Abstract
The objective of the present study was to investigate the effectiveness of the post-harvest treatments of abiotic elicitors, that is, calcium chloride (CaCl2) and salicylic acid (SA) on physicochemical and biochemical parameters in relation to activities of antioxidative enzymes in carrot to enhance shelf life. Carrot of variety Punjab Carrot Red was harvested, washed, surface dried and treated with CaCl2 (1, 1.5 and 2%) or SA (1, 1.5 and 2 mM) for 5 min, while distilled water was used as the control. Treated as well as untreated carrots were placed in open trays and stored under refrigerated (5 ± 1 °C, 90% RH) conditions for 63 days. Treatment of carrots with CaCl2 and SA showed a reduction in changes in physiological weight, color, total soluble solids, ascorbic acid, titratable acidity, total phenolics, carotenoids, antioxidant activity and TBA reactive compound as compared to untreated samples. Higher activities of antioxidative enzymes, that is, catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), peroxidase (POD), dehydro-ascorbate-reductase (DHAR) and monodehydro-ascorbate-reductase (MDHAR), were found in treated carrots as compared to untreated carrots during the whole storage period. SA treatment exhibited more usefulness in maintaining the quality of carrot than CaCl2 treatment. Among all the treatments, 1.5 mM SA exhibited the highest antioxidative enzyme activities and slowest changes in biochemical quality of carrot during storage. Thus, 1.5 mM SA can be used to extend the shelf life of carrot during refrigerated storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carrot (Daucus carota L.) is a globally important vegetable crop from the Apiaceae (Umbelliferae) family, grown extensively in India during the winter season. Because of its nutritious value and high bioactive component concentration, it has high importance and recognition. It is used as food as well as for medicinal purposes. It consists of nutrients like carbohydrate, protein, fibers that have cholesterol lowering properties, minerals like potassium, sodium, calcium, phosphorus, trace mineral molybdenum, aromatic compounds, vitamin A, thiamine, riboflavin, color and refreshing characteristics (Kaur et al. 2012). Like many other colored vegetables, carrot is a gold mine of antioxidants, that is, α-carotene, β-carotene, phytochemicals and glutathione. It increases resistance against blood and eye diseases (Isaac and Maalekuu 2013). Fresh grated roots are used in salads, and tender roots are pickled.
However, consumption and sales of vegetables produced are hindered on average between 10 and 40% due to improper post-harvest operations, storage and marketing. There is constant deterioration in quality of vegetables during storage due to post-harvest metabolic processes. During storage, several biochemical changes take place in vegetables that result in deterioration of quality. These detrimental changes include browning, weight loss, increased susceptibility to microbial spoilage, high respiration rate, off flavor development, acidification, reduced firmness and discoloration which are due to the activity of many enzymes (Luo et al. 2015a, b; Huang et al. 2017). The major cause of oxidative damage during senescence is generated through biochemical reactions resulting in production of reactive oxygen species (ROS) like superoxide (O2·‾), hydrogen peroxide (H2O2), hydroxide radicals (OH−) and singlet oxygen (O2) (Luo et al. 2011). The active oxygen species formed during stress damage cellular compartments including carbohydrates, lipids, proteins and nucleic acid (Blokhina et al. 2003). In plant cells, these damaging effects of free radicals ROS are protected by the antioxidant defense system which includes both enzymatic and non-enzymatic systems (Kabiri et al. 2012). The non-enzymatic compounds generally are the low molecular mass antioxidants, such as ascorbate, glutathione, β-carotene and α-tocopherol, whereas the enzymatic system includes ROS interacting enzymes such as superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.6) and the enzymes regenerating the reduced forms of antioxidants such as mono-dehydro-ascorbate reductase (MDHAR, EC 1.6.5.4), dehydro-ascorbate reductase (DHAR, EC 1.8.5.1) and glutathione reductase (GR, EC 1.6.4.2) (Luo et al. 2015a). Environment conditions around product may change the rate of biochemical reactions which in turn may change the level of these antioxidants and antioxidative enzymes; thus, quantification of these reactions during storage is important.
Research efforts are required to develop inexpensive and effective strategies that minimize undesirable changes and to deliver safe and quality products with better shelf life stability to the consumers. Several post-harvest treatments employ certain chemicals/plant growth hormones to hasten or delay ripening, to improve and maintain the color and quality by slowing down the metabolic activities of the perishables and to reduce losses thus increasing overall economics (Pila et al. 2010).
The calcium chloride (CaCl2) role in the physiology of plant tissue is well recognized. Calcium application strengthens the cell wall by forming cross-links or bridges, while protecting the functional and structural integrity of membranes and delaying membrane lipid catabolism thus extending the storage life of fresh vegetables (Yang et al. 2017; Tappi et al. 2016). Zhang et al. (2018b) also suggested that Ca2+ inactivates the enzyme polygalacturonase (PG), which is responsible for the breakdown of cell wall materials and components like pectins, thereby playing a critical role in maintaining vegetable quality. It also delayed senescence in vegetables with no detrimental effect on consumer acceptance (Lester and Grusak 2004). Salicylic acid (SA) (o-hydroxybenzoic acid) is known as a signal molecule in the induction defense mechanisms in plants. SA exhibits a high potential in controlling post-harvest losses of horticultural crops and can decrease ROS (Asghari and Aghdam 2010). It has been recently accepted that SA is a safe chemical used to control post-harvest quality or quantity losses of perishable crops (Supapvanich and Promyou 2013). The role of SA has been investigated against chilling injury in tomato (Ding et al. 2002), alleviation of browning of post-harvest bamboo shoot (Luo et al. 2012), delaying ripening in banana (Srivastava and Dwivedi 2000), shelf life enhancement in Kiwi fruit (Aghdam et al. 2011) and radish (Devi et al. 2018). With attention to consumer’s demand for healthy products, studies on the application of post-harvest treatments, along with cold storage, are today considered of strategic importance. In this context, the aim of this study was to evaluate the effect of post-harvest application of exogenous calcium and salicylic acid on extending the shelf life of carrot in terms of changes in activities of antioxidant enzymes and associated biochemical constituents during refrigerated storage.
