Abstract
The objectives of this study were to investigate the changes and the relationship between structure and physiochemical properties of low sodium salt substitutes (NaCl partially replaced by KCl, CaCl2, and MgCl2) on grass carp myofibrillar protein gels mediated by microbial transglutaminase during cold-set gels and to provide more information about the gel characteristics. The gel strength, water holding capacity, whiteness, rheological characteristics, differential scanning calorimeter (DSC), and Raman spectra of cold-set gels were determined. The Raman spectra data were fitted to four secondary structures (α-helix, β-sheet, β-turn, and random coil). The gel properties of cold-set gels varied both with the low sodium salt types and incubation time. Myofibrillar protein (MP) gels added with NaCl and KCl had significantly higher water holding capacity than the MgCl2, CaCl2, and control groups. Additionally, the results showed that the gel strength and G’ value increased with the incubating time. No significant difference was detected in whiteness between the NaCl group and partial substituted groups. Cold-set gels added with the same molar amount of NaCl and KCl had fairly similar gel properties. There is a strong correlation between structural properties and gel properties of MP gels determined by DSC during the cold-set gelation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the meat processing industry, cold-set gelation is a useful and special technique for producing meat products that can be sold in a raw state (Hong et al. 2012). A range of producing technologies for raw chilled meats were developed to solve some problems associated with conventional heat-induced cooking technology and pre-cooked meats that had loss of heat-sensitive nutrients, unacceptable discoloration, and warmed-over flavors. It makes lots of sense to modify fresh fish meat or myofibrillar proteins in a consumer acceptable way to enable performing of good texture and functional properties at low temperatures.
One of the most extensively used effective protein-gelling food additives to improve the covalent bonding between protein molecules in meat system is the microbial transglutaminase (MTGase) (Sun and Holley 2011). MTGase can catalyze inter- or intra-molecule cross-linkages in proteins via ε-(γ-Glu)-Lys bond formation (Dejong and Koppelman 2002). This function is crucial for modifying the physicochemical and functional properties of foods, especially for gelatin properties (Yi et al. 2017). Some studies have been reported that cold-set pretreated casein or soy proteins induced by MTGase were more susceptible to gelation than their nature forms (Alting et al. 2003; Peipei et al. 2016). Thus, it is expected that the use of MTGase as a protein-based binder might eliminate the drawback of cold-set meat gelation.
Cold-set gelation of myofibrillar protein (MP) gel or low-temperature surimi could be obtained by MTGase induction in a slow gelation process (Yi et al. 2017; Koob et al. 2009). Surimi gel formation is important for the texture properties of semi-solid food products, such as fish ball and sausages. Among proteins, MPs are likely to be more labile in response to environmental changes than plant proteins, owing to the differences in structure and thermal stability (Zhao et al. 2014; Tornberg 2005). The texture, flavor, and taste of meat products are significantly related to the gel-forming ability of MPs.
Salt concentrations and kinds can influence the type of gels formed; low sodium dosage induces a weak gel, whereas high dosage produces an opaque more aggregated gel. Nowadays, meat consumption has been criticized because it contains high levels of sodium. Consumers’ demands require dietary guidelines for developing low salt functional foods to reduce the intake of sodium salt. The reduction of sodium to develop healthier products is particularly challenging because it necessarily implies removing or partially replacing sodium chloride by other neutral salts. So the addition of MTGase to a MP gel system could mimic the conditions of low NaCl to induce gel formation as was achieved good gel structure properties and nutrition at low temperatures. However, there is no available information about the use of other chloride salts for partial substitution of NaCl on the cold-set gelation of fish MPs mediated by MTGase during the gelation process. The aim of this study was to investigate the cold-set gel properties of grass carp MPs added with low sodium additives, induced by MTGase and formed at 4 °C. The effects of salt (NaCl) and/or salt substitutes (KCl, MgCl2, and CaCl2) were measured at levels commonly used in meat systems.
Materials and Methods
Materials and Chemicals
Live adult grass carps (Ctenopharyngodon idellus) with an average weight of 1500 ± 50 g and an average mantle length of 35 ± 1 cm were harvested from a local aquaculture farm in Jinzhou, Liaoning, China. The fish were transported in ice with a fish/ice ratio of 1:2 (w/w) to the Seafood Processing Laboratory of Bohai University within 0.5 h. On arrival, grass carps were stunned by percussive stunning method (Sun et al. 2017) and washed in iced deionized water. All the experiments were carried out at the seafood processing lab. All operation processes were approved by the Animal Care Committee of Bohai University and commanded according to the guiding principle of the Liaoning Province Committee on Animal Care. The dorsal white muscle (without the skin, head, tail, bones, and blood) was collected and washed with cold distilled water and kept at 4 °C during the operation until the next step to avoid overheating. Sodium salt or sodium salt substitutes (NaCl, KCl, MgCl2, and CaCl2) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), and MTGase was obtained from Solarbio Bioscience and Technology Co. (Shanghai, China). All chemicals are at least of analytical grade.
