Abstract
A sonication-assisted, Agrobacterium-mediated, co-cultivation technique was used in an attempt to increase the transformation efficiency of flax. Hypocotyls and cotyledons excised from about 10-day-old flax seedlings grown in vitro were placed into a 10 mM MgSO4 solution, and inoculated with an A. tumefaciens vector bearing the mgfp5-ER gene driven by the CaMV 35S promoter. The explants were subjected to pulses of ultrasound delivered by a sonicator apparatus (35 kHz) for 0–150 s and co-cultivated for 2 h at 27°C. The dried hypocotyls and cotyledons were grown on a selective MS medium to promote shoot regeneration. An electron microscopic study showed that the sonication treatment resulted in thousands of microwounds on and below the surface of the explants. A stereo microscope Leica MZ 12 equipped with a GFP adaptor was used to assess the infection and transformation of plant tissues in real time. After only 48 h and for at least 30 days after bacteria elimination, signs of transgene expression could be seen as a bright fluorescence. Our results show that treatment with ultrasound facilitates an enhanced uptake of plasmid DNA into the cells of flax hypocotyls and cotyledons and that its efficiency depends on the duration of the treatment and the frequency used. SAAT could be a promising tool for enhancing transformation efficiency in flax.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flax, which is well known as a traditional source of fibre, is now cultivated also for good quality oil (linseed) and compounds favourable to human health. As an important economic fibre (flax) and oilseed (linseed) plant, it has in recent years been the focus of both applied and basic research effort in plant cell and biotechnology studies (Wróbel et al. 2004; Milam et al. 2005).
The use of Agrobacterium tumefaciens in transformation systems based on its Ti-plasmid has become a major tool for the insertion of foreign genes into plant cells which express desirable gene products. Transformed plants were already being recovered in 1985 by the use of the co-cultivation of leaf disks with Agrobacterium tumefaciens (Horsch et al. 1985), and since then many plant species have been successfully transformed. One of the main disadvantages of using Agrobacterium for plant transformation is the organism’s host specificity, resulting in low levels of transformation in certain plant species (Holford et al. 1992; Fullner et al. 1996). In some species, including flax (Linum usitatissimum L.), transformation methods have achieved only limited progress, leading mostly to a low efficiency in transformation protocols. Regeneration of flax plants transformed by A. rhizogenes was already reported in 1988 (Zhan et al. 1988) and the same year Jordan and McHughen (1988) published successful and verified transformation in flax using an A. tumefaciens-based vector construct. Since then numerous authors have made attempts to improve the transformation protocol for flax (e.g., Dong and McHughen 1993; Mlynárová et al. 1994; Rakouský et al. 1999), including also protoplast- (Ling and Binding 1997) and particle gun techniques (Wijayanto and McHughen 1999).
Trick and Finer (1997) reported that Sonication-Assisted Agrobacterium-mediated Transformation (SAAT) is an efficient Agrobacterium-based transformation technology for soybean, which enhances also the transient expression of a signal gene for β-Glucuronidase (GUS) in five other species. This technique involves subjecting the plant tissue to brief periods of ultrasound in the presence of agrobacteria. Since then, the ultrasound treatment has been successfully employed to enhance transformation efficiency in several plant species, both by direct DNA transfer to protoplasts and tissue cultures, and co-cultivation with vector bacteria (Joersbo and Brunstedt 1990, 1992; Zhang et al. 1991; Trick and Finer 2000; Zaragozá et al. 2004). Here, we focused our effort on SAAT and an evaluation of its suitability for flax transformation.