Materials and Methods
Collection and Storage of Carrot
This study was conducted in the laboratories of the Department of Processing and Food Engineering, Punjab Agricultural University, Ludhiana. The Punjab Carrot Red variety was raised following the recommended agronomic and cultural practices. Carrot was harvested and washed with distilled water to eliminate dirt and other pollutants. Samples were dipped in different concentrations of aqueous solutions of CaCl2 (1.0%, 1.5%, and 2.0%) and SA (1 mM, 1.5 mM and 2.0 mM/l) for 5 min, whereas carrots dipped in distilled water served as control. After surface drying, carrots were placed openly in corrugated cartons and stored in cold store at 5 ± 1 °C and 90 ± 4% relative humidity. Samples (5 kg) were placed in each tray, and five trays were kept for each treatment. After weekly intervals, a half kg sample was taken from each tray, pooled for each treatment and analyzed for quality parameters and activities of antioxidative enzymes.
Estimation of Physicochemical and Biochemical Quality Parameters
Physicochemical Quality Parameters
Physiological loss in weight was periodically calculated by percentage of differences between initial weight and final weight of tested carrot divided by their initial weight (Wang et al. 2014). Color of carrot was measured by using Miniscan XE plus Hunter lab Colorimeter (USA) as lightness (L) and redness (a) value after calibrating the colorimeter with standard white and black plates. The ‘L’ and ‘a’ values were recorded at D 65/10° and were compared to the standard values of fresh carrot (Znidarcic and Pozrl 2006). Total soluble solids (TSS) content was measured with a hand-held ‘ERMA’ refractometer and reported as Brix (Znidarcic and Pozrl 2006).
Biochemical Parameters
Estimation of Titratable Acidity (TA), Ascorbic Acid and Carotenoids
For estimation of TA, the carrot sample (1 g) was homogenized with 50 ml distilled water in pestle and mortar and was filtered. Then, the filtered extract (5 ml) was titrated with NaOH 0.1 N using phenolphthalein as an indicator until the appearance of a light pink color. The volume of NaOH used was noted and was expressed as percent malic acid (Wang et al. 2012). For estimation of ascorbic acid, the carrot sample (1 g) was crushed in pestle and mortar using metaphosphoric acid–acetic acid solution (10 ml) and was filtered. Ascorbic acid content was determined by titrating the standard ascorbic acid (0.2 mg/ml) with 2,6-dichlorophenol indophenol dye (Mau et al. 2005). The quantitative extraction of carotenoids was done as per the method described by Vimala and Poonghuzhali (2015) with some modifications. The carrot sample (1 g) was crushed in pestle and mortar and extracted using acetone (10 ml). The extract was covered with aluminum foil to prevent photo-bleaching. The combined mixture was finally placed on a shaker at 140 g for 30 min and then centrifuged at 13,000g for 15 min. A final volume of supernatant was made to 100 ml by adding acetone. Carotenoid content was determined by taking the absorbance at 450 nm, and results were expressed as mg/100 g FW. Carotenoids were calculated from a standard curve prepared simultaneously using high purity β-carotene (1–10 mg/ml).
Extraction and Estimation of Phenolic Compounds
Phenolic compounds were extracted by crushing a carrot sample (1 g) and refluxed with 80% methanol (5 ml) for 1 h. The refluxed sample was filtered, and the volume was made to 10 ml with 80% methanol and was used for estimation of phenolic compounds. For estimation of total phenols, the methanolic extract (0.5 ml) was evaporated to dryness and the residue was dissolved in 6.5 ml of distilled water. To this, Folin’s reagent (0.5 ml) was added and shaken thoroughly. After 5 min, a saturated solution of Na2CO3 (1 ml) was added and the reaction mixture was incubated at room temperature for 1 h. The absorbance of blue color was read at 760 nm against a blank. The concentration of total phenol was determined from a standard curve prepared simultaneously using gallic acid (10–50 µg) (Wang et al. 2013). For estimation of flavonoids, the methanolic extract (3 ml) was evaporated to dryness. The residue left was dissolved in 0.1 M methanolic solution of aluminum chloride (10 ml). Intensity of the yellow color so developed was read at 420 nm against a blank. The concentration of total soluble flavonoids was determined from a standard curve prepared simultaneously using rutin (40–200 µg/ml) (Popova et al. 2004).
Estimation of Total Antioxidant Activity
The method of Dasgupta and De (2006) was used for the estimation of total antioxidant activity. To the crushed carrot sample (1 g), distilled water (10 ml) was added. Samples were boiled for 1 h in boiling water bath and left overnight. To 2 ml of the above extract, 1 ml of total antioxidant activity reagent (0.6 M H2SO4, 28 mM sodium phosphate and 4 mM ammonium molybdate mixed in equal amounts before use) was added and the mixture was incubated at 95 °C for 90 min. After cooling, the intensity of blue color was read at 695 nm. The total antioxidant activity was measured as ascorbic acid equivalent (mg/g) and was determined from a standard curve prepared simultaneously using ascorbic acid (40–200 µg/ml).
Extraction and Estimation of Thiobarbituric Acid (TBA)-Reactive Compounds
TBA-reactive compounds were determined according to Hodges et al. (1999). The carrot sample (2 g) was homogenized in distilled water (4 ml). To 4 ml of this homogenate, 10% TCA (4 ml) was added and the solution was filtered through filter paper. To 4 ml of filtrate, 0.06M thiobarbituric acid (1 ml) was added and the solution was heated for 10 min at 100 °C. After cooling, the absorbance was read at 532 nm. The content of MDA was calculated using 1.56 as the extinction coefficient.
Extraction and Estimation of Antioxidant Enzymes
Extraction of CAT, SOD, GR and DHAR
Carrot (0.5 g) was homogenized in 5 ml of cold (4 °C) extraction buffer (0.1 M sodium phosphate buffer (pH 7.0) containing 1% (w/v) insoluble polyvinyl pyrrolidone and 1 mM EDTA) using a pre-chilled pestle and mortar. The mixture was then centrifuged at 13,000g for 10 min at 4 °C. The supernatant was collected from the centrifuged material and was analyzed for enzyme activities on a spectrophotometer (Rayleigh UV-2601). For assaying CAT enzyme, enzyme extract (0.1 ml) was added to chilled 0.1M sodium phosphate buffer pH 7.0 (1.9 ml). The reaction was started by adding H2O2 (1 ml) to the reaction mixture. The rate of decrease in absorbance at 240 nm was calculated at 30-s intervals for 3 min. Enzyme activity was expressed as µmoles of H2O2 decomposed /min/g FW using 0.0394 as the extinction coefficient (Omar et al. 2012).