Extraction of MPs
Myofibrillar proteins were isolated from the dorsal white muscle. All operations were performed in ice and kept at lower temperature. The fish muscle was chopped and the chopped flesh (200 g) was homogenized in 800 mL of cold extraction buffer (10 mM Tris-HCl, pH 7.2) using a digital high-speed dispersion homogenizer (FJ200-SH, Shanghai Specimen Model Factory, Shanghai, China). The final pH of mixture was adjusted to 7.2. The homogenate was subsequently centrifuged three times using a refrigerated high-speed centrifugal machine (Sorvall Stratos, Sigma Co., Ltd., Hesse, Germany) at 5000 rpm at 4 °C for 15 min and the sediment was collected for the next circle. The sediment was dissolved in three volumes of cold 10 mM Tris-HCl buffer (0.6 M NaCl, pH 7.2). All the supernatant was obtained and MPs were stored at 4 °C during all analyses.
Gel Sample Preparation
MP solution was adjusted to 40 mg/mL with 10 mM Tris-HCl buffer. The protein concentration was determined by Bradford (1976) and the protein marker was bovine serum albumin. The control sample with 0.6 mol/L NaCl was elaborated simultaneously to different concentrations of NaCl replacers. The amounts of NaCl replacer were determined in MP solutions based on the equivalent ionic strength in the experiment. The details are as follows: control group (without NaCl), NaCl group (normal sodium level) (0.6 mol/L NaCl), KCl group (0.4 mol/L NaCl + 0.2 mol/L KCl), MgCl2 group (0.4 mol/L NaCl + 0.1 mol/L MgCl2), and CaCl2 group (0.4 mol/L NaCl + 0.1 mol/L CaCl2). Fifteen milliliters of 40 mg/mL MP solutions with different chloride salts was placed in 20-mL tubes with a cork. All samples were added with 0.1% (w/v) MTGase. Each mixture was completely dissolved using a magnetic stirrer at 300 rpm for 20 min at 4 °C and then stored in glass cubes (21 mm diameter × 36 mm height) until analyzed. The cold-set gelation was carried out at 4 °C during a total of 10 days and then analyzed for thermal stability, cooking loss, water holding capacity, whitening, rheological properties, and gel strength as described subsequently. The experiment was done in triplicate, and each independent trial used a newly prepared MP sample.
Gel Strength
The gel strength of the cold-set gels in the glass tube was tested according to the method of Cai et al. (2017) using a TA-XT plus texture analyzer (Stable Micro Systems Ltd., Godalming, UK) with a standard cylindrical (P/0.5) stainless steel probe at a speed of 1.0 mm/s, using a 5-g trigger force, stopping at 40% of the thickness, and data acquisition at a rate of 250 points/s. The gels were stored at 4 °C in a refrigerator for a maximum of 10 days and changes of cold-set gels were analyzed at 2-day intervals. The control which had not been kept in cold storage was tested directly after keeping it at 4 °C for 1 h in casing tubes. Each cold-set gelation sample was repeated for three times. The peak force required to disrupt the gels was expressed as gel strength (N).
pH Measurement
The pH of the samples was measured using a combined and calibrated glass electrode pH meter (Leici PHS-3C, Shanghai Yidian Scientific Instruments Inc., Shanghai, China). The samples were equilibrated at room temperature for 2 h prior to analysis. All measurements were made on at least three random positions and each sample was measured in duplicate. The pH value was recorded every 2 days.
Water Holding Capacity
The water holding capacity (WHC) of cold-set gel samples was measured using the centrifugation method with some modifications (Han et al. 2017). The sample (10 g) was cut into small cubes (2 cm × 2 cm × 2 cm) and inserted into centrifuge tubes (50 mL) attached with absorbent cotton at the bottom and centrifuged at 3000 rpm at 4 °C for 10 min. WHC values were obtained from three measurements. All results were recorded every 2 days. WHC was expressed as the following equation:
Measurement of Whiteness
Surface color of low salt cold-set gels was performed using a chroma meter (CR-400, Konica Minolta, Tokyo, Japan). Before measuring, the chroma meter was calibrated on a standard white plate (Standard No. 18133042, Y = 86.8, X = 0.3175, and y = 0.3340). Color measurements were made on the surface of each sample in triplicate at three random locations. Whiteness was calculated using the following equation:
where L (lightness), a (redness/greenness), and b (yellow ness/blueness) are the difference between the corresponding color parameters of the cold-set gelation samples. A numerical whiteness of samples was measured at intervals of 2 days for 4 °C storage.
Measurement of Dynamic Rheological Properties
Dynamic rheological properties were tested using a Discovery rheometer (HR-1, TA Instruments Co. Ltd., Manchester, UK). A 40-mm parallel steel plate geometry with a 100,000 μm gap size was used, and the cold-set gels were surrounded by liquid paraffin to prevent drying. Changes in the storage modulus (G’) and loss modulus (G”) were recorded during the gelling process as a function of temperature. Gel samples were measured at temperatures ranging from 20 to 90 °C, at the heating rate of 5 °C/min, under controlled strain (0.5%) and a fixed frequency of 0.1 Hz in an oscillatory mode.