Material and methods
Flax (Linum usitatissimum L.) seeds of cvs. Biltstar, Venica, and the breeding line AGT 514/03 were obtained from Agritec Ltd., Šumperk (Czech Republic) and stored in a refrigerator at 4°C. Hypocotyls from about 10-day-old seedlings grown aseptically in vitro were cut into 8 mm pieces and placed for 1 h into a 10 mM solution of MgSO4. The same procedure was applied to the excised cotyledons. Before sonication the suspension was inoculated with A. tumefaciens strain LBA 4404 carrying binary vector pBIN m-gfp5-ER ER (kindly provided by J. Haseloff, MRC, Cambridge, UK) to a final O.D.600 0.4–0.8. The plasmid pBINmGFP5-ER contains the nos promoter driven nptII gene as a plant selection marker and a CaMV 35S driven mgfp5-ER gene, targeted to the endoplasmic reticulum. Target tissues were subjected to pulses of ultrasound for 0–150 s (ca. 35 kHz, 35 W) delivered by the Sonorex RK52 apparatus (Bandelin, Germany). Period of 0 s pulses (no ultrasound applied) was used throughout the experiments as a control variant. After sonication the suspension was co-cultivated for 2 h at 27°C. Hypocotyls and cotyledons were blotted dry on a sterile filter paper and placed on shoot regeneration MS medium containing BAP (0.1 μM) and NAA (0.005 μM) according to Tejklová (1992) for 2 days to co-culture in the dark at 22°C. A debacterization was carried out using sterile water and a solution of the antibiotic Claforan (1000 mg l−1). The explants were then placed on an MS regeneration medium supplemented with antibiotics to eliminate vector bacteria (500 mg l−1 Timentin and 250 mg l−1 Claforan) and to select transgenics (50 mg l−1 kanamycin). Monitoring of infection and transformation of hypocotyls and cotyledons was carried out in real time using a Leica MZ 12 stereo dissecting microscope equipped with a fluorescence module consisting of a 100 W mercury lamp and GFP excitation and emission filters integrated with the CCD camera. Transient gfp gene expression could already be seen 48 h after bacteria removal. The expression of GFP was monitored continuously up to 30 days following the SAAT with the aim of evaluating and selecting stable transformed tissues and plants. After a six to nine months period the polymerase chain reaction (PCR) following the procedure described by Hraška et al. (2008) was used to confirm the presence of the gfp transgene in regenerated shoots and to exclude the simultaneous occurrence of the vir gene as a consequence of persistance of the vector bacteria.
Results
The first set of experiments was carried out using a more powerful 60 W laboratory sonicator (Chirana, Czechoslovakia). Although this obsolete apparatus was shown later to be too strong for the purpose of our studies, some important data on the sensitivity of flax explants and the ultrasound dose effect on plant regeneration were obtained. The proper treatment of the hypocotyl explants stimulated in cv. Venica both bud formation and plant regeneration (data not shown). This could be very important, especially in the case of cultivars with a low regeneration ability, but further studies in this respect are needed. The sets of experiments which followed were aimed mainly at optimizing the sonication conditions (duration of ultrasound treatment, cultivar response, and frequency of stable transformations). For this purpose cv. Biltstar, showing a relatively high regeneration ability in vitro was chosen together with the breeding line AGT 514/03, both representing a linseed type of flax. Compared to the co-cultivation method—the most common for flax transformation—the SAAT seems to decrease in a more pronounced way the number of viable explants in some sensitive genotypes like breeding line AGT 514/03 (Tables 1, 2). In the case of AGT 514/03 the viability of both types of explants generally decreased with the duration of ultrasound treatment. But the most deleterious effect on plant tissues was caused by the presence of bacteria during a co-cultivation (variant C50). Data obtained for cv. Biltstar showed irregular variation, especially in the data related to the hypocotyls. It could be speculated that the level of experimental variation in SAAT experiments depends on the actual physiological conditions of the original material—plants because the plant parts are subjected to numerous stresses (dissection, sonication, bacteria treatment, etc.). For these reasons, only the healthiest plantlets grown for a substantial period of time under proper conditions, especially of illumination, should be used.
The first (mostly transient) signs of GFP expression could be seen after about two days following the elimination of vector bacteria, i.e. on ca. fifth day after SAAT (Fig. 1). Because our aim was to increase the efficiency of stable transformation, the period of detailed evaluation of explant fluorescence was postponed to day 30 (based on our experience with other plant species where transient expression diminished within 2–3 weeks after co-cultivation). The influence of the length of the sonication period on the efficiency of transformation for cv. Biltstar, as indicated by the fluorescence of the explants is shown in Fig. 2. Data obtained from PCR molecular analyses for the gfp transgene revealed its presence in regenerated plants, i.e., it confirmed the efficiency of SAAT and a double selection (based on resistance to Kn, GFP fluorescence)—see Fig. 3.