For estimation of SOD, the reaction mixture (3 ml) contained 200 mM methionine (0.2 ml), 2.25 mM nitro blue tetrazolium (0.1 ml), 1 mM EDTA (0.1 ml), 0.1 M Na2CO3 (0.1 ml), 0.1 M sodium phosphate buffer of pH-7.0 (1.5 ml) and distilled water (0.95 ml). The enzyme extract (0.1 ml) was added at last, and the reaction was started by adding 2 µM riboflavin (0.1 ml). The test tubes were then placed under normal sunlight for 30 min for the development of blue color. The reaction was stopped by keeping the tubes in the dark. The activity was expressed as the amount of enzyme required for the 50% inhibition of photochemical reduction in NBT according to the method described by Xing et al. (2008).
For estimation of GR, the enzyme extract (0.1 ml) was added to 1 mM NADPH (1 ml), 5 mM oxidized glutathione (1 ml) and 1.5 mM MgCl2 (0.9 ml) in 0.1M phosphate buffer of pH-7.0 (1 ml). The activity was observed spectrophotometrically by observing the decrease in O.D at 340 nm at 30-s interval for 3 min. The activity was expressed as µmol NADPH oxidized /min/mg FW using 6.22 as the extinction coefficient (Omar et al. 2012).
For estimation of DHAR, enzyme extract (0.1 ml) was added to 0.1M phosphate buffer of pH-7.0 (1.4 ml), 1 mM EDTA (0.1 ml), 2.5 mM glutathione reduced (0.2 ml) and 0.2 mM dehydro-ascorbate (0.2 ml). DHAR was assayed by measuring the reduction in dehydro-ascorbate at 265 nm at 30-s intervals for 3 min on a spectrophotometer. The activity was expressed as nmol/min/g FW using 14 as the extinction coefficient (Huang et al. 2013).
Extraction and Assay of POD
POD was extracted, and activity was analyzed using the method described by Agüero et al. (2008). A carrot sample (0.2 g) was homogenized in cold (4 °C) 0.1M Tris–HCl buffer of (pH-7.0) (2 ml) containing 1 mM EDTA, 1% (w/v) polyvinyl pyrrolidone and 10 µM β-mercaptoethanol using a pre-chilled pestle and mortar. The mixture was then centrifuged at 13,000g for 10 min, and the supernatant was analyzed for enzyme activity. For assaying the activity, chilled guaiacol (3 ml), enzyme extract (0.1 ml) was added. The reaction was started by adding H2O2 (0.1 ml) and the rate in decrease in absorbance at 470 nm was measured at 30 s interval for 3 min using a spectrophotometer. The activity is expressed as µmoles/min/g FW by using 26.6 as the extinction coefficient.
Extraction and Assay of MDHAR
MDHAR was extracted, and activity was analyzed using the method described by Huang et al. (2013). A sample (0.2 gm) was homogenized in cold (4 °C) 50 mM Hepes buffer of pH 7.6 (2 ml), using a pre-chilled pestle and mortar. The mixture was then centrifuged at 13,000g for 10 min. The supernatant was collected and analyzed for enzyme activity. The reaction mixture (2 ml) contained 50 mM Hepes buffer, pH 7.6 (1.5 ml), 1 mM NADPH (0.2 ml), 2.5 mM ascorbate (0.2 ml) and enzyme extract (0.1 ml). The reaction was started by adding 20 µl of ascorbate oxidase (1 mg/10 ml) to the reaction mixture. MDHAR was assayed by decrease in absorbance monitored at 340 nm at 30-s intervals for 3 min. The activity of enzyme was expressed in nmoles/min/g FW using 6.2 as the extinction coefficient.
Statistical Analysis
Experimental data were expressed as mean ± standard error of three replicates. The critical difference at 0.5% level was analyzed using a fully randomized design as factorial, with three replications in CPCS software. Two-way analysis of variance (ANOVA) was used to determine the significance of differences.
Results
Effect of CaCl2 and SA Treatment on Carrot Physicochemical and Biochemical Parameters
Physicochemical Parameters
Physiological loss in weight significantly (p ≤ 0.05) increased with an increase in storage period in all treated as well as control samples. Treatment with SA and CaCl2 was effective in minimizing weight loss. Reduction in weight was lower in 1.5% CaCl2 and 1.5 mM SA-treated samples as compared to the other concentrations of CaCl2 and SA, respectively (Table 1). After 49 days of storage, weight loss in control samples was 34.36%, whereas in 1.5% CaCl2 and 1.5 mM SA, it was 20.33 and 19.86%, respectively. With regard to color characteristics of carrot, the L* (indicates lightness) and a* (indicates redness) values decreased along the storage period. The decrease was more profound in the untreated than the treated samples indicating better retention of color in treated samples. The L* value of untreated, 1, 1.5 and 2% CaCl2 was 35.8, 41.5, 43.5 and 39.8, whereas that of the 1, 1.5 and 2 mM SA-treated samples was 42, 40.7 and 41.7, respectively, on day 49 (Table 1). The a* values of untreated, 1%, 1.5%, 2% CaCl2 were 15.5, 17.8 26.3 and 16.2 and in 1 mM, 1.5 mM and 2 mM, whereas those of SA-treated samples were 17.5, 26.4 and 17.4, respectively, on day 49 (Table 1). Irrespective of the chemical treatments, TSS of carrot increased gradually with the advancement of storage period. Treatments of carrot with SA and CaCl2 slowed down the increase in TSS. However, a slower increase in TSS content was found with SA treatment as compared to CaCl2. The TSS of carrot increased from 7.2° Brix at day 0 to 12.5° Brix in untreated, 11.8° Brix in 1% CaCl2, 11.5° Brix in 1.5% CaCl2 and 11.9° Brix in 2% CaCl2-treated carrot on day 49. Similarly, with SA treatment the TSS content increased to 11.6, 11.2 and 11.4° Brix with the treatment with 1, 1.5 and 2 mM SA after same period of treatment (Table 2). Among all the treatments, 1.5 mM SA was found as the most effective in maintaining changes in weight loss, color and TSS.
