Abstract
This study aimed to evaluate edible films and coatings based on carboxymethylcellulose (ECMC), alginate (EALG), and cassava starch (ECS) polymers that have been nano-emulsified by ultrasound, using food-grade sunflower oil as the lipid source and Tween 20 as an emulsifying agent. The formulations were characterized by particle size distribution, and molecular interactions in the edible films were analyzed using Fourier-transform infrared spectroscopy. Furthermore, the morphological behavior of the films was studied through scanning electron microscopy. Additionally, to assess their effectiveness as a green preservation technology for minimally processed foods, the formulations were applied as edible coatings on carrot slices, and peroxidase activity, total phenols, antioxidant capacity, and color changes during refrigerated storage were studied. FTIR spectra confirmed interactions between the compounds in the formulations of ECMC and ECS films. Instead, the spectrum of EALG was significantly similar to that of ALG, showing poor affinity between alginate and the oil in the nano-emulsion. ECS films showed a compact, smooth, and uniform surface, with oil drops inserted uniformly throughout the matrix. The biodegradable film based on ECS is considered a promising alternative for use as an edible film or coating for food preservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Edible films (EF) and coatings (EC) based on natural components, such as biopolymers, are used as covering, wrapping, and packaging materials. They represent one of the most promising approaches to ensure and prolong the quality and shelf life of various food products. When specifically applied to vegetables, these films and coatings can control respiration and senescence (Jafarzadeh et al., 2021). In addition, EF and EC materials are cost-effective, impart no odor or taste to the product, and serve as a protective barrier against external factors, including moisture, oxygen, and microorganisms (Armghan et al., 2022). They also offer an environmentally friendly option, as opposed to petroleum-based plastic films, as they can be readily degraded (Kaur et al., 2021). Among biopolymer-based edible film and coating materials, proteins, polysaccharides, and lipids have been successfully employed either individually or in combination (Sharma et al., 2022). Polysaccharide-based films, in particular, have garnered recent attention due to their remarkable ability to form a strong, cohesive, and continuous matrix (Vasco et al., 2022).
Promising polysaccharides for use in edible films and coatings include sodium carboxymethylcellulose, sodium alginate, and cassava starch. Carboxymethylcellulose is a biopolymer derived from cellulose, which is naturally insoluble (Nechita & Roman, 2020). This biopolymer is synthesized by mercerization and esterification of cellulose, resulting in a polymer with a cellulose backbone and carboxymethyl groups attached to at least one hydroxyl group (Pinto et al., 2022). Carboxymethylcellulose is important for industrial applications such as cosmetics, pharmaceuticals, and food (Sun et al., 2023).
Sodium alginate is an attractive compound that can create robust films due to its linear structure (Blanco-Pascual et al., 2014). It is an unbranched linear polymer composed of β-D-mannuronic acid units (M unit) and α-L-guluronic acid units (G unit), connected by 1,4-glycosidic bonds in three ways of MM, MG, and GG blocks. The rings of two structural units are connected with carboxyl groups and two hydroxyl groups in different orientations. Alginate’s ability to react with polyvalent metal cations, specifically calcium ions, through the “egg-box” phenomenon, allows for the production of films with good water barrier, mechanical strength, cohesion, and stiffness properties (Dave et al., 2023).
Cassava starch is another polysaccharide with significant potential for producing edible films. Cassava roots are one of the world’s primary sources of starch. In Argentina, cassava production has been steadily increasing, with an average of approximately 186,500 tons per year (FAOSTAT, 2017). Unlike some other starches, cassava starch can produce films and coatings that exhibit excellent flexibility and transparency (Vicentini & Cereda, 1999). From a molecular point of view, starch is a semi-crystalline polymer compound consisting of two principal polymer types: amylopectin, which is the main polymer in starch and has 1,4-linked glucose chains (with a polymerization degree < 100) joined by α-1,6-linked branch points, and amylose, which constitutes < 35% of starches and is composed of long α-1,4-linked linear chains (with a polymerization degree between 100 and 10,000) with rare α-1,6-branched points (Matheus et al., 2023). Few studies on the application of cassava starch films in foodstuffs have been reported in the literature. Adjouman et al. (2018) found that cassava-based coatings allowed the preservation of fresh tomatoes for up to one month while maintaining the different key quality parameters. Additionally, an emulsified coating formulation based on cassava starch was studied for potential application to cut apples for the maintenance of product quality (Chiumarelli & Hubinger, 2012).
However, due to the highly hydrophilic nature of these polysaccharides, the films and coatings they produce have poor water vapor barrier properties. An alternative solution to overcome this limitation is the incorporation of hydrophobic molecules, such as lipids and emulsifying agents, into the film composition.
Among hydrophobic substances, vegetable oils offer the advantage of being liquid at room temperature. This property allows them to interact more easily with biopolymers, as emulsions can be formed without the need for additional heat treatments (Ghanbarzadeh & Almasi, 2011). Sunflower oil (SO), in particular, is one of the most commonly used vegetable oils. It primarily consists of monounsaturated and polyunsaturated fatty acids, such as oleic and linoleic acids, with low percentages of saturated fatty acids. Additionally, it contains essential vitamins, including A, D, and, most notably, E (alpha-tocopherol) (Gunstond, 2011).
The main EF and EC properties are primarily influenced by their formation process. Smaller and more homogeneous droplets result in a more stable emulsion (Seifari & Ahari, 2020). Nano-sized emulsions can be achieved through mechanical (high-energy) or chemical (low-energy) methods. Mechanical treatments, such as ultrasonic emulsification, are commonly used to produce stable nano-emulsions with particles smaller than 300 nm. Ultrasonic emulsification generates high-intensity acoustic waves, causing droplets to collapse due to cavitation effects and resulting in nano-sized droplets (Sharma et al., 2022).
This study aims to evaluate edible films and coatings based on carboxymethylcellulose, alginate, and cassava starch polymers that have been nano-emulsified by ultrasound, using food-grade SO and Tween 20 as an emulsifying agent. The formulations were characterized by particle size distribution, and molecular interactions in the edible films were analyzed using Fourier-transform infrared (FTIR) spectroscopy. Furthermore, the morphological behavior of the films was studied through scanning electron microscopy (SEM). Additionally, to assess their effectiveness as a green preservation technology for minimally processed foods, the formulations were applied as edible coatings on carrot slices. This involved studying peroxidase activity, total phenols, antioxidant capacity, and color changes during refrigerated storage.
Materials and Methods
Materials
Sodium carboxymethylcellulose (CMCNa) (Marpal), alginic acid sodium salt (SA) (Sigma-Aldrich, 180947, MW 120–190 g/mol) and cassava starch (CS) (Grandiet) were used to form the films. The following reagents were incorporated: anhydrous calcium chloride CaCl2 (Anedra AG), glycerol (99.9% Biopack, Gly), commercial sunflower oil (SO) and Tween 20 (99.9% Biopack). Films formulated with polymers and plastizicer, but without oil were called CMC, ALG and CS.
