shoots were rooted in RM having no hygromycin B.
Selection and Regeneration of Transformed Plants from Cotyledonary Node Explants
The infected cotyledonary node explants after 5 days of co-cultivation were washed with sterile double-distilled water followed by liquid shoot induction medium containing 200 mg l−1 cefotaxime and 50 mg l−1 vancomycin. The blot-dried cotyledonary node explants were inoculated into SIM containing 200 mg l−1 cefotaxime and 50 mg l−1 vancomycin to induce the shoot buds (Fig. 2b). After 10 days, the explants were transferred to SIM containing 10 mg l−1 hygromycin B along with the aforesaid antibiotics and sub-cultured trice at 10 days interval for shoot regeneration (Fig. 2c–e). The hygromycin B-resistant multiple shoots developed from the cotyledonary nodes elongated (Fig. 2f) within 30 days in SEM containing 100 mg l−1 cefotaxime, 25 mg l−1 vancomycin, and 10 mg l−1 hygromycin B. The elongated shoots established well-developed roots (Fig. 2g) in RM containing 4 mg l−1 hygromycin B within 30 days of inoculation. The well-rooted plantlets were transferred to plastic cups (Fig. 2h) containing sterile sand, soil, and vermiculate (1:1:1 v/v/v). Upon growth, the plantlets were transferred to earthen pots (Fig. 2i) containing sterile sand, soil, and vermiculate (1:1:1 v/v/v) and grown in greenhouse under controlled conditions.
Optimization of Transformation Parameters
Influence of Agrobacterium Strain on Transformation Efficiency
The A. tumefaciens strains vary in their virulence capacity, and selection of an efficient strain is prerequisite to achieve maximum transformation efficiency. In the present study, significant difference was observed among the cotyledonary node explants (wounded using sterile hypodermic needle) infected with the three different Agrobacterium strains (LBA4404, EHA101, and EHA105) in terms of shoot induction, shoot elongation, and rooting on hygromycin B-containing medium (Table 1). In addition, there was a notable difference in the number of GUS-GFP-positive plants between the three strains tested (Table 1). Among the three Agrobacterium strains evaluated, EHA105 was proved to be the most effective strain to produce maximum number of GUS-GFP-positive plants with 4.6 % of transformation efficiency (Table 1) which was followed by EHA101 and LBA4404 with a transformation efficiency of 3 and 1.3 %, respectively (Table 1). The significant difference in the transformation efficiency might be due to the different chromosomal background of A. tumefaciens strains and activating potency of the genes in virulence region of the Ti plasmid [27–29, 34]. It was likely for these reasons: the strain EHA105 had stronger ability to infect cotyledonary nodes than EHA101 and LBA4404. Subramanyam et al. [21] assessed the influence of LBA4404, EHA101, and EHA105 on the transformation efficiency of banana and concluded that EHA105 is best over other two strains. Rajesh et al. [35] reported that the transformation efficiency of Podophyllum hexandrum was higher with EHA105 than EHA101 and LBA4404. In previous reports on soybean, the Agrobacterium strain EHA105 was successfully used in the genetic transformation for transformed plant production [5, 7, 11, 36, 37].
Influence of Sonication on the Transformation Efficiency
The meristematic cells are present deep inside the soybean cotyledonary nodes, and it is a well-known fact that the meristematic cells are the most active cells for the genetic transformation. Hence, it is necessary to create the way of travel for Agrobacterium to ease them reaching the meristematic cells region. Application of sonication was adopted as an efficient method to create the micro-wounds by cavitation through which the Agrobacterium could reach the meristematic cells and improves the transformation efficiency [38]. Sonication enhanced the DNA transfer in diverse plant species including dicots, monocots, and gymnosperms [39].
In the present investigation, the mean number of explants responded for shoot induction, multiple shoots produced, number of shoots that elongated, and rooted plantlets that survived on respective medium containing hygromycin B as well as the number of GUS-GFP-positive plants gradually increased with the increasing sonication duration (0–20 s) and at a optimum duration of 20 s, 33 % of infected cotyledonary node explants responded and resulted with a maximum transformation efficiency of 10.3 % (Table 2). Beyond 20 s, due to the severe wounding, the response of the cotyledonary node explants for shoot induction was reduced which ultimately resulted in low transformation efficiency (Table 2). The obtained maximum transformation efficiency using sonication (10.3 %) was significantly higher when compared to that of the transformation efficiency achieved by wounding cotyledonary nodes with sterile hypodermic needle (4.6 %). Trick and Finer [38] and Santarém et al. [40] successfully used sonication to transform the immature cotyledons of soybean and recovered the transformed plants through somatic embryogenesis. Ye et al. [41] applied 20 s sonication to transform the meristem explants of soybean. Solís et al. [39] applied 75 s of sonication to transform Chenopodium rubrum which produced 19.2 % of transformation efficiency. Subramanyam et al. [21] reported that among the various sonication durations analyzed, 6 min was found optimum to achieve maximum transformation efficiency of 34.9 % in banana. Chopra et al. [22] adopted 60 s sonication to obtain 68 % of transformed Lens culinaris Medik plants. Sonication has been successfully applied in Vigna unguiculata [20], Cicer arietinum [42], and Catharanthus roseus [43].