Biochemical Parameters
Titratable Acidity (TA), Ascorbic Acid and Carotenoids
An significant (p ≤ 0.05) increase in TA (% malic acid) content was observed with increase in storage period in both treated as well as untreated carrot showing maximum acidity of 0.348 in untreated, 0.295 in 1% CaCl2, 0.268 in 1.5% CaCl2, 0.308 in 2% CaCl2, 0.281in 1 mM SA, 0.255 in 1.5 mM SA and 0.308 in 2 mM SA on day 28 of storage and declined thereafter (Table 2). SA- and CaCl2-treated samples showed slower changes in TA as compared to untreated samples. However, the treatment of carrot with SA proved to be better in maintaining the TA as compared to CaCl2-treated and untreated carrot throughout the storage period. Among different CaCl2 and SA treatments, 1.5% CaCl2 and 1.5 mM SA treatments were more effective than their other concentrations, respectively. Ascorbic acid content of carrot first increased and then decreased with advancement in storage period irrespective of chemical treatments. An increase in ascorbic acid content was found up to day 42 in untreated, 1% CaCl2- and 2 mM SA-treated samples followed by a decline. However, with 1.5%, 2% CaCl2, 1 mM and 1.5 mM SA treatment the increase was up to 49 days of storage (Table 2). The slower changes in ascorbic acid content were observed in carrot with CaCl2 and SA treatments as compared to untreated samples. However, the change in ascorbic acid content was slowest in 1.5 mM SA-treated carrot. A decline in carotenoid content of carrot was observed with increase in storage period in all treated and untreated samples. The rate of decrease of the pigment was significantly slower in treated samples as compared to the untreated carrot. Among CaCl2-treated samples, the carotenoid decline was slowest in carrot treated with 1.5% CaCl2 followed by 1 and 2% CaCl2 treatments. Among SA, the decline rate of carotenoid content was slowest in carrot treated with 1.5 mM SA followed by 1 and 2 mM treatments (Table 4). SA at 1.5 mM concentration maintained the highest value of carotenoids after 63 days of storage.
Effect of CaCl2 and SA Treatment on Carrot Phenolic Compounds
Phenolics were notably dissimilar among the treatments (Table 3). Phenol and flavonoid contents of carrot first increased up to a certain period of storage and then declined thereafter with the increment in storage period. Increase in phenol content was observed up to day 42 in untreated, 1% CaCl2- and 2 mM SA-treated samples whereas for 1.5%, 2% CaCl2, 1 mM and 1.5 mM SA this increase was recorded up to day 49 (Table 3). Flavonoid content of carrot also increased up to day 21 of storage and then decreased. Carrot treated with CaCl2 and SA showed a slower rise or decline in phenolic content as compared to untreated ones. Among CaCl2-treated carrots, changes in flavonoid contents were slower with 1.5% CaCl2 treatment as compared to 1 or 2% CaCl2 treatment, whereas with SA treatment, the slower changes in content of flavonoids was found in 1.5 mM SA-treated carrot as compared to 1 or 2 mM SA-treated carrot.
Effect of CaCl2 and SA Treatment on Carrot Total Antioxidant Activity and TBA-Reactive Compounds
Total antioxidant activity comprises lipophylic and hydrophilic antioxidant activity. The total antioxidant activity increased throughout the storage period (Table 3). However, changes in antioxidant activity were smaller in 1.5% CaCl2- and 1.5 mM SA-treated carrot with regard to their further CaCl2 and SA concentrations. TBA reactive compounds of carrot were observed to increase up to day 14 and then decreased afterwards with the increase in storage period. During the whole storage period, the content of TBA reactive compounds was less in treated samples as compared to untreated samples. Among CaCl2, the slowest changes were found in samples treated with 1.5% CaCl2 with a mean value of 1.58 in carrot on day 49 whereas among SA treatment, the slowest increase was found in samples treated with 1.5 mM SA with a mean value of 1.50 in carrot on day 49, respectively, where control samples show TBA value of 1.69 during the same period (Table 4).
Effect of CaCl2 and SA Treatment on Carrot Antioxidant Enzymes
Normally in the beginning, all the treatments showed enhancement for activities of all the antioxidant enzymes in carrot and then decreased afterwards with the increase in storage period. During the whole storage period, carrot treated with SA and CaCl2 exhibited a higher increase in activity of all the enzymes than the untreated samples. The activity of enzyme CAT significantly (p ≤ 0.05) increased and reached a high level at day 35 in all the treatments except in samples treated with 1.5% CaCl2, 1 and 1.5 mM SA in which the increase in activity was for a longer storage period, that is, day 42 and then dropped at the end of the storage period (Table 5). The activity of enzyme SOD, POD and DHAR significantly (p ≤ 0.05) increased up to day 42 in all the treatments except in 1.5%, 2% CaCl2, 1 mM and 1.5 mM SA where the increase was witnessed up to day 49 and then decreased afterward. SOD, POD and DHAR activity remained lower during the storage period in untreated carrot than CaCl2- and SA-treated carrot (Tables 5, 6). GR activity reached a high level at day 35 in untreated, 1% CaCl2- and 2 mM SA-treated carrot, whereas 1.5%, 2% CaCl2-, 1 mM and 1.5 mM SA-treated carrot had higher GR activity up to day 49 and then decreased thereafter with the increase in storage period (Table 6). MDHAR in carrot showed a steady increase up to day 42 in all the treated as well as untreated carrot and then decrease thereafter with the increase in storage period (Table 6).
Discussion
Weight loss of carrot during storage might be due to the loss of water through transpiration and respiration as indicated by Petriccione et al. (2014). During transpiration, water is lost due to differences in vapor pressure of water in the atmosphere and the vegetable surface while during respiration with loss of one carbon atom one molecule of water is produced resulting in reduction in weight loss. SA and CaCl2 treatments significantly decreased percent weight loss during the storage period, and our results are in agreement with the findings of Yanmaz et al. (1999). Babu et al. (2015) reported that reduction in respiration and transpiration in loquat fruits by Ca2+ treatments. Reduction in weight loss in CaCl2-treated carrot might be due to the fact that CaCl2 had the ability to strengthen the cell walls of fruits and vegetables, resulting in prevention of excessive moisture loss which then retained weight of fruits and vegetables (Yang et al. 2017; Pila et al. 2010). Pectin is a major component of the cell wall and is related to tissue firmness because of its solubilization and depolymerization during ripening and storage (Zhang et al. 2018a). CaCl2 had the ability to stabilize the membrane system of fruits and vegetables by the formation of Ca-pectate which increases rigidity of the middle portion and the cell walls of the carrot root there by, maintaining the dry matter content by preventing the degradation of the cell wall (Zhang et al. 2018b). Researchers have reported that the uptake of exogenous calcium ions by fruits and vegetables is related to an increase in the fraction of ionically bound pectin, thus promoting the cell-to-cell adhesion and enhancing cell wall stability (Yang et al. 2017). Liu et al. 2017 also reported that during apricot storage, significant changes in the concentration and nanostructure of cell wall pectins revealed their disassembly and degradation which could be retarded by 1% w/v calcium chloride treatment. Previously, CaCl2 application had also been reported to slow down the rates of weight loss in different fruits and vegetables (Tareen et al. 2012; Pila et al. 2010). SA had been reported to decrease normal respiration rate and closing of stomata (Wolucka et al. 2005) which might be the reason for lower weight loss in SA-treated carrot. These data indicate that the treatments can effectively prevent moisture loss of carrot by coating its surface, the migration of moisture from the inner of vegetable to surface slowed down, leading to reduced rate of moisture loss and possibly reduced respiration rate.
