Abstract
Heat treatment reduces the quality of Antarctic krill (Euphausia superba) meat, thus greatly limiting its industrial application. It was found that L-Lys immersion pretreatment can effectively improve the quality of heat-treated Antarctic krill meat; the underlying mechanism is unclear. Therefore, this study aimed to investigate the effect of L-Lys concentrations (0, 25, 50, 100, and 200 mM) on the aggregation behavior and structure of Antarctic krill myofibrillar protein solution before and after heat treatment. Compared with the untreated group, L-Lys decreased the surface hydrophobicity and particle size of the heat-treated Antarctic krill protein by 2.38 times and 18.27 times while increasing the solubility by 3.59 times. Furthermore, L-Lys intervention inhibited the formation of disulfide bonds in myofibrillar protein of the heat-treated Antarctic krill, enhanced the intermolecular hydrogen bonding force, improved the orderliness of the secondary structure, and “exposed” the tyrosine residues of the protein molecule. As a result, the polarity of the microenvironment was enhanced while the tertiary structure of the protein was altered, thus inhibiting thermal aggregation. This study reveals the mechanism of L-Lys inhibition of thermal aggregation behavior of Antarctic krill myofibrillar protein. Our results provide insights into the development and utilization of Antarctic krill protein in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic krill (Euphausia superba) is a crustacean zooplankton found in Antarctic waters, with an estimated biomass of about 379 million tons (Atkinson et al., 2009). Antarctic krill is rich in proteins, including crude protein content of 64.44% and 11.9–15.4% in dried Antarctic krill and fresh Antarctic krill, respectively (Cavan et al., 2019). Therefore, Antarctic krill may be the future source of high-quality protein for humans since it contains many essential amino acids and has a higher biological value than milk or other animal proteins (Zheng et al., 2019). Antarctic krill also contains trace minerals, vitamins, enzymes, chitin, astaxanthin, etc. However, Sun et al. (2023) found that heat treatment rapidly deteriorates the quality of Antarctic krill meat due to severe moisture loss and increased hardness, which results in a rough texture and poor sensory acceptability.
The application of basic amino acids (as phosphorus-free additives) in the meat industry has received much attention in recent years. Basic amino acids are a safe, non-toxic, nutritious, healthy, and inexpensive small molecule green food additive, which interacts with proteins to change their structural and functional properties to improve the quality and sensory properties of meat and meat products (Zhang et al., 2017a). Cao et al. (2021) found that the L-Lys added to myofibrillar protein from pigs can significantly reduce the cooking loss of thermally induced gels, improve gel strength, and enhance gel properties. Furthermore, Bao et al. (2021) found that L-Arg or L-Lys can reduce the thawing loss of frozen pork and the cooking loss during the process. Zheng and Sciences (2017) found that L-Arg or L-Lys can induce smooth and dense microstructure of low-sodium emulsified pork sausage, thus improving the textural properties (hardness, adhesiveness, and chewiness). In conclusion, basic amino acids can improve the quality of meat products.
As a result, several studies have explored the mechanisms by which these basic amino acids improve food quality. For example, Fu et al. (2017) found that L-Lys can induce conformational and charge changes in myofibrillar proteins and interact with aromatic and acidic groups on myofibrillar proteins. As a result, L-Lys can enhance the solubility of myosin and the hydration capacity of myofibrillar proteins, thereby significantly improving the tenderness of chicken meat and reducing cooking losses. Wachirasiri et al. (2016) found that L-Lys can improve the quality of frozen white shrimp by affecting its pH. As a result, the interaction of polar and non-polar groups on the amino acid side chains with myofibrillar proteins is altered, thereby increasing the water holding capacity of myofibrillar proteins and improving the textural properties. Guo et al. (2015) found that L-Lys can induce the structural unfolding of myosin, expose more reactive sulfhydryl groups and other groups, and improve the interaction between myosin and water molecules, thus increasing its solubility. However, the effects of these basic amino acids on Antarctic krill have not been reported.
One of our previous study found that simple soaking treatment with L-Lys can improve the water holding capacity and textural properties of heat-treated Antarctic krill meat, and the improvements were better than in the 3% STPP (sodium tripolyphosphate)-treated group (Lin et al., 2022). However, it was unclear how L-Lys could improve the quality of Antarctic krill. Therefore, it is necessary to further explore how Lys affects key structural proteins of Antarctic krill. In this study, different L-Lys concentrations were used to determine the solubility, turbidity, and particle size of the myofibrillar protein before and after heat treatment and characterize the aggregation behavior of Antarctic krill myofibrillar protein. UV spectroscopy, intrinsic fluorescence spectroscopy, Fourier transforms infrared spectroscopy, and Raman spectroscopy were used to investigate the mechanism by which L-Lys improves myofibrillar protein aggregation in Antarctic krill. The results of the study can help to understand the mechanism of L-Lys to improve the meat quality of Antarctic krill and then promote the application of basic amino acids in the deep-processing industry of Antarctic krill as well as in the field of meat products.
Materials and Methods
Materials and Chemicals
The frozen Antarctic krill (Euphausia superba) was sourced from Dalian Liaoyu Group Co., Ltd. (Dalian, China). L-lysine was obtained from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Potassium bromide (KBr) was of spectral grade, while other chemicals were of analytical grade.
Extraction of Antarctic Krill Myofibrillar Proteins
Myofibrillar proteins were extracted from Antarctic krill (beheaded and peeled) as Sun et al. (2022) described. Briefly, the Antarctic krill meat was homogenized (T-25; IKA, Guangzhou, China) in an ice-water bath at 8000 rpm for 1 min after adding five times the volume of cooling low-salt phosphate buffer (0.05 M NaCl, 50 mM NaH2PO4/Na2HPO4, pH 7.0). The sample was centrifuged at 8000 × g for 10 min (4 °C) to remove the water-soluble protein and fat. This step was repeated thrice, and then, the precipitate was mixed with five times the volume of cooling high-salt phosphate buffer (0.6 M NaCl, 50 mM NaH2PO4/Na2HPO4, pH 7.0) and homogenized at 8000 rpm for 1 min. The sample was kept at 4 °C for 1 h to fully extract myofibrillar proteins. Finally, the suspensions were centrifuged at 8000 × g for 10 min (4 °C) to remove insoluble impurities. The bicinchoninic acid (BCA) method was used to determine the concentration of supernatant myofibrillar proteins. The supernatant was then diluted to the required concentration using the extracted buffer.