The carrots (Daucus carota) variety “Nantesa”, acquired in the regional market of the city of La Plata (Buenos Aires, Argentina), was used in the minimally processed carrots (MPC) step. Hydrogen peroxide (AQ), guaiacol (Sigma Aldrich), methanol (Anedra), sodium carbonate (BioPack), Folin-Ciocalteau reagent (BioPack) and 2,2-diphenyl-1-picrylhydrazyl (DPPH·) (Sigma Aldrich) were used for analytical determinations in the MPCs.
Preparation of Filmogenic Dispersions
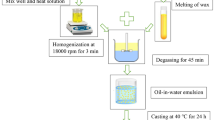
Aqueous dispersions of CMCNa (1% w/v) and SA (2.0% w/v) were obtained at 80 °C and 70 °C, respectively, with constant stirring until complete dissolution (20 and 10 min, respectively) (Table 1). The CS suspension (3.0% w/v) was obtained by gelatinization at 70 °C in a hot water bath with constant agitation for 20 min. Once the dispersions were obtained, glycerol was added as a plasticizing agent (30% w/w for CMC, 40% for ALG and 20% for CS). Finally, SO (0.25% w/v for CMC, 0.15% w/v for ALG, and 0.25% w/v for CS) and Tween 20 (0.2% w/v from Biopack) were added to obtain the nano-emulsions ECMC, EALG and ECS, respectively. All concentrations used were selected from previous tests (Vasco et al., 2018).
Preparation of Nano-emulsions, Films and Coatings
Nano-emulsified systems were prepared using an ultrasonic tip (Sonic Ruptor 400, Onmi International, USA) with a nominal power of 400 W and a frequency of 20 kHz. They were formed through the ultrasonic flow cell for 5 min at 40% sonication amplitude and their size particle and viscosity were analyzed immediately after processing.
The nano-emulsified films (ECMC, EALG and ECS) were casted onto acrylic plates, dried in an oven at 37 ºC up to constant weight, and then conditioned at 75% relative humidity for two weeks. The same procedure was applied to control formulations (CMC, ALG and CS). ALG and EALG were crosslinked with a CaCl2 solution (2%, w/v) (Vasco et al., 2022).
Carrots (Daucus carota v. Nantesa) were carefully selected according to their appearance (uniformity, free of defects, bruises and/or diseases). The whole samples were then brushed under running water and immersed in a chlorinated water bath (200 ppm) for one hour, in order to reduce the initial microbial load. They were then peeled and cut into 1 cm thick slices after discarding 3 cm of the crown. From the total obtained, approximately 6 slices were taken at random. Once the weight of each sample had been recorded, they were decontaminated by immersion in chlorinated water (50 ppm) for 1 min, dried with absorbent paper and thus ready for the application of the coating treatment.
The coating application was performed by immersing the carrot slices in the obtained nano-emulsions for 2 min and then draining the excess for 10 min. The slices introduced into the EALG formulation were immediately immersed in a CaCl2 (2%) solution for 2 min to allow cross-linking with calcium. Finally, all coated samples were oven dried with forced convection at 25 °C until a dry coating was visualized. The coated samples were then packed in polypropylene plastic trays wrapped with 25-micron film and stored at 4 °C for 20 days, alongside uncoated slices (used as a control group).
Characterization of Nano-emulsified Formulations
Particle Size and Polydispersity Index
The mean droplet size, intensity size distribution curves and polydispersity index (PdI) were used to characterize the dispersion of oil droplets in the nano-emulsions by using a dynamic light scattering equipment (Horiba SZ-100, Horiba Scientific, Japan). The oil droplet size and the homogeneity of the film-forming nano-emulsions were measured at 25 °C using a polystyrene cell and a dispersion angle of 90°. The refractive index of the SO used was 1.474. Measurements were performed in triplicate.
Characterization of Films
ATR-FTIR Technique
The FTIR spectra were recorded on films with and without emulsification treatment. Each sample was placed on the sample holder of an ATR-FTIR Thermo Nicolet iS10 spectrometer (Thermo Scientific, MA, USA). Spectra were recorded in the 4000 –400 cm−1 range by co-adding 64 scans with 4 cm−1 spectral resolution, using the OMNIC software version 8.3 (Thermo Scientific, MA, USA) at 20 °C. Measurements were performed in duplicate.
Microstructural Study
The microstructure of the films was evaluated by SEM. Microscopy tests were performed on the surface and the cross-section of the films with and without oil incorporation, using the cryo-fracture technique by immersing the samples in liquid nitrogen. The films were mounted on aluminum parts by using double-sided tape and coating them with a thin layer of gold using a sputtering system (SCD 005, Bal-Tec, Switzerland). SEM images were acquired with a scanning electron microscope (Quanta 200, FEI, The Netherlands), using an accelerating voltage of 10 kV for all samples.
Evaluation of Minimally Processed Coated Carrots
Peroxidase Enzyme Activity (PEA)
It was performed according to the method of Morales-Blancas et al. (2002). PEA was measured from the increase in absorbance at 470 nm as a function of time, with a Synergy HT Microplate Multi-Modal reader (Biotek). Enzyme activity was determined from the maximum slope of the absorbance curve as a function of time calculated by linear regression and the PEA results (%PEA) of the coated samples were normalized to the value of the control (uncoated) sample.
Total Polyphenol Content (TPP)
Methanolic extracts were prepared using the Folin-Ciocalteu (FC) reagent according to the method of Singleton et al. (1999) by spectrophotometric determination at 735 nm. The results of the coated samples (mg gallic acid equivalent per 100 g dry weight of the sample) were normalized to the value of the control sample. The remaining phenolic extract was stored at -20 °C for subsequent analysis of antioxidant capacity.
Antioxidant Capacity (AOX)
The antioxidant activity (AOX) of the coated samples was determined with 2,2-diphenyl-1-picryl-hydrazyl radical (DPPH·), according to Brand-Williams et al. (1995), by spectrophotometric determination at 515 nm at different time intervals. The assay was carried out until the reaction reached a plateau (90 min). The results were expressed as percentage inhibition of the DPPH- radical (I% 90’), calculated according to the following expression:
where AC corresponds to the absorbance of the control (without extract) and AE is the absorbance in the presence of extract.
Subsequently, the percentage of inhibition versus the sample concentration was plotted to determine the effective concentration at 50% (EC50). To do this, the extracts of each sample were diluted to 50%, 10%, and 1% v/v. Three measurements were taken for each of the extract concentrations (Molyneux, 2004). The EC50 values were calculated using linear regression, where the x-axis represents the concentrations of the extracts (mg/ml), and the y-axis represents the average percentage of antioxidant activity obtained from three independent measurements.