Color is one of the critical factors determining consumer acceptance of the quality of vegetables because it is an indicator of the degree of freshness, extent of fruit deterioration, even infestation of disease as well as contamination (Chen et al. 2011). Decrease in L* and a* color values during storage might be due to dehydration of external tissue layers which has been indicated as a main reason of surface discoloration (Devi et al. 2018). Better moisture retention in the treated samples over control could have aided in keeping the sample tissue exterior wet and therefore prevented surface discoloration. According to Jia et al. (2005), carrot having high L* and a* values was reported with fine visual quality. The significantly less overall color changes observed in all treated groups was highly due to the gas barrier properties of coating layers, which reduced the rate of oxygen diffusion into the fruits, resulting in less metabolic or other biochemical processes that could have caused undesirable color changes for carrot (Chong et al. 2015).
TSS content is a major quality parameter, which is correlated with texture and composition (Peck et al. 2006). It is a marker of sweetness and ripeness of fruits and vegetables. Increase in TSS might be attributable to an increase water loss and due to metabolic activity in them resulting in the conversion of starch to sugars (Abugoch et al. 2016). The more water lost, the more TSS value increases. Sobral et al. (2017) reported that higher total soluble solids (TSS) inhibit the decrease in ascorbic acid and have a positive effect on protection of fruit against oxidation, thus extending the shelf life of vegetables. The less increase in TSS of CaCl2- and SA-treated vegetables was attributed due to the slowing down of respiration and metabolic activities as indicated by Pila et al. (2010). TA is a measure of the concentration of organic acids present in fruits and vegetables, which are important in maintaining quality and also influencing stability thus considered as an indicator of maturity or spoilage (Hasib et al. 2012). During storage, the acid constitution of fruits and vegetables had been shown to influence by metabolic changes linked to ripening and enzymatic activities in live tissues (Bhattarai and Gautam 2006). In all treated as well as untreated samples, a decrease in TA during the later period of storage was probably due to metabolic conversion of organic acid into carbon dioxide and water during respiration as indicated by Tadesse and Abtew (2016). In the current study, CaCl2 and SA treatments slowed down changes in TA of carrot and were thus effective in delaying carrot ripening. The slower change in TA in treated carrot might be due to the reduced metabolic activities in these samples because of the coating layer acting as a protection against metabolic changes (Xin et al. 2017). According to Liu et al. (2009), increased levels of TA can be caused by increased respiration rate and production of high level CO2, which affects the glycolytic enzyme systems and results in accumulation of acids. The slightly lower TA observed in treated samples as compared to control sample was suggested to be due to the coating layer acting as a protection against spoilage microorganisms, which resulted in less off-flavors of fruits and lower amounts of acidic compounds produced within samples (Chong et al. 2015). However, TA was not affected by SA in mango (Ding et al. 2007) and by CaCl2 in peach (Manganaris et al. 2005). A decrease in carotene content in carrot during storage at 4 °C has also been reported by Yanmaz et al. (1999) and Lee et al. (2011).
Ascorbic acid is heat liable, and its retention is often monitored when evaluating post-harvest storage effects on storage and nutritional quality of fruits and vegetables (Mathooko 2003). Decline of vitamin C content in vegetables with increase in storage period has been observed by Arvanitoyannis et al. (2005). As suggested by Lee and Kader (2000), the decay of vitamin C content in vegetables during advanced period of storage was attributed to degradation of ascorbic acid which is due to oxidation that could take place because of the existence of catalysts and oxidizing enzymes. High maintenance of ascorbic acid content at the end of the storage period in treated samples might be due to a lowered rate of respiration, and ethylene production is in accordance with the results of Renhua et al. (2008). The plant pigment such as carotenoid not only contributes to the color appearance and but also exhibits anticarcinogenic and chemoprotective effects for human beings (Lee et al. 2013). Results showed that exogenous CaCl2 and SA treatment promoted the pigment formation, and this may also help to explain the higher accumulation of biomass in the CaCl2- and SA-treated group. A gradual increase in ascorbic acid content and in total phenolic content during storage might be responsible for the increase in the antioxidant activity of carrot as they are the major non-enzymatic antioxidants (Ali et al. 2011). Phenolic compounds are the secondary metabolites of plants, and they have the ability to act as antioxidants. A slower increase in total phenolics in carrot treated with CaCl2 and SA might be due to slow ethylene synthesis which in turn slowed down the activity of phenylalanine ammonia lyase enzyme, responsible for biosynthesis and accumulation of phenolic compounds (Yao and Tian 2005). Maria et al. (2010) also reported that phenolics gave a high contribution to the antioxidant activity and triggered antagonistic and synergistic effects in the antioxidant power of natural mixtures. Antioxidant activity increased at a slower rate in CaCl2- and SA-treated carrot, indicating that CaCl2 and SA treatments notably affected the metabolic process in carrot during storage.