Sample Preparation
Sample was prepared as described by Qin et al. (2015) with some modifications. L-Lys was added to the protein solution and equilibrated at 4 °C for 1 h. The final concentration of L-Lys in the myofibrillar protein solution was 0, 25, 50, 100, and 200 mM, while the protein concentration was 2.5 mg/mL. The samples were heated in a water bath at 95 °C for 10 min then cooled in an ice-water bath for 30 min. Notably, the samples were analyzed before and after heating.
Solubility
Solubility was determined as Liu et al. (2020) previously described, with minor modifications. Briefly, the samples were centrifuged at 8000 × g for 10 min (4 °C). Protein concentration of the supernatant was analyzed using the BCA method. The absorbance of the mixture was read at 562 nm by a microplate reader (Synergy H1, BioTek Instruments, Inc., Winooski, USA). Protein solubility was expressed as a percentage of the supernatant protein concentration relative to the protein concentration before centrifugation.
Turbidity
The turbidity of myofibrillar protein solution was analyzed as Barut Gök (2021) described with minor modifications. Briefly, the turbidity was measured at 340 nm using a UV-5200 spectrophotometer (Yuanxi Instrument Co., Shanghai, China) and expressed as an absorbance value.
Particle Size
A Zetasizer 3000HSA (Malvern Panalytical Ltd., UK) was used to determine the particle size at excitation wavelength and scattered light intensity detector angle of 633 nm and 173°, respectively. The particle size was measured ten times and averaged.
Fluorescent-Inverted Microscope
The morphology of protein samples after different treatments was assessed as described by Wang, Shi et al. (2017) with slight modifications. Briefly, 1 mL of the treated protein samples and 20 µL of 0.1% Nile Blue fluorescent dye were mixed and incubated in the dark for 15 min at room temperature (22 °C). The stained sample was put on a slide and quickly covered with a coverslip for visualization using an inverted fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
Surface Hydrophobicity
Surface hydrophobicity was measured as Lin et al. (2022) described, with minor modifications. Briefly, 200 µL of 1 mg/mL bromophenol blue (BPB) in distilled water was added into 1 mL sample, oscillated at room temperature for 20 min, and then centrifuged at 2000 × g for 15 min at room temperature (22 °C). A control had the same conditions except for the slurry that was replaced with the buffer. The absorbance of the supernatant was measured at 595 nm by a microplate reader (Synergy H1, BioTek Instruments, Inc., Winooski, USA) against a phosphate buffer blank. The amount of BPB bound was determined as follows:
where A represents absorbance at 595 nm.
Contents of Sulfhydryl Groups and Disulfide Bridge (S–S)
The content of total sulfhydryl groups in L-Lys-treated myofibrillar proteins before and after heating was determined as Zhang et al. (2017b) reported, with slight modifications. Specifically, 1 mL myofibrillar protein suspension was mixed with a 9 mL phosphate buffer (8 M urea, 10 mM EDTA, 50 mM NaH2PO4/Na2HPO4, pH 7.0), followed by the addition of 1 mL of 0.1% 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB). The mixture was then incubated at 40 ℃ for 25 min, and absorbance was recorded at 412 nm by a microplate reader (Synergy H1, BioTek Instruments, Inc., Winooski, USA). SH content was determined using the molar extinction coefficient of 13,600 L·M−1·cm−1. The content of reactive sulfhydryl group content (RSH) was determined using the same reaction buffer in the absence of urea as follows:
where A412, D, and C represent the absorbance at 412 nm, dilution of multiple samples, and protein concentration of the samples, respectively.
The content of the disulfide bridge was calculated as follows:
where M1 and M2 represent the contents of total sulfhydryl and reactive sulfhydryl groups, respectively.
Ultraviolet–Visible (UV–Vis) Absorption Spectra Measurements
The UV spectra were determined as Fadimu et al. (2021) previously described with slight modifications. Briefly, the samples were diluted to 1 mg/mL with the extraction buffer, and double-distilled water was used as the blank. An ultraviolet spectrophotometer (Lambda 35, Perkin Elmer, Massachusetts, USA) was used for analysis at 230–400 nm (acquisition interval; 0.5 nm).
Fluorescence Spectroscopy
The fluorescence spectra were determined by reference to the method of Chen et al. (2022), with minor modifications. An RF-6000 fluorescence spectrophotometer (Shimadzu Co, Tokyo, Japan) was used to determine the fluorescence spectra of the samples at excitation and emission bandwidths of 5 nm and 5 nm, respectively. Moreover, the excitation wavelength and the measurement range of emission spectra were set at 280 nm and 300–450 nm, respectively. The acquisition rate and data interval were 6000 nm/min and 1 nm, respectively.
Fourier Transform Infrared Spectroscopy
The Fourier transform infrared spectroscopy were assayed according to the method of Cueto et al. (2018). First, the L-Lys-treated Antarctic krill myofibrillar protein solution was freeze-dried. An appropriate amount of the freeze-dried powder was mixed with the dried KBr powder, ground, and pressed. An infrared spectrophotometer (Perkin Elmer, Salem, MA, USA) was used to obtain the sample spectral data at 4000–400 cm−1 and a resolution of 4 cm−1.
Raman Spectroscopy
The protein Raman spectroscopy was referred to Zielinska et al. (2022) with minor modifications. The Antarctic krill myofibrillar protein solutions were diluted to 0.5 mg/mL with the extraction buffer, put onto glass slides, and then analyzed using Raman spectroscopy (LabRAM HR, Horiba Scientific, Longjumeau, France) at an excitation of 532 nm (scan range; 400–2000 cm−1). Phenylalanine (1001 cm−1) was used as an internal standard for normalizing the data. The peak separation was calculated for the amide I band. The characteristic peaks from 1645 to 1658 cm−1 corresponded to the α-helix structure, while those from 1665 to 1680 cm−1 were related to the β-sheet structure.
Statistical Analysis
Each experiment was performed in triplicate. Data were presented as mean ± standard deviation (SD). Origin 2019 software (OriginLab Corp., Northampton, MA, USA) was used to plot the figures. ANOVA using the SPSS 25 statistical analysis program (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Duncan’s multiple range test of means was used to determine the significant differences. P < 0.05 was considered statistically significant.