In the present study, the number of regenerated shoots increased with the increasing sonication duration up to an optimum of 20 s. A possible explanation for improved regeneration is due to the formation of sufficient number of micro-wounds to travel the Agrobacterium to infect the meristematic cells which improved the regeneration of cotyledonary nodes on selection medium. In addition, sonication treatment was also proved to stimulate shoot regeneration in squash [44] and flax [45].
Combined Effect of Sonication and Vacuum Infiltration on the Transformation Efficiency
Even though the sonication created micro-wounds by cavitation across the explants for effective Agrobacterium infection, there is a necessity to force the Agrobacterium to the meristematic cells region. Vacuum infiltration was emerged as an effective method to improve the transformation efficiency by creating the negative atmospheric pressure to drive the Agrobacterium to the meristematic cells region [21, 46, 33, 23]. To date, there is no study demonstrating the application of vacuum infiltration for improving the transformation efficiency of soybean cotyledonary nodes. In addition, there is no report available describing the positive correlation between sonication and vacuum infiltration combination in improving the transformation efficiency of soybean.
In the present investigation, the cotyledonary node explants sonicated for 20 s were subjected to vacuum infiltration in Agrobacterium suspension at different vacuum pressures and different time durations. Among the various vacuum pressures (0, 100, 250, 500, or 750 mmHg) analyzed, 250 mmHg was found to be optimum, and beyond that, the mean number of cotyledonary node explants responded for shoot induction, the number of shoots elongated, and rooted plantlets survived on their respective medium containing hygromycin B as well as the number of GUS-GFP-positive plants gradually declined (Table 3). The vacuum duration also played a significant role in the transformation efficiency (Table 3). Among the three different time durations (1, 2, or 3 min) analyzed, 2 min was found to be optimum at 100 and 250 mmHg, where 45 and 58.3 % of infected cotyledonary node explants responded to shoot induction with a transformation efficiency of 15.6 and 18.6 %, respectively (Table 3). Conversely, 1 min was found to be optimum at 500 and 750 mmHg, where in 44 and 42.6 % of infected cotyledonary nodes responded to shoot induction with a transformation efficiency of 14.6 and 11.6 %, respectively (Table 3). Beyond 2 min (at 100 and 250 mmHg) or 1 min (500 and 750 mmHg), the vacuum infiltration negatively affected the explant survival which significantly reduced the transformation efficiency (Table 3). Hence, cotyledonary node explants sonicated for 20 s and vacuum-infiltered for 2 min at 250 mmHg in A. tumefaciens EHA105 suspension was found to be the optimum transformation regime to achieve maximum transformation efficiency of 18.6 % (Table 3). In the present investigation, the vacuum infiltration coupled with sonication showed significant improvement in transformation efficiency (18.6 %) than explants infected with sonication alone in which the maximum transformation efficiency was recorded to be 10.3 %.
In soybean, Franklin et al. [47] wounded mature cotyledons superficially using a sterile narrow-tipped surgical blade and infected the explants under a mild vacuum for 1 h and concluded that the effect of wounding and vacuum showed only little effect on regeneration. In another study, Paz et al. [48] reported that application of vacuum infiltration (24 in of Hg for 15–45 min) in half seeds of soybean reduced the number of explants expressing GUS transient activity and resulted with explants that failed to regenerate in the selection medium. Conversely, in the present study, application of vacuum infiltration significantly improved the transformation efficiency of soybean cotyledonary nodes wounded by means of 20 s sonication. It has been suggested that optimization of vacuum infiltration pressure and time duration along with sonication time period are prerequisite to achieve improved transformation rates, as evidenced in the present study. In similar studies, the combination of sonication and vacuum infiltration significantly improved the transformation efficiency from 28.6 to 39.4 % in banana [21] and 20 to 93 % in cowpea [20]. The combination of sonication and vacuum infiltration also significantly improved the transformation efficiency of radish [18], citrus [19], kidney bean [49], chickpea [50], and sugarcane [23].