Color
The color of the MPCs was assessed using a computer vision system (CVS), which consisted of a controlled lighting environment, a digital camera, a standard color table for calibration, and an image processor operating in the red, green, blue (RGB) color space. The RGB to CIELab conversion was achieved by employing a known theoretical model and various empirical mathematical models (Goñi & Salvadori, 2017). Color parameters were determined based on images of three samples for each treatment. The outcomes were quantified in terms of Chroma Index (Cab) and Whitening Index (WI) using the following equations:
where, L* is the luminance and a* and b* refer to the red-to-green and blue-to-yellow scales, respectively.
The color parameters were normalized to the values of the control samples (uncoated).
Statistical Analysis
Results are expressed as mean ± standard deviation and were analyzed by analysis of variance (ANOVA). Means were tested with Fisher’s least significant difference test for pairwise comparison, with a significance level α = 0.05, using the Statgraphics Centurion XVI software (Statgraphics, USA).
Results and Discussion
Characterization of the Nano-emulsified Formulations
Droplet Size and Polydispersity Index
Table 2 shows the droplet sizes found for the nano-emulsions studied (139.2 ± 22.8, 129.7 ± 17.3 and 181.9 ± 91.8 nm, for ECMC, ECS and EALG, respectively). As can be seen, the droplet sizes found for all the emulsions studied, especially for ECMC and ECS, were less than 200 nm. This is a critical value to consider a nano–emulsified system as stable (Tadros et al., 2004).
The nanometer size is achieved through the high-energy shock waves generated by sonication, which induce turbulence and the formation of microbubbles, facilitating the breakup of oil particles (Gupta et al., 2016; Marcet et al., 2018). However, the duration and power of sonication are important factors to consider when producing nano-emulsions. Some researchers have used ultrasound to create nano-emulsions, but the reported droplet sizes are generally larger than 200 nm (Jafari et al., 2007). Our findings indicate that the time and energy applied during the ultrasound-based nano-emulsification treatments effectively reduced the droplet size to the nanometer range, resulting in stable emulsions for the three polymers studied. Additionally, the inclusion of a surfactant (Tween 20) helped prevent the coalescence of newly formed droplets induced by the ultrasonic treatment. Similar results regarding particle size in various polymer-based nano-emulsions produced via ultrasound have been reported by Chu et al. (2020) and Branco et al. (2020).
The PdI value, which ranges from 0 (uniform distribution) to 1 (broader distribution), has been defined as a dimensionless measure of heterogeneity in the droplet size distribution. The PdI values here recorded for the different nano-emulsified formulations are presented in Table 2. ECS showed the lowest PdI and a narrow intensity peak (data not shown), which suggests a high efficiency of the ultrasound method in forming nano-emulsions of uniform droplet size distribution. In contrast, EALG showed the highest value of PdI and a greater amplitude in the intensity peak (data not shown), suggesting that the ultrasound method was not efficient in forming stable nano-emulsions.
Characterization of Films
ATR-FTIR Spectroscopy
The ATR-FTIR spectra of the CMC and ECMC films are displayed in Fig. 1a. The spectra of CMC films exhibited typical bands associated with functional groups of the polysaccharide. The FTIR spectra of CMC and ECMC shared a similar overall appearance, but the ultrasonic treatment had an impact on the original structure of the polymer film. This impact was evident in the reduced intensity and shifts in many of the peaks. This can be attributed to the influence of the treatment on the uniform dispersion of SO and Tween 20 within the polysaccharide matrix and their interaction with each other.
It was observed that the incorporation of oil and emulsifier into the polymeric matrix altered the hydrogen bond interactions between the polymer, glycerol, and water, leading to a shift of the original CMC peak located at 3274 cm−1 to a higher wavenumber (3292 cm−1) and a decrease in its intensity. Additionally, a new peak was detected in the 1740 cm−1 region, corresponding to the C=O stretching of ester groups between the oil molecules and Tween 20. These shifts and variations in intensities in the -OH stretching region of ECMC compared to CMC confirmed the formation of new interactions between the polymer and the oil in the nano-emulsion (Taleb et al., 2009). With the exception of the characteristic peak related to ester groups between oil and emulsifier molecules, the absence of additional peaks indicated that no chemical changes were induced by ultrasound during emulsification, implying that only physical alterations occurred during the treatment (Carpenter & Saharan, 2017; Romeira et al., 2021).
The FTIR spectra of CS and ECS are presented in Fig. 1b. The spectra indicated that ultrasonic emulsification had an impact on the native starch structure, as evidenced by shifts and variations in intensities compared to the control film. Notably, there was a slight decrease in the absorption intensities of the characteristic peaks. The peak located between 3000 and 3600 cm−1 showed a significant weakening while shifting towards a higher wavenumber (from 3265 cm−1 to 3277 cm−1). This shift suggested that the incorporation of SO and Tween 20 in the nano-emulsion formulation led to the disruption of the hydroxyl groups of the plasticized starch, resulting in a reduction in the hydrophilicity of the emulsified film compared to the control film. This could have implications for the water vapor permeability of the film. These findings align with those reported by (Oliveira Filho et al., 2020). Additionally, a band in the 1700 cm−1 region indicated the presence of ester functional groups resulting from C=O stretching vibrations, signifying interactions between triglycerides from SO and emulsifier groups, as observed by Hosseini et al. (2023). However, Marinopoulou et al. (2016) reported that the band observed at 1715 cm−1 corresponded to a complex formed between amylose and lipids rather than free lipid molecules. Figure 1b also showed a decrease in the intensity of bands within the region between 1400 and 800 cm−1 in ECS compared to the control film. This region is associated with the crystalline structure of starch and suggests a potential loss of crystallinity in the polymer. Ultrasonic emulsification has been known to induce structural disorganization of starch, causing damage to its crystalline structure (Zheng et al., 2013).
The ATR-FTIR spectra of ALG and EALG are shown in Fig. 1c. The spectra showed two small peaks between 2900 and 2800 cm−1 and a third peak near 1700 cm−1, which corresponded to stretching vibrations of functional groups associated with the SO and emulsifier (Pal et al., 2018). These results indicate that both the oil and Tween 20 would be present on the surface of the matrix after obtaining the nano-emulsified film. The remaining peaks observed were the same as those observed in the control spectrum (ALG) in terms of shift and/or intensities, indicating that virtually no changes occurred in these groups during nano-emulsification (Branco et al., 2020).
Microstructure of the Films
The microstructure of filmogenic matrices is critical for determining the properties of edible films, including their water barrier, mechanical characteristics, and oil retention within the emulsified matrix. This study investigated the microstructures of both control films (CMC, CS, and ALG) and nano-emulsified films (ECMC, ECS, and EALG).