Results and Discussion
Solubility Analysis
Solubility can reflect the degree of protein aggregation (Turgay-İzzetoğlu et al., 2022). The effect of L-Lys on the solubility of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 1a. The solubility of unheated myofibrillar protein without L-Lys intervention was 53.74%. However, the solubility significantly increased with increasing L-Lys concentration. For example, the solubility at 200 mM L-Lys was 64.63%. In Fig. S1, it can be seen that L-Lys increases the basicity of the myofibrillar protein solution, and this increased basicity inhibits the aggregation of myofibrillar filaments, which affects the solubility (Guo et al., 2015).
Furthermore, heat treatment decreased the solubility of myofibrillar proteins without L-Lys intervention to 12.18% due to changes in the secondary structure of the protein at high temperatures, which exposed non-polar groups within the molecule and reduced the affinity of the protein surface for water, leading to aggregation (Li et al., 2020). Compared with the untreated group, the solubility of heat-treated Antarctic krill myofibrillar protein significantly increased with increasing L-Lys concentration (to 43.72%, an increase of 3.59 times).
Turbidity Analysis
Turbidity reflects the size and number of insoluble suspended particles in a solution system, and thus it can be used as an indicator of protein aggregation (Igual et al., 2014). The turbidity changes in Antarctic krill myofibrillar protein before and after heat treatment at different L-Lys concentrations are shown in Fig. 1b. The turbidity of the unheated Antarctic krill myofibrillar protein solution decreased with increasing L-Lys concentration. The decrease in protein turbidity indicated increased solubility (Thorarinsdottir et al., 2002). This may be due to the fact that L-Lys is inhibiting the intermolecular self-assembly behavior of the protein at low temperatures, which affects the turbidity of myofibrillar proteins of the unheated group of Antarctic krill (Grewe & Schwarz, 2020).
Moreover, heat treatment increased the turbidity of Antarctic krill myofibrillar protein solutions without L-Lys intervention from 0.244 to 0.679, mainly due to protein aggregation. However, L-Lys might significantly decrease the turbidity of heat-treated Antarctic krill myofibrillar protein solutions by affecting covalent and non-covalent bonds, especially disulfide bonds. Disulfide bonds are the most dominant covalent bonds in protein cross-linking and aggregation and are stronger than electrostatic interactions at high temperatures, thus inhibiting protein unfolding or aggregation at high temperatures (Yu et al., 2017).
Particle Size Analysis
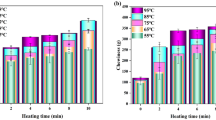
The particle size can reflect the degree of aggregation of the protein. Besides, particle size analysis is a macroscopic representation of the protein structure (Stefanović et al., 2017). The effect of L-Lys on the particle size of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 2a. The particle size of unheated Antarctic krill myofibrillar protein was 419.83 nm. However, 25 mM L-Lys significantly decreased the particle size of myofibrillar protein to 326.13 nm. Notably, the particle size of myofibrillar protein was not significantly different at 25 mM L-Lys and concentration ˃ 25 mM. Although L-Lys inhibits random aggregation between proteins by enhancing electrostatic repulsion, the aggregation cannot be increased indefinitely due to the balance of covalent and non-covalent interactions within the protein molecule (Wagner et al., 2020).
Effect of L-Lys on Antarctic krill myofibrillar protein before and after heat treatment; a particle size and b surface hydrophobicity. Data are presented as means ± standard deviation (n = 3). Different letters (a, b, c, A, B, C) indicate significant differences (P < 0.05) between different treatments
Heat treatment increased the particle size of Antarctic krill myofibrillar protein without L-Lys intervention to 7268.03 nm. However, the particle size of heat-treated Antarctic krill myofibrillar protein significantly reduced to 398.61 nm with increasing L-Lys concentration. Proteins form soluble aggregates under high temperatures through disulfide bonds, while they aggregate to form insoluble aggregates under hydrophobic interactions (Huang et al., 2021). These findings indicate that L-Lys may affect disulfide bond formation or hydrophobic interactions of the protein, thus affecting protein aggregation.
Surface Hydrophobicity Analysis
Surface hydrophobicity can be used to assess the physicochemical changes in proteins, indirectly reflecting the conformation of proteins and intermolecular interactions (Zhao et al., 2020). In this study, the effect of L-Lys on the surface hydrophobicity of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 2b. The untreated Antarctic krill myofibrillar protein had a BPB binding of 65.77 µg. However, L-Lys significantly decreased the BPB binding, indicating decreased surface hydrophobicity. Decreased surface hydrophobicity decreases protein aggregation and promotes solubility, consistent with the solubility results.
Furthermore, heat treatment increased the surface hydrophobicity of Antarctic krill myofibrillar protein to 115.06 µg. Firstly, heat causes peptide chain cleavage and structural unfolding of a protein molecule, thus exposing the hydrophobic groups within the molecule (Jia et al., 2016). Secondly, the hydrophobic interactions on the protein surface change from a hydrophilic to a hydrophobic state, causing aggregation of protein molecules and re-embedding of some of the hydrophobic groups (Vate & Benjakul, 2016). The superposition of the two eventually increases the surface hydrophobicity. Compared with the untreated group, the surface hydrophobicity of the heat-treated Antarctic krill myofibrillar protein significantly decreased with increasing L-Lys concentration. Notably, 200 mM L-Lys decreased the surface hydrophobicity by 2.38 times (48.38 µg). These results indicate that L-Lys can influence the protein aggregation state by affecting structural changes during thermal denaturation and decreasing hydrophobic interactions on the protein surface.
Macroanalysis of Aggregated States
The effect of L-Lys on the aggregation state of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 3a and b. The sample images were taken after the myofibrillar protein solution stood for 30 min. In the fluorescence image, the myofibrillar protein solution was oligomeric at 0 mM L-Lys (UH-0), and each blue spot was formed by aggregation of a few proteins (Fig. 3a). In this unheated state, there were fewer blue spots in the fluorescence image under the intervention of Lys, but there was no obvious change in the macro level of the sample solution.