Expression of a model transgene gfp in plant tissues can be evaluated non-invasively under stereo microscope Leica MZ12 in real time keeping the aseptic conditions of in vitro cultures. GFP presence and thus gfp transgene expression is visible already on the fifth day after SAAT. Numbers indicate the length of sonication (Sonorex RK52, 35 kHz) in seconds (cv. Biltstar). The ultrasound applied to an excess (150 s) has a deleterious effect on cell/tissue viability
Influence of the sonication period length on amount of flax hypocotyl and cotyledonary explants expressing the GFP; an indication of transformation efficiency. Evaluations were performed under a stereomicroscope, based on the presence/absence of fluorescent foci. Per each variant of ultrasound treatment and type of explant from 60 to 120 tissue samples were scored 30 days following the SAAT
Gel electrophoresis of PCR products of gfp gene sequence multiplication. An arrow indicated position of the product of expected mass. The polymerase chain reaction (PCR) following the procedure described by Hraška et al. (2008) was used to confirm the presence of the gfp transgene in regenerated shoots and to exclude the simultaneous occurrence of the vir gene as a consequence of persistance of the vector bacteria after 6–9 months to SAAT. MW = 100 bp molecular weight marker; PC = positive control (pBINmGFP5-ER plasmid); 0–150 = DNA isolated of double-selected flax plants regenerated from transformation experiments based on a simple co-cultivation (0) or sonication for 50–150 s (50–150) preceeding to the co-cultivation; NC = negative control (DNA from untransformed plants)
Discussion
For some period of time the SAAT has been only rarely used to enhance the efficiency of plant transformation procedures. Later, it was shown that SAAT increases both the transient expression and stable transformation of some species which are not easily transformable, e.g. of soybean (Trick and Finer 2000), black locust (Zaragozá et al. 2004) and Chenopodium rubrum (Flores Solís et al. 2007). Sonication could also stimulate massive explant growth and the regeneration response of some species recalcitrant in tissue cultures, such as squash (Ananthakrishnan et al. 2007). Our introductory experiments with cv. Venica indicated also the possibility of stimulation of regeneration in some flax cultivars. If ultrasound is applied to excess, it could cause hyperhydration of plant regenerants or loss of cell/tissue viability. This means that the intensity and period of sonication should be very carefully evaluated and optimized for each species, type of tissue and purpose of the experiment.
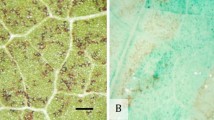
It is speculated that the microwounds produced by sonication could either disturb or eliminate the surface barrier preventing the regeneration of cells located deeper in the plant tissues (Ananthakrishnan et al. 2007) and/or allow Agrobacterium to efficiently infect deep within the tissue and stably transform. In our preliminary experiments performed with a powerful sonicator (source ca. 60 W) we discovered that only a brief period of treatment could be used. It was shown that 10 s of sonication made microcavities in cotyledons (enabling easier penetration of vector bacteria) while its application for periods of only 30 s caused serious damage to hypocotyls—see SEM photographs (Figs. 4, 5). It was clear that proper doses of ultrasound (5–20 s period for source 60 W, cv. Venica) may efficiently stimulate bud formation and plant regeneration of flax hypocotyl segments. Using the Sonorex RK52 sonicator we were able more precisely to apply ultrasound to reach reproducible results. Our results using real time microscopy studies indicated that intervals of about 50 s of sonication (35 kHz, 35 W) could substantially increase the efficiency of stable flax transformation (Fig. 6). Usually, the SAAT (50–100 s, 35 W) enhanced the proportion of fluorescing hypocotyls by 1/4 and cotyledons by (1/4)–(1/2) as compared to non-sonicated explants, depending on duration of ultrasound treatment and variety used. The frequency of stabile transformants showing an integration of the gfp gene into flax genome reached 0.88–2.73% as related to cultured viable hypocotyl segments in the best variants. This is the first report on successful SAAT application to flax, representing thus an additional alternative to the co-cultivation and biolistic method for flax transformation. On the other hand, more extensive and detailed studies are needed to establish reliable protocols for common flax and linseed cultivars.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- CaMV 35S:
-
Cauliflower mosaic virus promoter