The control films exhibited smooth, compact, and uniform surface and transverse microstructures, with slight unevenness observed in ALG films due to cross-linking between SA and calcium (Acevedo et al., 2012) (Fig. 2). In contrast, the nano-emulsified films, especially ECMC and EALG, displayed irregular and heterogeneous surface structures with differences in porosity and uniformity (Fig. 3). These variations in surface characteristics could be attributed to emulsion destabilization phenomena during film formation, such as flocculation, creaming, or oil droplet coalescence at the film-air interface (Hosseini et al., 2023). ECMC, in particular, showed the presence of aggregates of oil droplets on the film’s surface, indicating a coalescence phenomenon. The cross-sectional images of ECMC revealed a porous and spongy structure, suggesting that the oil droplets partially incorporated into the matrix, consistent with FTIR results. However, the droplet sizes in ECMC were larger, ranging from 3000 to 14,000 nm, possibly due to lipid agglomeration during the film formation process. Hosseini et al. (2023) related similar results to the low viscosity of the film-forming solutions and the weakening of the polysaccharide matrix due to the introduction of oil molecules between them. In this sense Perone et al. (2014) demonstrated that increasing the concentration of hydroxypropylmethylcellulose (2–6%) hindered the migration of oil to the surface, thereby enhancing the uniformity of the films.
EALG exhibited clear flocculation and coalescence of the dispersed phase, resulting in a rough and discontinuous surface with pores and cracks. The cross-sectional micrographs of EALG confirmed the presence of hollows and oil drop migration towards the surface. These destabilization effects can negatively impact the film’s barrier, optical, and mechanical properties, creating stress points where ruptures can occur.
In contrast, ECS films demonstrated a compact, smooth, and uniform surface with a regular lipid distribution and an absence of cracks or pores. This improved surface quality likely contributes to the good barrier properties of ECS films. The use of low power density promotes the formation of amylose-lipid complexes, as the treatment disrupts starch granules and partially depolymerizes amylose, increasing the probability of contact between these components and the lipid (Wu et al., 2012). However, the degree of interaction between the two compounds gradually decreases with increasing ultrasound amplitude, which can negatively affect the properties of the polymer films as a result of possible breakdown of the hydrophobic bonds between amylose molecules and fatty acids (Liu et al., 2018).
Evaluation of Minimally Processed Coated Carrots
Peroxidase Enzyme Activity
The determination of PEA in carrot tissues is of utmost importance since peroxidase is one of the main enzymes involved in the surface lignification process of minimally processed vegetables. Additionally, it contributes to the aged and discolored appearance (Guo et al., 2020). The evolution of PEA in the coated carrot samples (ECMC, ECS, and EALG) is presented in Fig. 4. An increase in PEA was observed in the ECMC and EALG samples during storage, compared to the initial values. Other authors have observed the trend of increasing of PEA with storage time, irrespective of the presence of coating (Souza et al., 2015; Guo et al., 2020). The peeling and cutting operations involved in the processing of minimally processed products are known to induce damage to plant tissue, resulting in physiological and biochemical changes. When this occurs, vegetables can adjust their metabolism to heal damaged tissues and activate defense mechanisms to prevent further damage (León et al., 2001). Despite the recorded increases, the observed behaviors among the samples have been diverse (Fig. 4).
In the case of the ECMC samples, the PEA increased significantly on day 10, even exceeding the activity of the control samples at that time (value > 1). Subsequently, the values remained unchanged until the end of storage. This could indicate that the ECMC was not effectively preventing the reduction of available oxygen needed to trigger enzymatic reactions, likely due to the rupture and/or destabilization of the nano-emulsion during the formation of the coating as observed in the microstructure of the film in the "Microstructure of the Films" section (Fig. 3). Additionally, these results could also be influenced by the solubilization effect of the EC over the evaluation time. This was corroborated in previous studies characterizing water affinity and barrier properties of these same formulations, which revealed a solubility of approximately 57% for this nano-emulsified matrix (Vasco et al., 2022). Therefore, during storage, the solubilization of the EC could have been generated, allowing the observed increase in PEA values. There is research evidence in line with our results, warning about the increase of PEA with the application of various ECs by acting as semi-permeable membranes attached to the products (Murmu & Mishra, 2017).
In the case of the ECS samples, these were the only ones whose initial PEA values remained unchanged throughout storage. These results are consistent with those observed by Ojeda et al. (2014), who studied enzymatic behavior in sweet potatoes minimally processed with various CS-based ECs during refrigerated storage. As previously mentioned, the higher density and uniformity shown by the ECS coatings were probably due to the stability of the emulsion during the drying process, favored by the nanometer size of the droplets (Table 2). Also, the interactions between amylose molecules and lipids likely enabled the formation of inclusion complexes as a result of the US treatment. Other factors for the decrease in PEA values have also been described, such as the indirect effect of the lack of phenolic compounds (substrate), or a direct effect on an unknown receptor involved in peroxidase enzime synthesis (Ponce et al., 2008; Pan et al., 2013).
A significantly different trend was observed in the EALG samples, whose PEA notably increased from day 10, remaining unchanged until day 20. The values were even much higher than those of the control samples (PEA < 1). This same trend had been observed in a previous study for a similar SA-Ca+2-based formulation, where a different technology than US was used to obtain non-nanometer emulsions (Vasco et al., 2018). A possible explanation could be the interaction between Ca+2 from EC and the peroxidase enzyme present in plant tissue. Although the function of these ions in C=O has not yet been determined, they are known to influence enzyme activity and its structural configuration. Ca+2 can act in tissue in two ways: as a structural component of cell walls and membranes and as a cofactor of several enzymes. In a study on plant tissues, a direct effect between the presence of Ca+2 ions and PEA has been observed (Medda et al., 2003). These authors indicated that Ca+2 removal generated a remarkable reduction in enzyme activity, while addition produced the opposite effect; PEA increased threefold. Similarly, Kubo et al. (2018) found that the addition of CaCl2 to a model fruit juice solution significantly increased the thermal stability of peroxidase enzime, further indicating that a concentration of 0.2 g/100 mL CaCl2 resulted in a 0.7% increase in PEA. Regarding this, Badui (2006) states that many cations and anions, including calcium, act as activators. The activating effect is due to the fact that sometimes these cations are part of the active site, are required for the interaction of the enzyme with the substrate, or help maintain the structural conformation by interacting with some region of the enzyme. Recently, the importance of calcium has been highlighted both for promoting enzymatic activity and for maintaining the structural integrity of the protein around its heme group. The loss of calcium results in reorientation and deformations of the heme group, which, in turn, leads to a loss of activity. Therefore, the addition of CaCl2 can reinforce the presence of ions necessary for preserving the structural stability of the heme group (Kubo et al., 2018). Other studies examining alginate-based coatings without calcium have reported positive outcomes in terms of oxidative or enzymatic retardation. This is attributed to the effective formation of a surface layer that restricts the transfer of oxygen from the surrounding atmosphere (Souza et al., 2015). In our results, at the end of storage, there was a significant decrease in enzyme activity in the EALG samples, although it always remained above the control sample. The same trend was observed in a previous study (Devi et al., 2019). The reduction in activity was likely caused by decreased metabolism in carrots resulting from senescence. At stages beyond senescence, the enzyme loses the ability to remove toxic substances (H2O2) and consequently decreases its activity (Ng et al., 2005).