Effect of L-Lys on Antarctic krill myofibrillar protein microscopic morphology a before and b after heat treatment. UH-0: 0 mM Lys and unheated; UH-25: 25 mM Lys and unheated; UH-50: 50 mM Lys and unheated; UH-100: 100 mM Lys and unheated; UH-200: 200 mM Lys and unheated; H-0: 0 mM Lys and heated; H-25: 25 mM Lys and heated; H-50: 50 mM Lys and heated; H-100: 100 mM Lys and heated; H-200: 200 mM Lys and heated
Antarctic krill myofibrillar protein aggregated in large quantities without the intervention of L-Lys by heated, thus forming large fluorescent blue spots (H-0). Moreover, the protein solution showed obvious precipitation at the macro level. After L-Lys intervened, the fluorescence image showed more dispersion in the field of view and appeared as small particles. It can be seen that the protein sedimentation decreased with increasing L-Lys concentration and then disappeared and showed a suspended colloidal state. Macroscopic changes verified the effects of L-Lys on the solubility, turbidity, and particle size changes of Antarctic krill myofibrillar protein after heat treatment.
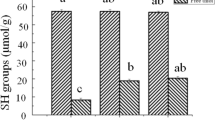
Total Sulfhydryl (–SH) and Disulfide Bond (S–S) Content Analysis
The sulfhydryl group is the most reactive functional group in myofibrillar proteins and plays a key role in the myofibrillar proteins (Liang et al., 2017). Total sulfhydryl groups include sulfhydryl groups embedded within the protein molecule and sulfhydryl groups free on the protein surface (Chen et al., 2019). The effect of L-Lys on the sulfhydryl content of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 4. The decrease in solubility may be due to cross-linking between myofibrillar proteins caused by the formation of disulfide bonds (Xia et al., 2019). High temperatures also promote the formation of a new disulfide bond in the protein, thus decreasing solubility (Ding et al., 2014). Figure 4a and b show the L-Lys did not significantly affect the sulfhydryl and total sulfhydryl group contents of non-heat-treated Antarctic krill myofibrillar protein. This indicates that no disulfide bonds were formed, and thus, the physicochemical properties of Antarctic krill myofibrillar protein were not altered. This also confirms that L-Lys did not significantly affect the solubility, turbidity, and particle size of non-heat-treated Antarctic krill myofibrillar protein.
Effect of L-Lys on Antarctic krill myofibrillar protein before and after heat treatment; a free sulfhydryl groups and b total sulfhydryl groups. Data are presented as means ± standard deviation (n = 3). Different letters (a, b, c, A, B, C) indicate significant differences (P < 0.05) between different treatments
However, L-Lys significantly reduced the free and total sulfhydryl contents of heat-treated Antarctic krill myofibrillar protein (Fig. 4a and b). Heat treatment unfolds the structure of the protein, thus exposing the sulfhydryl groups inside the protein molecule, which are easily oxidized to form disulfide bonds (Buamard & Benjakul, 2017). The change in the disulfide bond content after heat treatment is shown in Table 1. The disulfide bond content was 9.13 µmol/g without Lys intervention, but it could be significantly reduced to 6.75 µmol/g under the influence of Lys (100 mM), indicating that L-Lys inhibits the production of disulfide bonds. L-Lys inhibits the oxidation of protein sulfhydryl groups induced by hydroxyl radicals, thus preventing the formation of related oxides, such as intramolecular and intermolecular disulfide bonds (Zhang et al., 2021). L-Lys can also affect the spatial structure of the protein when exposed to heat, further masking the internal sulfhydryl groups and decreasing total sulfhydryl content (Bao et al., 2022).
Ultraviolet–Visible Absorption Spectra Analysis
Thermal aggregation of proteins is associated with conformation changes in the protein, which can be determined using UV–Visible absorption spectroscopy (Panpipat & Chaijan, 2020). Protein molecule contains several chromogenic groups, including tyrosine residues and tryptophan residues, which can absorb light in the UV region at a certain wavelength to produce a corresponding UV absorption spectrum (Zhang et al., 2020). In this study, the effect of L-Lys on the UV absorption spectra of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 5a and b. The ultraviolet absorption peaks of most amino acids are concentrated at 200 ~ 220 nm (Wang, Luo, et al., 2017). The UV absorption peak appeared at around 280 nm due to superimposed UV absorption of various aromatic amino acid residues; the absorption peak of Lys in solution had no effect on the UV absorption peak. The intensity of the UV absorption peak increased with increasing L-Lys concentration, mainly due to the “exposure” of tryptophan and tyrosine residues in the protein molecule caused by conformational changes. These results indicate that L-Lys can affect the tertiary structure of Antarctic krill myofibrillar protein (Guo et al., 2015). Notably, UV absorption significantly increased in the heat-treated group than in the unheated group.
Effect of L-Lys on UV absorption spectra of Antarctic krill myofibrillar protein a before and b after heat treatment. Effect of L-Lys on endogenous fluorescence spectra of Antarctic krill myofibrillar protein c before and d after heat treatment. UH-0: 0 mM Lys and unheated; UH-25: 25 mM Lys and unheated; UH-50: 50 mM Lys and unheated; UH-100: 100 mM Lys and unheated; UH-200: 200 mM Lys and unheated; H-0: 0 mM Lys and heated; H-25: 25 mM Lys and heated; H-50: 50 mM Lys and heated; H-100: 100 mM Lys and heated; H-200: 200 mM Lys and heated
Fluorescence Spectroscopy Analysis
The transformation of the protein structure can alter the fluorescence of the protein in the solution. The effect of L-Lys on the endogenous fluorescence spectrum of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 5c and d. The λmax intensity of the non-heat-treated protein without L-Lys intervention was 329 nm. However, λmax gradually increased and redshifted with increasing L-Lys concentration (340 nm). Furthermore, λmax in the heat-treated group increased from 330 to 345 nm. The change in λmax can represent changes in the polarity of the microenvironment of tryptophan and tyrosine residues of the protein. In this study, L-Lys caused a red shift before and after heat treatment, indicating that L-Lys affects the microenvironment of tryptophan and tyrosine in the protein by increasing its regional polarity and reducing environmental hydrophobicity (Mao et al., 2016). However, the redshift in λmax was greater in the heated group than in the non-treated group, indicating that heat significantly affects the protein structure and microenvironment, consistent with surface hydrophobicity analysis. Furthermore, L-Lys decreased the fluorescence intensity of Antarctic krill myofibrillar protein, probably because the fluorescence of tryptophan and tyrosine is very sensitive to microenvironmental polarity, indicating that changes in the microenvironment of the protein solution can quench the fluorescence (Qiu et al., 2014).