The anti-oxidation enzyme system was activated to scavenge the accumulated ROS (Li et al. 2017; Xu et al. 2017). Under ordinary conditions in plants, the total sum of ROS is determined by the balance between several ROS producing pathways and the capability of the enzymatic and non-enzymatic mechanisms to deal with them, whereas under stress conditions, the plant’s capability to remove ROS is slower as compared to its formation, and this could be the consequence leading to oxidative damage (Laspina et al. 2005). SOD has a lead role in the antioxidative defense mechanism. The superoxide radicals thus formed due to oxidative damage are converted into hydrogen peroxide by this enzyme (Ruth et al. 2002). The increase in activity of the SOD enzyme could be correlated with the increase in activity of CAT and POD activity as they both are the key components of cellular antioxidant systems. Hydrogen peroxide produced by SOD is disintegrated by CAT and POD enzymes that are localized in the peroxisomes and cytosol, into water and oxygen. Both enzymes, that is, CAT and POD, use hydrogen peroxide as a common substrate. Moreover, exogenous CaCl2 and SA significantly (p ≤ 0.05) decreased the H2O2 content by rising higher activities of POD and SOD compared with control group. POD catalyzes the oxidation of phenolic compounds to form quinone compounds. During storage, the increase in activities of POD and CAT was higher and was for longer storage periods, thus protecting the carrot from the damaging effect of reactive oxygen species, therefore, prolonging the shelf life of carrot. Elevated CAT and POD in treated samples would lower H2O2 levels, which in turn lessens the degree of lipid peroxidation (TBARS) and membrane damage as compared to untreated samples. Decline in activities of these enzymes at higher stages of senescence is related to the loss of H2O2 scavenging capacity (Ng et al. 2005) indicated by higher increase in the TBA reactive compounds. These enzymes were reported to extend food freshness by protecting the integrity of membranes and were also related with stress tolerance and gene encoding the enzyme reported to up regulate post-harvest (Xing et al. 2008). In the antioxidant system, these enzymes, that is, CAT, SOD and POD, could be induced by the second messengers such as Ca2+ and ROS resulting in increased activity by calcium (Chen et al. 2018).
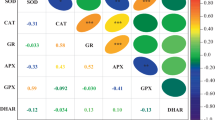
The increased GR activity could be related to the fact that during storage, the SH content of vegetables increased as GR is the enzyme that reduces glutathione disulfide to the sulfhydryl form which is an important cellular antioxidant and protects the thiol groups on enzymes and regenerate ascorbate that further reduces oxidative stress (Mittler et al. 2004). GR also may remove H2O2 by maintaining additional constructive levels of glutathione (reduced) and oxidized within chloroplasts (Jiang and Huang 2001). Meanwhile, DHAR uses the glutathione as the reducing agent to reduce dehydro ascorbate to ascorbate while monodehydro-ascorbate (MDHA), a primary product of ascorbate peroxidase can return into the ascorbate pool by MDHAR (Chanjirakul et al. 2006). The increase in DHAR and MDHAR activities could be correlated with the increase in content of ascorbate as also indicated by Jimenez et al. (2002). The increased DHAR and MDHAR activity and lower oxidation level in the fruit and vegetable ascorbate pool are correlated with decreased loss in firmness (Stevens et al. 2008). Our results also indicated possible involvement of antioxidative enzymes, that is, POD, SOD and CAT, GR, DHAR and MDHAR in scavenging the ROS and thus maintaining the shelf life of carrot for longer periods of storage in CaCl2- and SA-treated carrot as compared to untreated carrot.
Conclusion
Treatment of carrot with CaCl2 and SA along with storage at 5 °C slowed down the changes in physiological and biochemical parameters as compared to untreated carrot, respectively. Higher enhancement in the activities of antioxidative enzymes, that is, SOD, CAT, POD, GR, DHAR and MDHAR, was also found in treated carrot during storage. In conclusion, among all the treatments studied, 1.5% SA maintained the quality of carrot for a longer period probably by reducing the oxidative stress to a larger extent due to enhanced activities of antioxidative enzymes and thus can be used to extend the shelf life of carrot.
References
Abugoch L, Tapia C, Plasencia D, Pastor A, Castro-Mandujano O, López L, Escalona VH (2016) Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J Sci Food Agric 96:619–626
Aghdam MS, Motallebiazar A, Mostofi Y, Modhaddam JF, Ghasemnezhad M (2011) Methyl salicylate affects the quality of Haward Kiwi fruits during storage at low temperature. J Agric Sci 3(2):149–156. https://doi.org/10.5539/jas.v3n2p149
Agüero MV, Ansorena MR, Roura SI, del Valle CE (2008) Thermal inactivation of peroxidase during blanching of butternut squash. Lebensm Wiss Technol 41(3):401–407
Ali S, Masud T, Abbasi KS (2011) Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern Areas of Pakistan. Sci Hortic 30(2):386–393. https://doi.org/10.1016/j.scienta.2011.05.040
Arvanitoyannis IS, Khan EM, Christakou EC, Bletsos F (2005) Effect of grafting and modified atmosphere packing (MAP) on eggplant quality parameters during storage. J Food Agric Environ 3:204–252. https://doi.org/10.1111/j.1365-2621.2004.00919.x
Asghari M, Aghdam MS (2010) Impact of salicylic acid on postharvest physiology of horticultural crops. Food Sci Technol 2:502–509
Babu I, Ali MA, Shamim F, Yasmin Z, Asghar M, Khan AR (2015) Effect of calcium chloride application on quality characteristics and post harvest performance of loquat fruit during storage. IJAR 3:602–610
Bhattarai SR, Gautam DM (2006) Effect of harvesting method and calcium on post harvest physiology of tomato. Nepal Agric Res J 7:37–41
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Chanjirakul K, Wang S, Wang C, Siriphanich J (2006) Effect of natural volatile compounds on antioxidant capacity and antioxidant enzymes in raspberries. Post-harvest Biol Technol 40:106–115. https://doi.org/10.1016/j.postharvbio.2006.01.004
Chen F, Liu H, Yang H, Lai S, Cheng X, Xin Y, Yang B, Hou H, Yao Y, Zhang S, Bu G, Deng Y (2011) Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem 126:450–459