Total Polyphenol Content (TPP)
Figure 5 presents the results of TPP retention as a function of refrigerated storage time for all the coated carrot samples. As shown, during the refrigerated storage of the samples, both ECMC and ECS exhibited similar behavior to their respective controls (values close to 1) (Fig. 5), indicating that neither of these two treatments influenced the TPP contents in the carrot slices. Similar findings have been reported in other coating systems applied to fruits (Bilbao-Sainz et al., 2018).
In contrast, the study of the evolution of TPP content in the EALG samples during storage yielded significantly higher values than those found in the remaining samples. Significant increases in retention were recorded during the refrigerated storage period (Fig. 5). These results align with those reported in previous studies on the application of SA-Ca+2 coatings formulated without the use of ultrasound on fruits and vegetables (Vasco et al., 2018), as well as with other EC materials applied to cut carrots (Song et al., 2017). Mustafa et al. (2014) also reported similar findings in their study involving tomatoes coated with another nano-emulsified biopolymer. The authors attributed this effect to an enhanced defense mechanism, presumably resulting from the improved delivery of biopolymer particles to tissue cells. The increase in TPP content in plant tissue may be associated with the activation of the defense mechanism against oxidative stress induced by low temperatures, including the upregulation of defense enzymes such as peroxidase enzime and the accumulation of phenolic compounds and lignins. This response is attributed to the effects of cutting and cold storage, particularly the presence of calcium in the EALG, which, as previously discussed, acts as an enzymatic cofactor (Medda et al., 2003; Simões et al., 2009). In a recent study, the damage caused by the processing and storage of minimally processed carrots was analyzed comprehensively through physiological and transcriptomic (cellular RNA) analyses. It was concluded that the injury accelerates primary metabolisms in carrots, including respiratory and energy metabolisms, to meet the demand for phenolic antioxidant production. In other words, the accumulation of phenolic compounds in carrots is induced by the processing (Han et al., 2017).
In our case, since the increase in TPP was only observed in EALG, the effect appears to be more closely linked to the increase in PEA due to the presence of Ca+2, which is triggered after carrot processing.
Antioxidant Activity
Figure 6 shows the changes in AOX activity, evaluated as the concentration required to remove 50% of the radical, CE50 of the coated MPCs normalized with their control sample in relation to storage time. It was observed that, during storage, the ECMC samples showed an increase in AOX, which was evidenced by a decrease in EC50, although only at time 10 (Fig. 6). On the other hand, for ECS samples, AOX remained unchanged throughout the analysis period. Similar results were reported by Thomas et al. (2016) for strawberry samples coated with CS. A particularly different case is again observed for EALG samples, which recorded an increase in AOX at the end of refrigerated storage (day 30). It has been reported that the antiradical capacity of some minimally processed products may increase during refrigerated storage (Zaro, 2014). Specifically, in the case of EALG carrot tissue, the trend of increased AOX during prolonged storage would be due to the injury produced by slicing and the presence of calcium, further suggesting that the AOX capacity of sliced carrots is mainly derived from the contribution of TPPs. In the presence of increased free radicals and consequent oxidative stress, tissues develop a number of defense mechanisms to scavenge these reactive compounds, including phenol synthesis and production of non-enzymatic antioxidants, as well as activation of antioxidant enzymes, such as peroxidase (Han et al., 2017). Regarding the latter, we previously commented on the possible effect of CaCl2 on EALG samples and its potential intervention in increasing enzymatic activity, related to the effects on phenolic compounds and antioxidants visualized. These results are in agreement with those reported by Devi et al. (2019) for carrots treated with CaCl2 and stored under refrigeration. Despite the higher AOX and TPP values found in the EALG-coated carrot samples, lower quality was observed in these samples due to the elevated enzyme activity and its relationship with tissue lignification (Fig. 4). Similar results have been reported by Ranjitha et al. (2017) in their study of EC based on cellulose emulsions and applied to MPC. In that study, an improvement of TPP and AOX levels was observed in the coated samples, although the acceptability was very low. The latter was due to the low color and flavor retention, which are determining indicators in the purchase of the product by the consumer.
Color
Figure 7a and b display the color results expressed as WI (Whiteness Index) and Cab, respectively, for all the coated carrot samples. The application of the treatments involving EC nano-emulsification and drying had a notable effect on the initial color of the ECMC slices. These samples exhibited a higher WI value than those recorded in ECS and EALG (Fig. 7a). This difference was attributed to the longer drying time required for the formation of the polymeric matrix in the ECMC coating, which led to surface dehydration and a whitish appearance. The microstructure analysis of ECMC films confirmed the substantial instability and heterogeneity of these nano-emulsions during drying, characterized by the presence of cracks and pores, which facilitated moisture loss (Fig. 2). As a result of lacking a uniform and continuous protective barrier, surface dehydration and whitening of the tissue occurred (Deng et al., 2017). While in carrots, the development of a whitish layer is usually associated with the presence of lignin as a natural tissue healer, this phenomenon is also strongly linked to surface dehydration (Edelenbos et al., 2020).
Conversely, the treatment applied to the ECS samples did not affect the initial color. In contrast to ECMC and ECS, the EALG samples seemed to enhance their initial color as indicated by a decrease in WI and higher Cab values compared to their initial values (Fig. 7a and b). Other studies have also reported an enhancement of the initial color of fresh fruits and vegetables with the application of emulsified ECs based on SA and Ca+2 (Trigo et al., 2012). The formation of a gel between SA and the ion imparts gloss and moisture to the surface of the slices, which is characteristic of the gelled coating. These effects could be responsible for intensifying the native color properties of the samples.