Fourier Transform Infrared Spectroscopy Analysis
Infrared spectroscopy can be used to reflect changes in the conformation of proteins and thus is crucial for the analysis of protein structures (Hassoun et al., 2021). The effect of L-Lys on the infrared spectra of Antarctic krill myofibrillar protein before and after heat treatment is shown in Fig. 6a and b. A characteristic absorption peak appeared at around 3418 cm−1 (3600–3300 cm−1), mainly characterizing O–H stretched vibrations; N–H stretched vibrations with intramolecular and intermolecular hydrogen bonds formed by the O–H group in the bound water with the C = O in the amino acid. The amide I band (1700–1600 cm−1) mainly characterizes C = O stretched vibrations, while the amide II band (1600–1500 cm−1) mainly characterizes C–N stretched vibrations or N–H bend vibrations (Zhao et al., 2008). It could be seen that the peaks of the unheated and heated Antarctic krill myofibrillar proteins appeared at 3415 cm−1 and 3418 cm−1, respectively. L-Lys led to a weak red shift of the maximum absorption peak, indicating that the hydrogen bond has changed slightly (Zheng et al., 2019). Moreover, in the heat treatment group, the red shift of the absorption peak was greater, indicating that L-Lys plays a greater role. The absorption peaks of the amide I and amide II bands at 1660 cm−1 and 1540 cm−1 were shifted towards lower wave numbers after L-Lys intervention, indicating that L-Lys enhances intermolecular interactions by affecting hydrogen bonds. Meanwhile, the change of the peak shape of amide I band also showed that L-Lys has an effect on the conformation of myofibrillar protein of Antarctic krill.
Effect of L-Lys on infrared spectra of Antarctic krill myofibrillar protein a before and b after heat treatment. Effect of L-Lys on Raman spectra of Antarctic krill myofibrillar protein c before and d after heat treatment. UH-0: 0 mM Lys and unheated; UH-25: 25 mM Lys and unheated; UH-50: 50 mM Lys and unheated; UH-100: 100 mM Lys and unheated; UH-200: 200 mM Lys and unheated; H-0: 0 mM Lys and heated; H-25: 25 mM Lys and heated; H-50: 50 mM Lys and heated; H-100: 100 mM Lys and heated; H-200: 200 mM Lys and heated
Raman Spectroscopy Analysis
Raman spectroscopy detects structural changes in proteins based on changes in the shape, displacement, and relative intensity of the spectral peaks in a detailed manner (Cai et al., 2018). The effects of L-Lys on the Raman spectra of Antarctic krill myofibrillar protein before and after heat treatment are shown in Fig. 6c and d, respectively. The amide I band (1700–1600 cm−1) reflects the conformational changes of the protein. With the intervention of L-Lys, the peak shape of amide I band in the heat treatment group has changed greatly, which further indicated that L-Lys might influence the conformation of protein during thermal denaturation. The relative contents of α-helix and β-sheet are shown in Tables 2 and 3. The change in the relative content of α-helix and β-sheet in unheated Antarctic krill myofibrillar protein α before heat treatment was not significantly different after L-Lys intervention. In contrast, L-Lys significantly increased the relative content of the heat-treated Antarctic krill myofibrillar protein α-helix and slightly changed the β-sheet content. These results suggest that L-Lys promotes the formation of tighter α-helix structures of heat-treated Antarctic krill myofibrillar proteins, thus enhancing the orderliness of the protein secondary structure. This explains how L-Lys significantly reduces the cooking loss and improves the textural characteristics of krill in Antarctic krill (Lin et al., 2022).
A range of 450–600 cm−1 generally represents the change of disulfide bonds or aliphatic residues in protein, and proteins are generally accompanied by the formation and change of disulfide bonds under heat induction. The normalized intensity of the peaks at 450–600 cm−1 increased after heating, indicating that disulfide bonds are generated during heating (Fig. 6c and d). Furthermore, the peak appeared at 510 cm−1 without L-Lys intervention, indicating a fully distorted conformation of S–S. However, the overall relative intensity of the peaks in the heat-treated group decreased after L-Lys intervention, indicating decreased disulfide bond production. Besides, new absorption peaks appeared at 540 cm−1 in the unheated and heat-treated groups after 200 mM L-Lys treatment, indicating trans-twisted-trans conformation of S–S (Ngarize et al., 2004). The variation in wave number suggested that L-Lys intervention can influence the conformation of some disulfide bonds. Furthermore, the changes in peak intensity in the heat-treated group indicate that L-Lys can inhibit the formation of disulfide bonds in the protein under high temperatures, consistent with disulfide bond content analysis.
The relative intensity (I760) reflects the polarity of the microenvironment where the tryptophan residue is located. The relative intensity decreases as the tryptophan residue shifts from an encapsulated hydrophobic to a polar microenvironment. The effect of L-Lys on the relative intensities of some Raman spectra bands of Antarctic krill myofibrillar protein after heat treatment is shown in Tables 2 and 3. L-Lys significantly decreased the I760 of the samples before and after heating, indicating that the tryptophan residues were gradually exposed to a polar microenvironment, consistent with endogenous fluorescence spectra analysis (Yang et al., 2021).
Peaks at 830 cm−1 and 850 cm−1 were related to vibrations of tyrosine residues para-substituted to the benzene ring. Moreover, the ratio (I850/I830) was influenced by the tyrosine microenvironment and the hydrogen bonding on the phenolic hydroxyl group. A lower I850/I830 value (0.7 to 1.0) indicates that tyrosine may be internal or acts as a donor for strong hydrogen bonds. Besides, I850/I830 value ˃ 1 indicates that the tyrosine residue is exposed on the protein surface and interacts with the solvent as a hydrogen bond donor or acceptor (Du et al., 2021). In this study, I850/I830 value was 1.27 before heat treatment and 1.53 after heat treatment, indicating that heating increased tyrosine exposure. Moreover, L-Lys increased I850/I830, indicating enhanced hydrogen bonding.