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- Kn:
-
Kanamycin
- SEM:
-
Scanning electron microscopy
- NAA:
-
Naphthalene acetic acid
- gfp :
-
Green fluorescent protein gene
- mgfp5-ER :
-
Modified gene for Green fluorescent protein
- nptII :
-
Neomycin Phosphotransferase II gene
- nos (promoter):
-
Nopaline Synthase promoter
References
Ananthakrishnan G, Xia X, Amutha S, Singer S, Muruganantham M, Yablonsky S, Fischer E, Gaba V (2007) Ultrasonic treatment stimulates multiple shoot regeneration and explant enlargement in recalcitrant squash cotyledon explants in vitro. Plant Cell Rep 26:267–276. doi:10.1007/s00299-006-0235-1
Dong JZ, McHughen A (1993) An improved procedure for production of transgenic flax plants using Agrobacterium tumefaciens. Plant Sci 88:61–71. doi:10.1016/0168-9452(93)90110-L
Flores Solís JI, Mlejnek P, Studená K, Procházka S (2007) Application of sonication-assisted Agrobacterium-mediated transformation in Chenopodium rubrum L. Plant Soil Environ 49:255–260
Fullner KJ, Cano LJ, Nester EW (1996) Pilus assembly by Agrobacterium T-DNA transfer genes. Science 273:1107–1109. doi:10.1126/science.273.5278.1107
Holford P, Hernandez N, Newbury HJ (1992) Factors influencing the efficiency of T-DNA transfer during co-cultivation of Antirrhinum majus with Agrobacterium tumefaciens. Plant Cell Rep 11:196–199
Horsch RB, Fry JE, Hoffman NL, Einchholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hraška M, Heřmanová V, Rakouský S, Čurn V (2008) Sample topography and position within plant body influence the detection of the intensity of green fluorescent protein (GFP) fluorescence in the leaves of transgenic tobacco plants. Plant Cell Rep (in press). doi:10.1007/s00299-007-0431-7
Jordan MC, McHughen A (1988) Glyphosate tolerant flax plants from Agrobacterium mediated gene transfer. Plant Cell Rep 7:281–284
Joersbo M, Brunstedt J (1990) Direct gene transfer to plant protoplasts by mild sonication. Plant Cell Rep 9:207–210. doi:10.1007/BF00232181
Joersbo M, Brunstedt J (1992) Sonication: a new method for gene transfer to plants. Physiol Plant 85:230–234. doi:10.1034/j.1399-3054.1992.850215.x
Ling HQ, Binding H (1997) Transformation in protoplast cultures of Linum usitatissimum and L. suffruticosum mediated with PEG and with Agrobacterium tumefaciens. J Plant Physiol 151:479–488
Millam S, Obert B, Preťová A (2005) Plant cell and biotechnology studies in Linum usitatissimum – a review. Plant Cell Tissue Organ Cult 82:93–103. doi:10.1007/s11240-004-6961-6
Mlynárová L, Bauer M, Nap JP, Preťová A (1994) High efficiency Agrobacterium-mediated gene transfer to flax. Plant Cell Rep. 13:282–285. doi:10.1007/BF00233320
Rakouský S, Tejklová E, Wiesner I, Wiesnerová D, Kocábek T, Ondřej M (1999) Hygromycin B − an alternative in flax transformant selection. Biol Plant 42:361−369. doi:10.1023/A:1002457000944
Tejklová E (1992) Long-term in vitro shoot-tip culture and plant regeneration in flax. Rost. Výroba (Praha) 28:1009–1022 (In Czech)
Trick HN, Finer JJ (1997) SAAT: sonicated-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336. doi:10.1023/A:1018470930944
Trick HN, Finer JJ (2000) Use of Agrobacterium expressing green fluorescent protein to evaluate colonization of sonication-assisted Agrobacterium-mediated transformation-treated soybean cotyledons. Lett Appl Microbiol 30:406–410
Wijayanto T, McHughen A (1999) Genetic transformation of Linum by particle bombardment. InVitro Cell Dev-Pl 35:456–465
Wróbel M, Zebrowski J, Szopa J (2004) Polyhydroxybutyrate synthesis in transgenic flax. J Biotechnol 107:41–54. doi:10.1016/j.jbiotec.2003.10.005
Zaragozá C, Muñoz-Bertomeu J, Arrillaga I (2004) Regeneration of herbicide-tolerant black locust transgenic plants by SAAT. Plant Cell Rep 22:832–838. doi:10.1007/s00299-004-0766-2
Zhan XC, Jones DA, Kerr A (1988) Regeneration of flax plants transformed by Agrobacterium rhizogenes. Plant Mol Biol 11:551–559. doi:10.1007/BF00017455
Zhang LJ, Cheng LM, Xu N, Zhao NM, Li CG, Jing Y, Jia SR (1991) Efficient transformation of tobacco by ultrasonication. Biotechnology 9:996–997
Acknowledgements
The authors are grateful for the financial support received from Ministry of Education, Youth and Sport of the Czech Republic (grants 1M06030, 1PO5ME800). Linguistic revision was kindly performed by John McAvoy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beranová, M., Rakouský, S., Vávrová, Z. et al. Sonication assisted Agrobacterium-mediated transformation enhances the transformation efficiency in flax (Linum usitatissimum L.). Plant Cell Tiss Organ Cult 94, 253–259 (2008). https://doi.org/10.1007/s11240-007-9335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9335-z