Chen L, Zhou Y, He Z, Liu Q, Lai S, Yang H (2018) Effect of exogenous ATP on the postharvest properties and pectin degradation of mung bean sprouts (Vigna radiata). Food Chem 251:9–17. https://doi.org/10.1016/j.foodchem.2018.01.061
Chong JX, Lai S, Yang H (2015) Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 53:195–205. https://doi.org/10.1016/j.foodcont.2014.12.035
Dasgupta N, De B (2006) Antioxidant activity of some leafy vegetables of India. A comparative study. Food Chem 101:471–474. https://doi.org/10.1016/j.foodchem.2006.02.003
Devi J, Bhatia S, Alam MS, Dhillon TS (2018) Effect of calcium and salicylic acid on quality retention in relation to antioxidative enzymes in radish stored under refrigerated conditions. J Food Sci Technol 55(3):1116–1126. https://doi.org/10.1007/s13197-017-3027-4
Ding CK, Wang CY, Gross KC, Smith DL (2002) Jasmonate and salicylate induce the expression of pathogenesis-related protein genes and increase resistance to chilling injury in tomato fruit. Planta 214:895–901. https://doi.org/10.1007/s00425-001-0698-9
Ding ZS, Tian SP, Zheng XL, Zhou ZW, Xu Y (2007) Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol Plant 130:112–121. https://doi.org/10.1111/j.1399-3054.2007.00893.x
Hasib A, Jaouad A, Mahrouz M, Khouili M (2012) HPLC determination of organic acids in Morocan apricots. J Food 3:207–211. https://doi.org/10.1080/11358120209487729
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Huang GJ, Deng JS, Chen HJ, Huang SS, Shih CC, Lin YH (2013) Dehydroascorbate reductase and monodehydroascorbate reductase activities of two metallothionein-like proteins from sweet potato (Ipomoea batatas [L.] Lam. ‘Tainong 57’) storage roots. Bot Stud 54:7. https://doi.org/10.1186/1999-3110-54-7
Huang H, Ge Z, Limwachiranon J, Lia L, Li W, Luo Z (2017) UV-C treatment affects browning and starch metabolism of minimally processed lily bulb. Postharvest Biol Technol 128:105–111. https://doi.org/10.1016/j.postharvbio.2017.02.010
Isaac O, Maalekuu BK (2013) Effect of some postharvest treatments on the quality and shelf life of three cultivars of carrot (Daucus carota L.) during storage at room temperature. Am J Food Nutr 3:64–72. https://doi.org/10.5251/ajfn.2013.3.2.64.72
Jia HJ, Araki A, Ang G, Okamoto (2005) Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica Batsch). Post-harvest Biol Technol 35:61–68
Jiang YW, Huang BR (2001) Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41:436–442
Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux PM (2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214: 751–758. https://doi.org/10.1007/s004250100667
Kabiri R, Farahbakhsh H, Nasibi F (2012) Salicylic acid ameliorates the effects of oxidative stress induced by water deficit in hydroponic culture of Nigella sativa. J Stress Physiol Biochem 8:13–22
Kaur M, Sharma HK, Bala J (2012) Kinetic changes in quality attributes of stored carrot-pineapple blended juice. Indian Food Pack 66(5):32–43
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330. https://doi.org/10.1016/j.plantsci.2005.02.007
Lee SK, Kader AA (2000) Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol 20:207–220
Lee EJ, Yoo KS, Patil BS (2011) Total carotenoid, anthocyanin and sugar contents in sliced or whole purple (cv. Eetaweet) and orange carrots during 4 weeks cold storage. Hortic Environ Biotechnol 52:402–407. https://doi.org/10.1007/s13580-011-0227-0
Lee J, Hwang YS, Lee JD, Chang WS, Choung MG (2013) Metabolic alterations of lutein, β-carotene and chlorophyll a during germination of two soybean sprout varieties. Food Chem 141:3177–3182. https://doi.org/10.1016/j.foodchem.2013.06.006
Lester GE, Grusak MA (2004) Field application of chelated calcium: postharvest effects on cantaloupe and honeydew fruit quality. Hortic Technol 14:29–38
Li D, Ye Q, Jiang L, Luo Z (2017) Effects of nano-TiO2 packaging on postharvest quality and antioxidant activity of strawberry (Fragaria × ananassa Duch.) stored at low temperature. J Sci Food Agric 97:1116–1123
Liu H, Chen F, Yang H, Yao Y, Gong X, Xin Y, Ding C (2009) Effect of calcium treatment on nanostructure of chelate soluble pectin and physicochemical and textural properties of apricot fruits. Food Res Int 42(8):1131–1140. https://doi.org/10.1016/j.foodres.2009.05.014
Liu H, Chen F, Lai S, Tao J, Yang H, Jiao Z (2017) Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem 225:87–97. https://doi.org/10.1016/j.foodchem.2017.01.008
Luo Z, Chen C, Xie J (2011) Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Post-harvest Biol Technol 62:115–120. https://doi.org/10.1016/j.postharvbio.2011.05.012
Luo Z, Wu X, Xie Y, Chen C (2012) Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. Food Chem 131:456–461. https://doi.org/10.1016/j.foodchem.2011.09.007
Luo Z, Li D, Du R, Mou W, Mao L (2015a) Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant capacity and proline content. Sci Hortic 183:144–151. https://doi.org/10.1016/j.scienta.2014.12.021
Luo Z, Wang Y, Jiang L, Xu X (2015b) Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT Food Sci Technol 60:1155–1161. https://doi.org/10.1016/j.lwt.2014.09.021
Manganaris GA, Vasilakakis M, Mignani I, Diamantidis G (2005) Effect of calcium additivies on physiochemical aspects of cell wall pectin and sensory attributes of canned peach (Prunus persica L. cv. ‘Andross’). J Sci Food Agric 85(10):1773–1778. https://doi.org/10.1002/jsfa.2182
Maria H, Concepcion SM, Sonia PT (2010) Flavonoid–flavonoid interaction and its effects on their antioxidant activity. Food Chem 121:691–696. https://doi.org/10.1016/j.foodchem.2009.12.097
Mathooko FM (2003) A comparison of modified atmosphere packaging under ambient conditions and low storage on quality of tomato fruit. Agric J Food Agric Nutr Dev 3:57–72. https://doi.org/10.4314/ajfand.v3i2.19140
Mau JL, Tsai SY, Tseng YH, Huang SJ (2005) Antioxidant properties of methanolic extracts from Ganoderma tsugae. Food Chem 93:641–649. https://doi.org/10.1016/j.foodchem.2004.10.043
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network in plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Ng TB, Gao W, Li L, Niu SM, Zhao L, Liu J, Shi LS, Fu M, Liu F (2005) Rose (Rosa rugosa) flower extract increases the activities of antioxidant enzymes and their gene expression and reduces lipid peroxidation. Biochem Cell Biol 83:78–85. https://doi.org/10.1139/o04-100