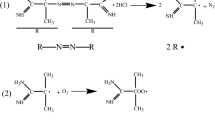
These results indicate that L-Lys can inhibit heat-induced aggregation of Antarctic krill proteins. The heated natural Antarctic krill proteins aggregated, probably due to sulfhydryl disulfide bond interchanges in the structural domain of the head of myosin (Fig. 7). In contrast, L-Lys enhanced the formation of hydrogen bonds of Antarctic krill myofibrillar protein during heat treatment (Table 3), thus forming a more dense α-helix structure (Table 3) and inhibiting the generation of disulfide bonds (Table 1). Moreover, L-Lys enhanced the microenvironmental polarity of Antarctic krill myofibrillar protein, reduced the hydrophobic interactions of proteins (Fig. 2b), altered the conformation of the protein, reduced particle size (Fig. 1a), and inhibited the thermal aggregation of myofibrillar protein.
Conclusion
Compared with the untreated group, L-Lys enhanced the formation of hydrogen bonds of Antarctic krill myofibrillar protein during heat treatment, formed a tighter α-helix structure, inhibited the generation of disulfide bonds, enhanced the microenvironmental polarity of Antarctic krill myofibrillar protein, reduced surface hydrophobicity by 2.38 times, decreased the hydrophobic interaction of the protein, affected the protein conformation, and decreased particle size by 18.27 times, thus inhibiting thermal aggregation of myofibrillar proteins. Also, L-Lys increased the solubility of Antarctic krill myofibrillar proteins after heat treatment (by 3.59 times); the increased solubility results in improved water-holding capacity and tenderness of heat-treated Antarctic krill. This study provides a theoretical basis and technical support for the development and utilization of Antarctic krill protein and the expansion of L-Lys application.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article.
Data Availability
The data that support the findings of this study are available upon reasonable request.
References
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J., & Loeb, V. (2009). A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Research Part I: Oceanographic Research Papers, 56(5), 727–740. https://doi.org/10.1016/j.dsr.2008.12.007
Bao, P., Chen, L., Wang, Y., Hu, Y., & Zhou, C. J. M. S. (2021). Quality of frozen porcine Longissimus lumborum muscles injected with L-arginine and L-lysine solution. Meat Science, 179, 108530. https://doi.org/10.1016/j.meatsci.2021.108530
Bao, P., Chen, L., Hu, Y., Wang, Y., & Zhou, C. (2022). l-Arginine and l-lysine retard aggregation and polar residue modifications of myofibrillar proteins: Their roles in solubility of myofibrillar proteins in frozen porcine Longissimus lumborum. Food Chemistry, 393, 133347. https://doi.org/10.1016/j.foodchem.2022.133347
Barut Gök, S. (2021). UV-C Treatment of apple and grape juices by modified UV-C reactor based on Dean vortex technology: Microbial, physicochemical and sensorial parameters evaluation. Food and Bioprocess Technology, 14(6), 1055–1066. https://doi.org/10.1007/s11947-021-02624-z
Buamard, N., & Benjakul, S. (2017). Cross-linking activity of ethanolic coconut husk extract toward sardine (Sardinella albella) muscle proteins. Journal of Food Biochemistry, 41(2), e12283. https://doi.org/10.1111/jfbc.12283
Cai, L., Feng, J., Cao, A., Tian, H., Wang, J., Liu, Y., Gong, L., & Li, J. (2018). Effect of partial substitutes of NaCl on the cold-set gelation of grass carp myofibrillar protein mediated by microbial transglutaminase. Food and Bioprocess Technology, 11(10), 1876–1886. https://doi.org/10.1007/s11947-018-2149-7
Cao, Y., Li, B., Fan, X., Wang, J., Zhu, Z., Huang, J., & Xiong, Y. L. (2021). Synergistic recovery and enhancement of gelling properties of oxidatively damaged myofibrillar protein by l-lysine and transglutaminase. Food Chemistry, 358, 129860. https://doi.org/10.1016/j.foodchem.2021.129860
Cavan, E. L., Belcher, A., Atkinson, A., Hill, S. L., Kawaguchi, S., McCormack, S., Meyer, B., Nicol, S., Ratnarajah, L., Schmidt, K., Steinberg, D. K., Tarling, G. A., & Boyd, P. W. (2019). The importance of Antarctic krill in biogeochemical cycles. Nature Communications, 10(1), 4742. https://doi.org/10.1038/s41467-019-12668-7
Chen, M., Wang, L., Xie, B., Ma, A., Hu, K., Zheng, C., Xiong, G., Shi, L., Ding, A., Li, X., Qiao, Y., Sun, Z., & Wu, W. (2022). Effects of high-pressure treatments (ultra-high hydrostatic pressure and high-pressure homogenization) on bighead carp (Aristichthys nobilis) myofibrillar protein native state and its hydrolysate. Food and Bioprocess Technology, 15(10), 2252–2266. https://doi.org/10.1007/s11947-022-02878-1
Chen, X., Xiong, Y. L., & Xu, X. (2019). High-pressure homogenization combined with sulfhydryl blockage by hydrogen peroxide enhance the thermal stability of chicken breast myofibrillar protein aqueous solution. Food Chemistry, 285, 31–38. https://doi.org/10.1016/j.foodchem.2019.01.131
Cueto, M., Farroni, A., Rodríguez, S. D., Schoenlechner, R., Schleining, G., & del Pilar, B. M. (2018). Assessing changes in enriched maize flour formulations after extrusion by means of FTIR, XRD, and chemometric analysis. Food and Bioprocess Technology, 11(8), 1586–1595. https://doi.org/10.1007/s11947-018-2113-6
Ding, Y., Liu, R., Rong, J., & Xiong, S. (2014). Heat-induced denaturation and aggregation of actomyosin and myosin from yellowcheek carp during setting. Food Chemistry, 149, 237–243. https://doi.org/10.1016/j.foodchem.2013.10.123
Du, Y.-N., Han, J.-R., Yin, Z.-K., Yan, J.-N., Jiang, X.-Y., & Wu, H.-T. (2021). Conjugation of (–)-epigallocatechin-3-gallate and protein isolate from large yellow croaker (Pseudosciaena crocea) roe: Improvement of antioxidant activity and structural characteristics. Journal of the Science of Food and Agriculture, 101(14), 5948–5955. https://doi.org/10.1002/jsfa.11247