GUS Histochemical Analysis
In GUS histochemical analysis, an intense blue color was observed in the putatively transformed cotyledonary node explants (Fig. 3a), regenerated multiple shoots (Fig. 3c), stem (Fig. 3e), leaves (Fig. 3i), hand-cut sections of stem (Fig. 3g), leaf (midrib) (Fig. 3k), flowers (Fig. 4a), and floral parts (Fig. 4b–f and l–q). It indicates that gus gene was integrated and expressed in the putatively transformed soybean genome. Conversely, there was no blue coloration observed in WT counter parts such as cotyledonary node explants (Fig. 3b), regenerated multiple shoots (Fig. 3d), stem (Fig. 3f), leaves (Fig. 3j), hand-cut sections of stem (Fig. 3h), leaf (midrib) (Fig. 3l), flowers (Fig. 4g), and floral parts (Fig. 4h–k and r–t) upon GUS staining.
Histochemical analysis of gus gene expression in different stages of direct organogenesis. a Transient expression of the gus gene in cotyledonary node explants; b non-transformed cotyledonary node explants; c stable expression of the gus gene in regenerated shoots of cotyledonary node explants; d regenerated shoots of non-transformed soybean cotyledonary node; e putatively transformed soybean stem showing gus gene expression; f wild-type soybean stem; g putatively transformed stem cross-section showing gus gene expression; h wild-type soybean stem cross-section; i putatively transformed soybean leaves showing gus gene expression; j leaves of wild-type soybean plant; k putatively transformed leaf midrib cross-section showing gus gene expression; l wild-type soybean leaf midrib cross-section
Histochemical analysis of gus gene expression in flower and floral parts. a Putatively transformed soybean flower showing gus gene expression; b–f and l–q expression of gus gene in floral parts [b gynoecium, c calyx, d standard petal, e and f wings, l androecium with 9 + 1 arrangement of stamens (black arrow 9 stamens fused in bundles; blue arrow separate single stamen), m and n stamens, o anther, p and q pollen grains]; g wild-type soybean flower; h–k and r–t floral parts of wild-type plants (h gynoecium, i calyx, j standard petal, k wings, r androecium, s stamens, and t pollen grains)
GFP Visual Assay
In the present study, hand-cut tissue sections of stem, and floral reproductive parts such as androecium, stamens, anthers, and pollen grains from putative transformants along with WT plants were examined for gfp gene expression. An intense green color was observed in the hand-cut sections of stem (Fig. 5a–c) and reproductive parts such as androecium (Fig. 5e), stamens (Fig. 5f, g), anthers (Fig. 5h), and pollen grains (Fig. 5i) from the putative transformants. It indicates that the gfp gene was successfully integrated and expressed in soybean genome. The gfp gene expression was not detected in non-transformed control stem sections, and in addition, the control tissue sections showed only red auto fluorescence due to the presence of chlorophyll, indicating the absence of endogenous GFP expression (Fig. 5d). There was no GFP expression in floral reproductive parts such as androecium, stamens, anthers, and pollen grains from WT plants (data not shown).
GFP visual assay on putatively transformed soybean plants. a–c Putatively transformed soybean stem cross-sections showing gfp gene expression; d wild-type soybean stem cross-section showing red auto fluorescence; e androecium showing gfp gene expression (black arrow 9 stamens fused in bundles; white arrow separate single stamen); f and g stamens showing gfp gene expression; h single anther showing gfp gene expression; i pollen grains showing gfp gene expression
Molecular Analysis of Putative Transformants
To confirm the transgene integration into the soybean genome, the genomic DNA was isolated from randomly selected five GUS-GFP-positive plants and WT plants by adopting the protocol developed by Dellaporta et al. [31]. The genomic DNA samples along with pCAMBIA1304 plasmid were subjected to PCR using the hpt II gene primers which specifically amplify 407 bp fragment of the hpt II gene coding region. The presence of the amplified fragment of 407 bp in the putatively transformed plant genomic DNA samples (Fig. 6a, lanes 3–7) and pCAMBIA1304 plasmid (Fig. 6a, lane 2) confirmed the presence and integration of hpt II gene into the soybean genome. The DNA from WT plant did not show any amplified fragment (Fig. 6a, lane 8).