Omar SA, Elsheery NI, Kalaji HM, Xu ZF, Song-Quan S, Carpentier R, Lee CH, Allakhverdiev SI (2012) Dehydroascorbate reductase and glutathione reductase play an important role in scavenging hydrogen peroxide during natural and artificial dehydration of Jatropha curcas seeds. J Plant Biol 55:469–480. https://doi.org/10.1007/s12374-012-0276-7
Peck GM, Andrews PK, Reganold JP, Fellman JK (2006) Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hortic Sci 41(1):99–107
Petriccione M, Sanctis FD, Pasquariello MS, Mastrobuoni F, Rega P, Scortichini M, Mencarelli F (2014) The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Tech 8:394–408. https://doi.org/10.1007/s11947-014-1411-x
Pila N, Gol NB, Rao TUR (2010) Effect of post harvest treatments on physiochemical characteristics and shelf life of tomato (Lycopersicon esculentum Mill.) fruits during storage. Am Euras J Agric Environ Sci 9(5):470–479
Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, Marcazzan GL, Bogdanov S (2004) Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal 15:235–240. https://doi.org/10.1002/pca.777
Renhua HR, Xia RL, Yunmei U, Liming Yougjie X (2008) Effect of pre harvest salicylic acid spray treatment on post harvest antioxidants in the pulp and peel of Cara cara navel orange. J Sci Food Agric 88:229–236. https://doi.org/10.1002/jsfa.3076
Ruth GA, Neval E, Lenwood SH (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 372:1331–1341. https://doi.org/10.1093/jexbot/53.372.1331
Sobral MMC, Nunes C, Maia A, Ferreira P, Coimbra MA (2017) Conditions for producing long shelf life fruit salads processed using mild pasteurization. LWT-Food Sci Technol 85:316–323. https://doi.org/10.1016/j.lwt.2016.11.055
Srivastava MK, Dwivedi UN (2000) Ripening of banana fruit by salicylic acid. Plant Sci 158:87–96. https://doi.org/10.1016/S0168-9452(00)00304-6
Stevens R, Page D, Gouble B, Garchery C, Zamir D, Causse M (2008) Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ 31:1086–1096. https://doi.org/10.1111/j.1365.3040.2008.01824.x
Supapvanich S, Promyou S (2013) Efficiency of salicylic acid application on postharvest perishable crops. In: Hayat S (ed) Salicylic acid: plant growth and development. Springer Netherlands, Amsterdam. https://doi.org/10.1007/978-94-007-6428-6_15
Tadesse TN, Abtew WG (2016) Effect of hot water treatment on reduction of chilling injury and keeping quality in tomato (Solanum lycopersicum L.) fruits. J Stored Prod Postharvest Res 7:61–68. https://doi.org/10.5897/JSPPR2016.0221
Tappi S, Tylewicz U, Romani S, Siroli L, Patrignani F, Rosa M, Rocculi P (2016) Optimization of vacuum impregnation with calcium lactate of minimally processed melon and shelf-life study in real storage conditions. J Food Sci 81(11):2734–2742. https://doi.org/10.1111/1750-3841.13513
Tareen MJ, Abbasi NA, Hafiz IA (2012) Effect of salicylic acid treatments on storage life of each fruits cv. ‘Florda King’ Pak J Bot 44(1):119–124. https://doi.org/10.1016/j.scienta.2012.04.027
Vimala T, Poonghuzhali TV (2015) Estimation of pigments from seaweeds by using acetone and DMSO. IJSR 4(10):1850–1854
Wang H, Chen F, Yang H, Chen Y, Zhang L, An H (2012) Effects of ripening stage and cultivar on physicochemical properties and pectin nanostructures of jujubes. Carbohydr Pol 89:1180–1188. https://doi.org/10.1016/j.carbpol.2012.03.092
Wang J, Zhao YM, Tian YT, Yan CL, Guo CY (2013) Ultrasound-assisted extraction of total phenolic compounds from Inula helenium. Sci World J. 1–5. https://doi.org/10.1155/2013/157527
Wang Y, Xie X, Long L (2014) The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem 160:22–30. https://doi.org/10.1016/j.foodchem.2014.03.073
Wolucka BA, Goossens A, Inze D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56:2527–2538. https://doi.org/10.1093/jxb/eri246
Xin Y, Fusheng Chena F, Lai S, Yang H (2017) Influence of chitosan-based coatings on the physicochemical properties and pectin nanostructure of Chinese cherry. Postharvest Biol Technol 133:64–71. https://doi.org/10.1016/j.postharvbio.2017.06.010
Xing Z, Wang Z, Tan Q (2008) Effect of different packaging films on postharvest quality and selected enzyme activities of Hypsizygus marmoreus mushrooms. J Agric Food Chem 56:11838–11844. https://doi.org/10.1021/jf8024387
Xu Y, Luo Z, Charles MT, Rolland D, Roussel D (2017) Pre-harvest UV-C irradiation triggers VOCs accumulation with alteration of antioxidant enzymes and phytohormones in strawberry leaves. J Plant Physiol 218:265–274. https://doi.org/10.1016/j.jplph.2017.09.002
Yang H, Wu Q, Li Ying Ng LY, Wang S (2017) Effects of vacuum impregnation with calcium lactate and pectin methyl esterase on quality attributes and chelate-soluble pectin morphology of fresh-cut papayas. Food Bioprocess Technol 10:901–913. https://doi.org/10.1007/s11947-017-1874-7
Yanmaz R, Halloran N, Kasam MU, Agaoglu YS (1999) The effect of different storage condition and packages size on storage of carrot. J Agr Sci 5:1–6
Yao H, Tian S (2005) Effects of pre and postharvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol Technol 35:253–262. https://doi.org/10.1016/j.postharvbio.2004.09.001
Zhang L, Chen F, Lai S, Wang H, Yang H (2018a) Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT Food Sci Technol 96:604–611. https://doi.org/10.1016/j.lwt.2018.06.011
Zhang L, Zhao S, Lai S, Chen F, Yang H (2018b) Combined effects of ultrasound and calcium on the chelate-soluble pectin and quality of strawberries during storage. Carb Pol 200:427–435. https://doi.org/10.1016/j.carbpol.2018.08.013
Znidarcic D, Pozrl T (2006) Comparative study of quality changes in tomato C V “Malike” (Lycopersicon esculentum Mill.) whilst stored at different temperatures. Acta Agric Sol Veronica 87:235–243
Acknowledgements
Authors are thankful to Dr. Tarsem Singh Dhillon, Department of Vegetable Sciences, Punjab Agricultural University, Ludhiana for providing us carrot crop. The authors also acknowledge the financial support provided by ICAR under All India Coordinated Research project on Postharvest Engineering and Technology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest, they have informed consent, and research did not involve human and/or animal participation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devi, J., Bhatia, S. & Alam, M.S. Abiotic Elicitors Influence Antioxidative Enzyme Activities and Shelf Life of Carrot During Storage Under Refrigerated Conditions. J Plant Growth Regul 38, 1529–1544 (2019). https://doi.org/10.1007/s00344-019-09954-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09954-5