Fadimu, G. J., Gill, H., Farahnaky, A., & Truong, T. (2021). Investigating the impact of ultrasound pretreatment on the physicochemical, structural, and antioxidant properties of lupin protein hydrolysates. Food and Bioprocess Technology, 14(11), 2004–2019. https://doi.org/10.1007/s11947-021-02700-4
Fu, Y., Zheng, Y., Lei, Z., Xu, P., & Zhou, C. (2017). Gelling properties of myosin as affected by L-lysine and L-arginine by changing the main molecular forces and microstructure. International Journal of Food Properties, 20, S884-S898. https://doi.org/10.1080/10942912.2017.1315593
Guo, X. Y., Peng, Z. Q., Zhang, Y. W., Liu, B., & Cui, Y. Q. (2015). The solubility and conformational characteristics of porcine myosin as affected by the presence of l-lysine and l-histidine. Food Chemistry, 170, 212–217. https://doi.org/10.1016/j.foodchem.2014.08.045
Grewe, J., & Schwarz, U. S. (2020). Mechanosensitive self-assembly of myosin II minifilaments. Physical Review E, 101(2), 022402. https://doi.org/10.1103/PhysRevE.101.022402
Hassoun, A., Aït-Kaddour, A., Sahar, A., & Cozzolino, D. (2021). Monitoring thermal treatments applied to meat using traditional methods and spectroscopic techniques: A review of advances over the last decade. Food and Bioprocess Technology, 14(2), 195–208. https://doi.org/10.1007/s11947-020-02510-0
Huang, M., Mao, Y., Li, H., & Yang, H. (2021). Kappa-carrageenan enhances the gelation and structural changes of egg yolk via electrostatic interactions with yolk protein. Food Chemistry, 360, 129972. https://doi.org/10.1016/j.foodchem.2021.129972
Igual, M., Contreras, C., Camacho, M. M., & Martínez-Navarrete, N. (2014). Effect of thermal treatment and storage conditions on the physical and sensory properties of grapefruit juice. Food and Bioprocess Technology, 7(1), 191–203. https://doi.org/10.1007/s11947-013-1088-6
Jia, D., Huang, Q., & Xiong, S. (2016). Chemical interactions and gel properties of black carp actomyosin affected by MTGase and their relationships. Food Chemistry, 196, 1180–1187. https://doi.org/10.1016/j.foodchem.2015.10.030
Li, Z., Sun, Q., Zheng, Y., Wang, J., Tian, Y., Zheng, B., & Guo, Z. (2020). Effect of two-step microwave heating on the gelation properties of golden threadfin bream (Nemipterus virgatus) myosin. Food Chemistry, 328, 127104. https://doi.org/10.1016/j.foodchem.2020.127104
Liang, Y., Guo, B., Zhou, A., Xiao, S., & Liu, X. (2017). Effect of high pressure treatment on gel characteristics and gel formation mechanism of bighead carp (Aristichthys nobilis) surimi gels. Journal of Food Processing and Preservation, 41(5), e13155. https://doi.org/10.1111/jfpp.13155
Lin, J., Zhang, Y., Li, Y., Sun, P., Ren, X., & Li, D. (2022). Improving the texture properties and protein thermal stability of Antarctic krill (Euphausia superba) by L-lysine marination. Journal of the Science of Food and Agriculture, 102(9), 3916–3924. https://doi.org/10.1002/jsfa.11741
Liu, H., Zhang, H., Liu, Q., Chen, Q., & Kong, B. (2020). Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrasonics Sonochemistry, 67, 105160. https://doi.org/10.1016/j.ultsonch.2020.105160
Mao, W., Li, X., Fukuoka, M., Liu, S., Ji, H., & Sakai, N. (2016). Study of Ca2+-ATPase activity and solubility in the whole kuruma prawn (Marsupenaeus japonicus) meat during heating: Based on the kinetics analysis of myofibril protein thermal denaturation. Food and Bioprocess Technology, 9(9), 1511–1520. https://doi.org/10.1007/s11947-016-1739-5
Ngarize, S., Adams, A., & Howell, N. K. (2004). Studies on egg albumen and whey protein interactions by FT-Raman spectroscopy and rheology. Food Hydrocolloids, 18(1), 49–59. https://doi.org/10.1016/S0268-005X(03)00041-9
Panpipat, W., & Chaijan, M. (2020). Effect of atmospheric pressure cold plasma on biophysical properties and aggregation of natural actomyosin from threadfin bream (Nemipterus bleekeri). Food and Bioprocess Technology, 13(5), 851–859. https://doi.org/10.1007/s11947-020-02441-w
Qin, H., Xu, P., Zhou, C., & Wang, Y. (2015). Effects of l-arginine on water holding capacity and texture of heat-induced gel of salt-soluble proteins from breast muscle. LWT - Food Science and Technology, 63(2), 912–918. https://doi.org/10.1016/j.lwt.2015.04.048
Qiu, C., Xia, W., & Jiang, Q. (2014). Pressure-induced changes of silver carp (Hypophthalmichthys molitrix) myofibrillar protein structure. European Food Research and Technology, 238(5), 753–761. https://doi.org/10.1007/s00217-014-2155-6
Stefanović, A. B., Jovanović, J. R., Dojčinović, M. B., Lević, S. M., Nedović, V. A., Bugarski, B. M., & Knežević-Jugović, Z. D. (2017). Effect of the controlled high-intensity ultrasound on improving functionality and structural changes of egg white Proteins. Food and Bioprocess Technology, 10(7), 1224–1239. https://doi.org/10.1007/s11947-017-1884-5
Sun, P., Lin, J., Ren, X., Zhang, B., Liu, J., Zhao, Y., & Li, D. (2022). Effect of heating on protein denaturation, water state, microstructure, and textural properties of Antarctic krill (Euphausia superba) meat. Food and Bioprocess Technology, 15(10), 2313–2326. https://doi.org/10.1007/s11947-022-02881-6
Sun, P., Zhang, X., Ren, X., Cao, Z., Zhao, Y., Man, H., & Li, D. (2023). Effect of basic amino acid pretreatment on the quality of canned Antarctic krill. Food and Bioprocess Technology, 16(8), 1690–1702. https://doi.org/10.1007/s11947-023-03027-y
Thorarinsdottir, K. A., Arason, S., Geirsdottir, M., Bogason, S. G., & Kristbergsson, K. (2002). Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chemistry, 77(3), 377–385. https://doi.org/10.1016/S0308-8146(01)00349-1