Detection of hpt II integration in putatively transformed soybean plants genome. a PCR amplification of the hpt II gene from the genomic DNA of putatively transformed soybean plants. Lane 1 100 bp plus DNA ladder; lane 2 pCAMBIA1304 plasmid as a positive control; lanes 3–7 transformed soybean plants genomic DNA carrying the hpt II gene; lane 8 wild-type soybean genomic DNA as a negative control. b Southern blot analysis of transformed soybean plants. Lane 1 pCAMBIA1304 plasmid as a positive control; lanes 2–6 transformed soybean genomic DNA samples; lane 7 wild-type soybean plant genomic DNA as a negative control. DNA samples were digested with EcoRI restriction enzyme and PCR-amplified product of 407 bp hpt II gene was used as a probe
Further, to confirm the transgene integration and copy number, Southern blot hybridization was performed on the genomic DNA isolated from PCR-positive plants and WT plant. The genomic DNA and pCAMBIA1304 plasmid were digested with EcoRI which cuts once within the T-DNA (between the hpt II gene and gfp-gus fusion gene) and hybridized with alkaline phosphatase-labeled 407 bp PCR-amplified product of hpt II gene. The presence of a single EcoRI restriction site downstream of the hpt II gene within the T-DNA region of pCAMBIA1304 ensured that any hybridization fragments produced were due to an upstream EcoRI restriction site in the plant genome and subsequently corresponded to the number of integrated T-DNA sequences, and the integrated T-DNA fragments would be greater than 2.1 kb. All the five PCR-positive plants were found positive for hpt II gene, and furthermore, the hybridization patterns were non-identical due to different transformation events (Fig. 6b, lanes 2–6). The DNA from WT plant used as a negative control showed no hybridization (Fig. 6b, lane 7), while pCAMBIA1304 generated hybridization signal (Fig. 6b, lane 1). The transformed plants (Fig. 6b, lanes 2–6) exhibited simple hybridization patterns that ranged from single integration event to three loci, and in general, most fragments were greater than 2.1 kb (Fig. 6b).
Influence of Genotype on Transformation Efficiency
It is a well-known fact that the Agrobacterium-mediated genetic transformation is genotype dependent, and each genotype responds differently with different transformation efficiencies. Hence, in the present study, the Agrobacterium-mediated genetic transformation protocol developed using soybean cv. PK 416 was adopted to screen another four cultivars including Co 2, JS 90-41, Hara Soy, and Co 1 (Table 4). Among the five cultivars evaluated, PK 416 showed better transformation efficiency of 18.6 %, followed by Co 1, Hara Soy, Co 2, and JS 90-41 (Table 4). The transformation method based on sonication and vacuum infiltration developed in the present investigation yielded fruitful transformation events with all tested cultivars which proved that the developed method could be useful to transform diverse soybean cultivars.
In conclusion, a highly efficient and reproducible Agrobacterium-mediated genetic transformation protocol was developed for soybean cotyledonary nodes by evaluating various parameters influencing the Agrobacterium-mediated genetic transformation efficiency such as Agrobacterium strains, sonication duration, vacuum infiltration pressure, and vacuum duration. The method developed in the present study has several advantages over the previous reports of soybean. In previous studies on soybean cotyledonary node transformation, the usage of surgical blade or gauge needle was mostly reported for infecting cotyledonary nodes which requires skilled workers and takes a long time to complete the infection procedure. In the present study, cotyledonary node explants wounded using a sterile hypodermic needle and infected with A. tumefaciens strain EHA105 resulted with a low transformation efficiency of 4.6 %. Since the meristematic region is present deep inside the cotyledonary node explant used in the present study, wounding by surgical blade or gauge needle may not be sufficient for efficient gene transfer into the target cells. In addition, infecting with blade or a needle has a possibility in damaging the meristem region and, in turn, may affect the transformation efficiency of cotyledonary node explants. The protocol developed in the present study does not require skilled workers to transform cotyledonary node explants. The protocol also provides the possibility of infecting more number of explants within a short period of time. In addition, combination of sonication and vacuum infiltration treatments used in the present study greatly aided in efficient T-DNA transfer into the meristematic cells found deep in the cotyledonary node explants and resulted with an improved transformation efficiency of (18.6 %). This is the first report describing the positive influence of combined effect of sonication and vacuum infiltration on the soybean cotyledonary node transformation. We strongly believe that the protocol developed in the present study has great potential to be used in diverse soybean cultivars to transfer and express agronomically and economically important traits.