During storage, color parameters varied among the samples studied. Specifically, for the ECMC samples, the WI and Cab parameters were less affected. This effect may be attributed to the maintenance of a wet surface, generated as a result of the high solubility presented by this coating, as reported in our previous studies (Vasco et al., 2022). This surface moisture helped prevent surface discoloration (Devi et al., 2019). On the other hand, the ECS samples showed no significant color variation during storage, possibly due to the formation of a continuous and semi-permeable nano-emulsified film on the carrot surface, limiting surface water losses (Chiabrando & Giacalone, 2013). These results align with those obtained for enzyme activity, which remained relatively stable during the storage of ECS samples (Fig. 4). In this case, the lignification process associated with PEA may not have developed (Song et al., 2017). Romeira et al. (2021) reported color changes and fungal growth on the tenth day of storage for papaya fruits treated with a coating derived from unemulsified potato starch. The extended shelf life observed in our study, as indicated by the analyzed quality parameters, may suggest the efficacy of the ultrasonication (US) treatment in facilitating the dispersion of nano-emulsified starch and oil. In the case of EALG samples, color retention was evident during the initial 15 days of storage, aligning with findings reported by Rodrigues et al. (2018) for yacon treated with a comparable alginate-based formulation. However, a significant decrease in color parameters was observed after day 20 (Fig. 7a and b). As previously explained, the increase in WI could be related to surface tissue dehydration or to the formation of lignin, a process involving the peroxidase enzyme. The accumulation of hydrophobic lignin promotes increased light reflectance, resulting in the development of a white color (Song et al., 2017). Our results are in agreement with this observation, not only with regard to enzymatic parameters but also with the results obtained for TPP (Figs. 4 and 5). This relationship may be based on the process of TPP synthesis in response to the stress levels of the samples. It is essential to note that, according to Simões et al. (2009), dehydration and structural alterations of the most superficial cell layers are the primary causes of discoloration in carrots, which may not be directly related to the lignification process but rather to the synthesis of non-structural phenolic compounds. Avena-Bustillos et al. (1994) suggested that white discoloration in peeled carrots is mainly due to dehydration and that other authors may have misconstrued the phenomenon, as dehydration and lignification can occur independently and may not necessarily result in discoloration. Understanding the mechanism of involvement of physiological responses in the development of discoloration and its inhibition remains a complex issue (Kowalczyk et al., 2020). These observations also correlate with the microstructure results of the EALG film, which exhibited a discontinuous and heterogeneous coated surface (Fig. 2). In this context, EALG could be considered an EC with limited capacity to act as a barrier to the environment. The characteristics of the EC, combined with the action of Ca+2 present in the EC and its relationship with PEA, comprise a series of factors that contribute to the deterioration of MPCs coated with EALG.
Conclusions
The physical modifications that occurred in the starch molecules due to the effect of the ultrasound treatment, combined with the characteristics provided by the incorporation of sunflower oil (SO) to obtain the ECS (ultrasound-treated) nano-emulsified films, demonstrated that ECS offers several advantages over ECMC and EALG. SEM allowed the observation of a uniform distribution of oil droplets throughout the ECS film. Ultrasound favorably contributed to enhancing the desired compatibility between SO, Tween 20, and the polymer, resulting in the formation of a lipid-amylose inclusion complex and ensuring the stability of the nano-emulsion during the pouring and drying of the nano-emulsified film.
In fact, when analyzing the formulations applied as edible coatings in minimally processed carrots, it was observed that not all nano-emulsions were capable of preserving the overall quality of the vegetable. In this context, EALG exhibited high enzymatic activity, which, although it led to an increase in the levels of antioxidant polyphenolic compounds, was attributed to the stress induced in the tissue by the presence of the edible coating, specifically Ca+2. This resulted in a loss of sensory quality and accelerated senescence of the cut product. Conversely, ECMC also showed high PPO activity, likely due to emulsion destabilization during the edible coating formation and/or a solubilization effect, leading to a decline in product quality. On the other hand, the ECS-based coating effectively preserved all the quality parameters examined. This is likely attributable to the improved physical and chemical properties observed in the filmogenic characterization.
Hence, the biodegradable film based on ECS is considered a promising alternative for use as an edible film or coating for food preservation.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not aplicable.
References
Acevedo, C. A., López, D. A., & Tapia, M. J. (2012). Using RGB image processing for designing an alginate edible film. Food and Bioprocess Technology, 5, 1511–1520.
Adjouman, Y. D., Nindjin, C., Kouassi, K. N., Tetchi, F. A., N’Guessan, G. A., & Sindic, M. (2018). Effect of edible coating based on improved cassava starch on post-harvest quality of fresh tomatoes (Solanum lycopersicum L). International Journal of Nutritional Science and Food Technology, 4(1), 1–10.
Armghan Khalid, M., Niaz, B., Saeed, F., Afzaal, M., Islam, F., Hussain, M., & Al-Farga, A. (2022). Edible coatings for enhancing safety and quality attributes of fresh produce: A comprehensive review. International Journal of Food Properties, 25(1), 1817–1847.
Avena-Bustillos, R. J., Cisneros-Zevallos, L. A., Krochta, J. M., & Saltveit, M. E. (1994). Application of casein-lipid edible film emulsions to reduce white blush on minimally processed carrots. Postharvest Biology and Technology, 4(4), 319–329.
Badui, S. D. (2006). Química de los alimentos. México: Pearson Educación.
Bilbao-Sainz, C., Chiou, B. S., Punotai, K., Olson, D., Williams, T., Wood, D., & McHugh, T. (2018). Journal of Food Science, 83(7), 1880–1887.
Blanco-Pascual, N., Montero, M. P., & Gómez-Guillén, N. (2014). Development of antioxidant film from unrefined extracts of brown algae laminaria digitata and ascophyllum nodosum. Food Hydrocolloids, 37, 100–110.
Branco, I. G., Sen, K., & Rinaldi, C. (2020). Effect of sodium alginate and different types of oil on the physical properties of ultrasound-assisted nanoemulsions. Chemical Engineering and Processing: Process Intensification, 53(8), 107942.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30.
Carpenter, J., & Saharan, V. K. (2017). Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: Effect of process parameters and their optimization. Ultrasonics Sonochemistry, 35, 422–430.
Chiabrando, V., & Giacalone, G. (2013). Effect of different coatings in preventing deterioration and preserving the quality of fresh-cut nectarines (cv Big Top). CyTA - Journal of Food, 11(3), 285–292.
Chiumarelli, M., & Hubinger, M. D. (2012). Stability, solubility, mechanical and barrier properties of cassava starch – carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocolloids, 28(1), 59–67.
Chu, Y., Cheng, W., Feng, X., Gao, C., Wu, D., Meng, L., & Tang, X. (2020). Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packaging and Shelf Life, 25, 100547.
Dave, P. K., Rao, T. R., & Thakkar, V. R. (2023). Development of Films and Coatings from Alginates. Biopolymer-Based Films and Coatings (pp. 101–120). CRC Press.
de Oliveira Filho, J. G., de Oliveira Noble Bezerra, C. C., Albiero, B. R., Oldoni, F. C. A., Miranda, M., Egea, M. B., & Ferreira, M. D. (2020). New approach in the development of edible films: The use of microemulsions or nanoemulsions of carnauba wax in films based on arrowroot starch. Food Packaging and Shelf Life, 26, 100589.