Turgay-İzzetoğlu, G., Çokgezme, Ö. F., Döner, D., Ersoy, C., Çabas, B. M., & İçier, F. (2022). Cooking the chicken meat with moderate electric field: Rheological properties and image processing of microstructure. Food and Bioprocess Technology, 15(5), 1082–1100. https://doi.org/10.1007/s11947-022-02800-9
Vate, N. K., & Benjakul, S. (2016). Combined effect of squid ink tyrosinase and tannic acid on heat induced aggregation of natural actomyosin from sardine. Food Hydrocolloids, 56, 62–70. https://doi.org/10.1016/j.foodhyd.2015.12.009
Wachirasiri, K., Wanlapa, S., Uttapap, D., & Rungsardthong, V. (2016). Use of amino acids as a phosphate alternative and their effects on quality of frozen white shrimps (Penaeus vanamei). LWT - Food Science and Technology, 69, 303–311. https://doi.org/10.1016/j.lwt.2016.01.065
Wagner, J., Biliaderis, C. G., & Moschakis, T. (2020). Whey proteins: Musings on denaturation, aggregate formation and gelation. Critical Reviews in Food Science and Nutrition, 60(22), 3793–3806. https://doi.org/10.1080/10408398.2019.1708263
Wang, K.-Q., Luo, S.-Z., Zhong, X.-Y., Cai, J., Jiang, S.-T., & Zheng, Z. (2017). Changes in chemical interactions and protein conformation during heat-induced wheat gluten gel formation. Food Chemistry, 214, 393–399. https://doi.org/10.1016/j.foodchem.2016.07.037
Wang, S., Shi, Y., Tu, Z., Zhang, L., Wang, H., Tian, M., & Zhang, N. (2017). Influence of soy lecithin concentration on the physical properties of whey protein isolate-stabilized emulsion and microcapsule formation. Journal of Food Engineering, 207, 73–80. https://doi.org/10.1016/j.jfoodeng.2017.03.020
Xia, M., Chen, Y., Guo, J., Feng, X., Yin, X., Wang, L., Wu, W., Li, Z., Sun, W., & Ma, J. (2019). Effects of oxidative modification on textural properties and gel structure of pork myofibrillar proteins. Food Research International, 121, 678–683. https://doi.org/10.1016/j.foodres.2018.12.037
Yang, Y., Wang, Q., Tang, Y., Lei, L., Zhao, J., Zhang, Y., Li, L., Wang, Q., & Ming, J. (2021). Effects of ionic strength and (−)-epigallocatechin gallate on physicochemical characteristics of soybean 11S and 7S proteins. Food Hydrocolloids, 119, 106836. https://doi.org/10.1016/j.foodhyd.2021.106836
Yu, N., Xu, Y., Jiang, Q., & Xia, W. (2017). Molecular forces involved in heat-induced freshwater surimi gel: Effects of various bond disrupting agents on the gel properties and protein conformation changes. Food Hydrocolloids, 69, 193–201. https://doi.org/10.1016/j.foodhyd.2017.02.003
Zhang, F., Jiang, S., Feng, X., Wang, R., Zeng, M., & Zhao, Y. (2020). Physicochemical state and in vitro digestibility of heat treated water-soluble protein from Pacific oyster (Crassostrea gigas). Food Bioscience, 34, 100528. https://doi.org/10.1016/j.fbio.2020.100528
Zhang, D., Zhang, Y., Huang, Y., Chen, L., Bao, P., Fang, H., & Zhou, C. (2021). l-Arginine and l-lysine alleviate myosin from oxidation: Their role in maintaining myosin’s Emulsifying Properties. Journal of Agricultural and Food Chemistry, 69(10), 3189–3198. https://doi.org/10.1021/acs.jafc.0c06095
Zhang, M., Qiu, W., Zhang, R., Row, K., Cheng, Y., & Jin, Y. (2017a). Effect of amino acids on microwave dielectric properties of minced Antarctic krill (Euphausia superba). Food and Bioprocess Technology, 10(10), 1809–1823. https://doi.org/10.1007/s11947-017-1952-x
Zhang, Z., Yang, Y., Zhou, P., Zhang, X., & Wang, J. (2017b). Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chemistry, 217, 678–686. https://doi.org/10.1016/j.foodchem.2016.09.040
Zhao, Q., Yu, X., Zhou, C., Yagoub, A. E. A., & Ma, H. (2020). Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT, 124, 109192. https://doi.org/10.1016/j.lwt.2020.109192
Zhao, X., Chen, F., Xue, W., & Lee, L. (2008). FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocolloids, 22(4), 568–575. https://doi.org/10.1016/j.foodhyd.2007.01.019
Zheng, Y. J. I. J. O. N., & Sciences, F. (2017). Effects of L-lysine/L-arginine on the physicochemical properties and quality of sodium-reduced and phosphate-free pork sausage. International Journal of Nutrition and Food Sciences, 6(1), 12. https://doi.org/10.19026/ajfst.6.91
Zheng, H., Beamer, S. K., Matak, K. E., & Jaczynski, J. (2019). Effect of κ-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chemistry, 278, 644–652. https://doi.org/10.1016/j.foodchem.2018.11.080
Zielinska, S., Cybulska, J., Pieczywek, P., Zdunek, A., Kurzyna-Szklarek, M., Staniszewska, I., Liu, Z.-L., Pan, Z., Xiao, H.-W., & Zielinska, M. (2022). Structural morphology and rheological properties of pectin fractions extracted from okra pods subjected to cold plasma treatment. Food and Bioprocess Technology, 15(5), 1168–1181. https://doi.org/10.1007/s11947-022-02798-0
Funding
This work was supported by the National Natural Science Foundation of China (32372364) and the National Key Research and Development Project of Dalian (Grant No. 2022YF16SN033).
Author information
Authors and Affiliations
Contributions
Junxin Lin: methodology, investigation, and writing—original draft. Peizi Sun: conceptualization, methodology, and investigation. Yanfen Zhao: investigation and methodology. Xiaoping Du: investigation. Xiang Ren: investigation. Hao Man: investigation and methodology. Dongmei Li: conceptualization, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Sun, P., Zhao, Y. et al. Effect of L-Lysine on Heat-Induced Aggregation Behavior of Antarctic Krill (Euphausia superba) Myofibrillar Protein. Food Bioprocess Technol 17, 1448–1461 (2024). https://doi.org/10.1007/s11947-023-03205-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03205-y