Abbreviations
- MS salts:
-
Murashige and Skoogs salts
- B5 vitamins:
-
Gamborg vitamins
- BA:
-
N 6-Benzylaminopurine
- GA3 :
-
Gibberellic acid
- MES:
-
2-(N-Morpholino) ethanesulfonic acid
- IBA:
-
Indole-3-butyric acid
- CaMV 35S:
-
Cauliflower mosaic virus 35S promoter
- hpt II:
-
Hygromycin phosphotransferase II gene
- npt II:
-
Neomycin phosphotransferase II gene
- gfp-gus :
-
Green fluorescent protein-β-glucuronidase fusion gene
References
FAOSTAT (2009) Agricultural data. Available on http://faostat.fao.org/ site/339/default.aspx.
Shan, Z., Raemakers, K., Tzitzikas, E. N., Ma, Z., & Visser, R. G. (2005). Plant Cell Reports, 24(9), 507–512.
Hinchee, M. A. W., Connor-Ward, D. V., Newell, C. A., McDonnell, R. E., Sato, S. J., Gasser, C. S., et al. (1988). Nature Biotechnology, 6(8), 915–922.
Meurer, C. A., Dinkins, R. D., & Collins, G. B. (1998). Plant Cell Reports, 18(3), 180–186.
Zhang, Z., Xing, A., Staswick, P., & Clemente, T. E. (1999). Plant Cell Tissue and Organ Culture, 56(1), 37–46.
Clemente, T. E., La Vallee, B. J., Howe, A. R., Conner-Ward, D., Rozman, R. J., Hunter, P. E., et al. (2000). Crop Science, 40(3), 797–803.
Donaldson, P., & Simmonds, D. (2000). Plant Cell Reports, 19(5), 478–484.
Xing, A., Zhang, Z., Sato, S., Staswick, P., & Clemente, T. (2000). In Vitro Cellular & Developmental Biology. Plant, 36(6), 456–463.
Olhoft, P. M., & Somers, D. A. (2001). Plant Cell Reports, 20(8), 706–711.
Olhoft, P. M., Lin, K., Galbraith, J., Nielsen, N. C., & Somers, D. A. (2001). Plant Cell Reports, 20(8), 731–737.
Olhoft, P. M., Flagel, L. E., Donovan, C. M., & Somers, D. A. (2003). Planta, 216(5), 723–735.
Paz, M. M., Shou, H., Guo, Z., Zhang, Z., Banerjee, A. K., & Wang, K. (2004). Euphytica, 136(2), 167–179.
Zeng, P., Vadnais, D. A., Zhang, Z., & Polacco, J. C. (2004). Plant Cell Reports, 22(7), 478–482.
Xue, R. G., Xie, H. F., & Zhang, B. (2006). Biotechnology Letters, 28(19), 1551–1557.
Liu, S. J., Wei, Z. M., & Huang, J. Q. (2008). Plant Cell Reports, 27(3), 489–498.
Ye, X., & Qin, H. (2008). Frontiers Agricultural China, 2(2), 156–161.
Kim, W. S., Chronis, D., Juergens, M., Schroeder, A. C., Hyun, S. W., Jez, J. M., et al. (2011). Planta, 235(1), 13–23.
Park, B. J., Liu, Z., Kanno, A., & Kameya, T. (2005). Plant Cell Reports, 24, 494–500.
De Oliveira, M. L. P., Febres, V. J., Costa, M. G. C., Moore, G. A., & Otoni, W. C. (2009). Plant Cell Reports, 28, 387–395.
Bakshi, S., Sadhukhan, A., Mishra, S., & Sahoo, L. (2011). Plant Cell Reports, 30, 2281–2292.
Subramanyam, K., Subramanyam, K., Sailaja, K., Srinivasulu, M. V., & Lakshmidevi, K. (2011). Plant Cell Reports, 30(3), 425–436.
Chopra, R., Aparna, & Saini, R. (2012). Scientia Horticulturae, 143, 127–134.
Mayavan, S., Subramanyam, K., Arun, M., Rajesh, M., Dev, G. K., Sivanandhan, G., et al. (2013). Plant Cell Reports, 32, 1557–1574.
Di, R., Purcell, V., Collins, G. B., & Ghabrial, S. A. (1996). Plant Cell Reports, 15(10), 746–750.
Murashige, T., & Skoog, F. (1962). Physiologia Plantarum, 15(3), 473–497.
Gamborg, O. L., Miller, R. A., & Ojiama, K. (1968). Experimental Cell Research, 50, 151–158.