Deng, Z., Jung, J., Simonsen, J., & Zhao, Y. (2017). Cellulose nanomaterials emulsion coatings for controlling physiological activity, modifying surface morphology, and enhancing storability of postharvest bananas (Musa acuminate). Food Chemistry, 232, 359–368.
Devi, J., Bhatia, S., & Alam, M. S. (2019). Abiotic inducers influence the activities of the antioxidant enzyme and the shelf life of carrots during storage under refrigerated conditions. Journal of Plant Growth Regulation, 38, 1529–1544.
Edelenbos, M., Wold, A. B., Wieczynska, J., & Luca, A. (2020). Roots: Carrots. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce (pp. 597–603). Academic Press.
FAOSTAT. (2017). Food and Agriculture Organization Corporate Statistical Database. http://www.fao.org/faostat/es/#home. Consultation date: May 2020.
Ghanbarzadeh, B., & Almasi, H. (2011). Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. International Journal of Biological Macromolecules, 48, 44–49.
Goñi, S. M., & Salvadori, V. O. (2017). Color measurement: Comparison of colorimeter vs. computer vision system. Journal of Food Measurement and Characterization, 11(2), 538–547.
Gunstond, F. D. (2011). Vegetable Oils in Food Technology: Composition, Properties and Uses (second edition). UK: John Wiley and Sons Publication.
Guo, Y., Wu, B., Guo, X., Ding, F., Pan, Z., & Ma, H. (2020). Effects of power ultrasound enhancement on infrared drying of carrot slices: Moisture migration and quality characterizations. LWT-Food Science and Technology, 126, 109312.
Gupta, A., Eral, H. B., Hatton, H. B., & Doyle, T. A. (2016). Nanoemulsions: Formation, properties and applications. Soft Matter, 12, 2826–2841.
Han, C., Jin, P., Li, M., Wang, L., & Zheng, Y. (2017). Physiological and transcriptomic analysis validates previous findings of changes in primary metabolism for the production of Phenolic antioxidants in wounded carrots. Journal of Agricultural and Food Chemistry, 65, 7159–7167.
Hosseini, A. R., Zahabi, N., & Pazhouhandeh, F. (2023). Development and characterization of an active transparent biodegradable tara gum film incorporated with lavandula angustifolia essential oil. Food and Bioprocess Technology. https://doi.org/10.21203/rs.3.rs-2975200/v1
Jafari, S. M., He, Y., & Bhandari, B. (2007). Production of submicron emulsions using ultrasound and microfluidization techniques. Journal of Food Engineering, 82(4), 478–488.
Jafarzadeh, S., Nafchi, A. M., Salehabadi, A., Oladzad-Abbasabadi, N., & Jafari, S. M. (2021). Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Advances in Colloid and Interface Science, 291, 102405.
Kaur, G., Sharma, S., & Mir, S. A. (2021). Nanobiocomposite films: A greener alternate for Food Packaging. Food and Bioprocess Technology, 14, 1013–1027.
Kowalczyk, D., Skrzypek, T., & Łupina, K. (2020). Effect of carboxymethyl cellulose/candelilla wax edible coating incorporated with ascorbic acid on the physicochemical and sensory qualities of prepackaged minimally processed carrots (Daucus carota L.) during cold storage. Journal of Food Processing and Preservation, 44(9), e14713.
Kubo, M. T. K., Rojas, M. L., Curet, S., Boillereaux, L., & Augusto, P. E. D. (2018). Peroxidase inactivation kinetics is affected by the addition of calcium chloride in fruit beverages. LWT- Food Science and Technology, 89, 610–616.
León, J., Red, E., & Sánchez-Serrano, J. J. (2001). Signaling of wounds in plants. Journal of Experimental Botany, 52, 1–9.
Liu, P., Wang, R., Kang, X., Cui, B., & Yu, B. (2018). Effects of ultrasonic treatment on amylose-lipid complex formation and properties of sweet potato starch-based films. Ultrasonics Sonochemistry, 44, 215–222.
Marcet, I., Álvarez, C., & Paredes, B. (2018). Transparent and edible films from ultrasound-treated egg yolk granules. Food and Bioprocess Technology, 11, 734–747.
Marinopoulou, A., Papastergiadis, E., Raphaelides, S. N., & Kontominas, M. G. (2016). Morphological characteristics, oxidative stability and enzymic hydrolysis of amylose-fatty acid complexes. Carbohydrate Polymers, 141, 106–115.
Matheus, J. R. V., de Farias, P. M., Satoriva, J. M., de Andrade, C. J., & Fai, A. E. C. (2023). Cassava starch films for food packaging: Trends over the last decade and future research. International Journal of Biological Macromolecules, 225, 658–672.
Medda, R., Padiglia, A., Longu, S., Bellelli, A., Arcovito, A., Cavallo, S., Pedersen, J. Z., & Floris, G. (2003). Critical role of Ca2+ ions in the reaction mechanish of Euphorbia characias peroxidase. Biochemistry, 42(29), 8909–8918.
Molyneux, P. (2004). Activity, the use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant. Journal of Science and Technology, 26(2), 211–219.
Morales-Blancas, E. F., Chandia, V. E., & Cisneros-Zeballos, L. (2002). Thermal inactivation kinetics of peroxidase and lipoxygenase from broccoli, green asparagus and carrots. Journal of Food Science, 67, 146–154.
Murmu, S. B., & Mishra, H. N. (2017). The effect of edible coating based on arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chemistry, 245, 820–828.
Mustafa, M. A., Ali, A., & Manickam, S. (2014). Ultrasound-assisted chitosan–surfactant nanostructure assemblies: Towards maintaining postharvest quality of tomatoes. Food and Bioprocess Technology, 7, 2102–2111.
Nechita, P., & Roman, M. (2020). Review on polysaccharides used in coatings for food packaging papers. Coatings, 10(6), 566.
Ng, T. B., Gao, W., Li, L., Niu, S. M., Zhao, L., Liu, J., Shi, L. S., Fu, M., & Liu, F. (2005). Rose (Rosa rugosa) flower extract increases the activities of antioxidant enzymes and their gene expression and reduces lipid peroxidation. Biochemistry and Cell Biology, 83, 78–85.
Ojeda, G. A., Sgroppo, S. C., & Zaritzky, N. E. (2014). Application of edible coatings in minimally processed sweet potatoes (I pomoea Batatas L.) to prevent enzymatic browning. International Journal of Food Science and Technology, 49(3), 876–883.
Pal, N., Samanta, K., & Mandal, A. (2018). A novel family of non-ionic gemini surfactants derived from sunflower oil: Synthesis, characterization and physicochemical evaluation. Journal of Molecular Liquids. https://doi.org/10.1016/j.molliq.2018.11.111.
Pan, S. Y., Chen, C. H., & Lai, L. S. (2013). Effect of tapioca starch/decolorized hsian-tsao leaf gum-based active coatings on the qualities of fresh-cut apples. Food and Bioprocess Technology, 6(8), 2059–2069.
Perone, N., Torrieri, E., & Cavella, S. (2014). Effect of rosemary oil and HPMC concentrations on film structure and properties. Food Bioprocess and Technology, 7, 605–609.
Pinto, E., Aggrey, W. N., Boakye, P., Amenuvor, G., Sokama-Neuyam, Y. A., Fokuo, M. K., Karimaie, H., Sarkodie, K., Adenutsi, C. D., Ersuah, S., & Rockson, M. A. D. (2022). Cellulose processing from biomass and its derivatization into carboxymethylcellulose: A review. Scientific African, 15, e01078.
Ponce, A. G., Roura, S. I., Valle, C. E., & Moreira, M. R. (2008). Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biology and Technology, 49, 294–300.
Ranjitha, K., Rao, D. S., Shivashankara, K. S., Oberoi, H. S., Roy, T. K., & Bharathamma, H. (2017). Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innovative Food Science and Emerging Technologies, 42, 91–100.
Rodrigues, F. J., Cedran, M. F., & Garcia, S. (2018). Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf-life of fresh-cut yacon (smallanthus sonchifolius). Food and Bioprocess Technology, 11, 1605–1614.
Romeira, K. M., Abdalla, G., & Gonçalves, R. P. (2021). Residual starch packaging derived from potato washing slurries to preserve fruits. Food and Bioprocess Technology, 14, 2248–2259.
Seifari, F. K., & Ahari, H. (2020). Active edible films and coatings with enhanced properties using nanoemulsion and nanocrystals. Food & Health, 3(1), 15–22.
Sharma, K., Babaei, A., & Oberoi, K. (2022). Essential oil nanoemulsion edible coating in food industry: A review. Food and Bioprocess Technology, 15, 2375–2395.
Simões, A. D. N., Tudela, J. A., Allende, A., Puschmann, R., & Gil, M. I. (2009). Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Biology and Technology, 51(3), 364–370.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 99, 152–178. Academic press.
Song, Z., Li, F., Guan, H., Xu, Y., Fu, Q., & Li, D. (2017). Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control, 74, 34–44.
Souza, M. P., Vaz, A. F. M., & Cerqueira, M. A. (2015). Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food and Bioprocess Technology, 8, 647–654.
Sun, J., Wang, L., Chen, H., & Yin, G. (2023). Preparation and application of edible film based on sodium carboxymethylcellulose-sodium alginate composite soybean oil body. Coatings, 13(10), 1716.
Tadros, T., Izquierdo, P., Esquena, J., & Solans, C. (2004). Formation and stability of nano-emulsions. Advances in Colloid and Interface Science, 108–109, 303–318.
Taleb, M. F. A., El-Mohdy, H. L. A., & El-Rehim, H. A. A. (2009). Radiation preparation of PVA/CMC copolymers and their application in removal of dyes. Journal of Hazardous Materials, 168(1), 68–75.
Thomas, A. B., Nassur, R., de Boas, C. M. R., & de Lima, A. C. V. O (2016). Cassava starch edible coating incorporated with propolis on bioactive compounds in strawberries. Ciência E Agrotecnologia, 40(1), 87–96.
Trigo, J. M., Albertini, S., Spoto, M. H. F., Sarmento, S. B. S., Reyes, A. E. L., & Sarriés, G. A. (2012). Efeito de revestimentos comestíveis na conservação de mamões minimamente processados. Brazilian Journal of Food Technology, 15(2), 125–133.
Vasco, F., Campañone, L., & Agnelli, M. (2018). Evaluación y caracterización de films y recubrimientos de almidón de mandioca. Congreso latinoamericano de ingeniería y ciencias aplicadas, CLICAP. http://fcai.uncuyo.edu.ar/upload/02-trabajo-completo-alimentos.pdf. 46TCA 556 - 562.
Vasco, M. F., Campañone, L. A., & Gamboa-Santos, J. (2022). Formulation of edible films based on carboxymethylcellulose, cassava starch and alginate using high intensity ultrasound emulsification treatments. Journal of Food Processing and Preservation. https://doi.org/10.1111/jfpp.16417.
Vicentini, N. M., & Cereda, M. P. (1999). Uso de filmes de fécula de mandioca em pós-colheita de pepino (Cucumis sativus L). Brazilian Journal of Food Technology, 12, 87–90.
Wu, X., Chen, Y., Lv, X. C., Du, Z. L., & Zhu, P. X. (2012). Effect of stearic acid and sodium stearate on cast cornstarch films. Journal of Applied Polymer Science, 124(5), 3782–3791.
Zaro, MJ. (2014). Análisis de factores que afectan la acumulación, distribución y estabilidad de antioxidantes de naturaleza fenólica en berenjena (Solanum melongena L.), Ph Thesis, Universidad Nacional de La Plata, Argentina.
Zheng, J., Li, Q., Hu, A., Yang, L., Lu, J., Zhang, X., & Lin, Q. (2013). Dual-frequency ultrasound effect on structure and properties of sweet potato starch. Starch - Stärke, 65(7–8), 621–627.
Acknowledgements
This work has been founded by the National Agency for Scientific and Technological Promotion, (Argentina, PICT 0923/17 project, Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA)), and the National University of La Plata. We thank the Physical Metallurgy Research Laboratory of the Department of Mechanics of the Faculty of Engineering of the Universidad Nacional de La Plata, Buenos Aires, Argentina.
Funding
This study was funding by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación with the project PICT-2017-0923 “Aplicación de técnicas de conservación y deshidratación de alimentos para la obtención de productos de excelente calidad final”.
Author information
Authors and Affiliations
Contributions
L.C: writing—original draft; writing—review and editing, conceptualization, methodology, formal analysis, funding acquisition, project administration, M.F.V: writing—original draft; writing—review, investigation, methodology, J.L.: conceptualization, formal analysis, M.A.P: writing—review and editing, formal analysis.
Corresponding author
Ethics declarations
Ethical Approval
Not aplicable.
Consent to Participate
Not aplicable.
Consent for Publication
Not aplicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vasco, M.F., Lamarra, J., Pereyra, M.A. et al. Study of Nanoemulsified Films and Coatings for Enhanced Preservation of Minimally Processed Carrots During Refrigerated Storage. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03422-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03422-z