Hoekema, A., Hirsch, P. R., Hooykaas, P. J. J., & Schilperoort, R. A. (1983). Nature, 303, 179–180.
Hood, E. E., Helmer, G. C., Fraley, R. T., & Chilton, M. D. (1986). Journal of Bacteriology, 168, 1291–1301.
Hood, E. E., Gelvin, S. B., Melchers, L. S., & Hoekema, A. (1993). Transgenic Research, 2, 208–218.
Jefferson, R. A., Kavanagh, T. A., & Bevan, N. W. (1987). The EMBO Journal, 6, 3901–3907.
Dellaporta, S. L., Wood, J., & Hicks, J. B. (1983). Plant Molecular Biology Reporter, 1, 19–21.
Olhoft, P. M., & Phillips, R. L. (1999). Marcel Dekker. New York, 111–148.
Mariashibu, T. S., Subramanyam, K., Arun, M., Mayavan, S., Rajesh, M., Theboral, J., et al. (2013). Acta Physiologiae Plantarum, 35, 41–54.
Wang, G.L., & Fang, H.J. (1998). Science Publisher, Beijing.
Rajesh, M., Jeyaraj, M., Sivanandhan, G., Subramanyam, K., Mariashibu, T. S., Mayavan, S., et al. (2013). Plant Cell Tissue and Organ Culture, 114, 71–82.
Wang, G., & Xu, Y. (2008). Plant Cell Reports, 27(7), 1177–1184.
Chen, W., Song, K., Cai, Y., Li, W., Liu, B., & Liu, L. (2011). Plant Molecular Biology Reporter, 29, 866–874.
Trick, H. N., & Finer, J. J. (1997). Transgenic Research, 6, 329–337.
Solís, J. I. F., Mlejnek, P., Studená, K., & Procházka, S. (2003). Plant, Soil and Environment, 49(6), 255–260.
Santarém, E. R., Trick, H. N., Essig, J. S., & Finer, J. J. (1998). Plant Cell Reports, 17, 752–759.
Ye, X., Williams, E. J., Shen, J., Esser, J. A., Nichols, A. M., Petersen, M. W., et al. (2008). Transgenic Research, 17, 827–838.
Indurker, S., Misra, H. S., & Eapen, S. (2010). Physiology Molecular Biology Plants, 16(3), 273–284.
Wang, Q., Xing, S., Pan, Q., Yuan, F., Zhao, J., Tian, Y., et al. (2012). BMC Biotechnology, 12, 34.
Ananthakrishnan, G., Xia, X., Amutha, S., Singer, S., Muruganantham, M., Yablonsky, S., et al. (2007). Plant Cell Reports, 26, 267–276.
Beranová, M., Rakouský, S., Vávrová, Z., & Skalický, T. (2008). Plant Cell, Tissue and Organ Culture, 94, 253–259.
Subramanyam, K., Rajesh, M., Jaganath, B., Vasuki, A., Theboral, J., Elayaraja, D., et al. (2013). Applied Biochemistry and Biotechnology, doi:10.1007/s12010-013-0359-z.
Franklin, G., Carpenter, L., Davis, E., Reddy, C. S., Al-Abed, D., Abou Alaiwi, W., et al. (2004). Plant Growth Regulation, 43(1), 73–79.
Paz, M. M., Martinez, J. C., Kalvig, A. B., Fonger, T. M., & Wang, K. (2006). Plant Cell Reports, 25(3), 206–213.
Liu, Z., Park, B. J., Kanno, A., & Kameya, T. (2005). Molecular Breeding, 16, 189–197.
Sanyal, I., Singh, A. K., Kaushik, M., & Amla, D. V. (2005). Plant Science, 168, 1135–1146.
Acknowledgments
The authors are thankful to the Department of Biotechnology (DBT) of Ministry of Science and Technology, New Delhi, Government of India for the financial support (BT/PR9622/AGR/02/464/2007). The corresponding author is thankful to University Grants Commission (UGC), Govt. of. India for providing fellowship under UGC–BSR scheme. Subramanyam and Sivanandhan are thankful to Council of Scientific and Industrial Research (CSIR), Govt. of India for the award of Senior Research Fellowship (SRF) to carry out their doctoral work.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arun, M., Subramanyam, K., Mariashibu, T.S. et al. Application of Sonication in Combination with Vacuum Infiltration Enhances the Agrobacterium-Mediated Genetic Transformation in Indian Soybean Cultivars. Appl Biochem Biotechnol 175, 2266–2287 (2015). https://doi.org/10.1007/s12010-014-1360-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1360-x