Differential Scanning Calorimeter
The endothermal transitions of cold-set gel samples were measured using a differential scanning calorimeter (DSC Q2000, TA Instruments, New Castle, DE, USA) according to the methods of Ali et al. with some modifications (Ali et al. 2015). Temperature and heat flow calibration was performed using the indium thermogram. Gel sample (5–10 mg) was accurately weighed and placed in a stainless steel pan, hermetically sealed with a pressing machine (TZERO™, New Castle, DE, USA). A hermetically sealed empty aluminum pan was used as a reference. The program temperature was scanned over 20–90 °C using a heating rate of 5 °C/min. The peak transition temperature (Tmax) was estimated from the thermogram by the software (Universal Analysis 2000, Version 4.5A, TA Instruments).
Raman Spectra Measurement
Raman measurements were made on the cold-set gels using a Raman spectrometer instrument (LabRAM HR Evolution, Bruker, Germany) equipped with an argon ion laser providing radiation at 514.5 nm (Bruker, Germany) and a microscope with a × 50 lens. Raman spectra were recorded at room temperature (~ 25 °C). The samples were placed on microscope slides under the following conditions: laser power: 100 mW, a sweep range of 3300–400 cm−1, spectral resolution: 2.0 cm−1, number of sample scans: 3, exposure times: 60, data collected: 1 cm−1, speed: 120 cm−1/min. The spectral data from the scans of samples in the Raman spectrophotometer of phenylalanine band located near 1003 cm−1 was used as internal standard to normalize the spectra, as it has been reported to be insensitive to the microenvironment (Kang et al. 2014). The results of Raman spectra were smoothed, baselines corrected and normalized. The secondary structures of the MP gels were determined as percentages of α-helix, β-sheet, β-turn, and random coil or unordered conformations (Kang et al. 2014). With this aim, the water spectrum was subtracted from the spectra by following the same criteria as that described previously (Alix et al. 1988). All analyses were performed in triplicate and averaged for the reported results.
Statistical Analysis
SPSS 19 for Windows (SPSS Inc., Chicago, IL, USA) was used. Statistical significance of data was subjected to one-way analysis of variance and difference of mean values was evaluated using Duncan’s multiple range tests with a confidence level of P < 0.05.
Results and Discussion
Gel Strength
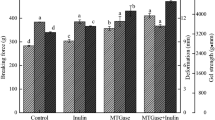
Table 1 presents the effects of partial substitutes of NaCl on gel strength of MP gels with different incubation times. In this study, the control group showed the highest cold-set gel strength among all treatments, indicating that 0.6 mol/L salt addition to MP gelatin system, which was chemically unfolded, could have induced salting-out effect at the relatively low salt concentration during cold-set gelation. The addition of neutral salts decreased the gel strength of the MP gels. But acceptable cold-set gel strength was proposed to be as high as possible to make the low sodium dosage of cold-set MP gels considered as a raw state, such as the control and NaCl groups. As the incubation time increased, the gel strength of cold-set gels added with different substitutes of NaCl increased significantly (P < 0.05). Meanwhile, a longer incubation time was also important for the gel network structure formation of MP gels set at 4 °C, except for the control group, which reached a maximum at the incubation time of day 4. These results were in agreement with Mahawanich et al. (2010), who reported that the storage time of cold-set gelation had significant effects on the gel strength with different storage times (Mahawanich et al. 2010). Therefore, the use of low sodium salt system to form a cold-set gel could be extended to utilize the incubation time and not required a further cooking procedure.
These results indicated that MP gels of NaCl substitutes resulted in a significant interactive effect with increasing incubation time. In addition, NaCl could not be partially replaced by CaCl2 and MgCl2 due to the decreased gel strength. However, partial replacement of NaCl with KCl may be capable of producing a gel with strength similar to that produced using NaCl alone. NaCl replaced by KCl could be acceptable with less than 50% substitution. Above this level, unacceptable saltiness and bitterness attributes between the control and the treated groups appeared. Lorenzo et al. (2015) reported similar results in dry fermented sausages when more than 50% partial replacement happened. The increase in gel strength of NaCl and KCl groups can be attributed to the increase in protein solubility over incubation time. The former research also indicated that MP gel strength increased with the amount of proteins solubilized in the brine gel system (Xiong and Brekke 1989). The exposure of hydrophobic groups and individual active groups through chemical interactions might be contributing another factor to this change in gel strength. During cold-set gel incubation, the MP molecules unfolded and interacted via disulfide and covalent bonds between protein molecules and saline ions (Liu et al. 2011). The observed general gel-enhancing pattern by MTGase was consistent with that reported by Koob et al. (2009). In the presence of MTGase, neutral salts had the potential to interact with MPs through the carbonyl-amine cross-linking. The greater accessibility of glutamine and lysine residues by MTGase is due to structure unfolding, and pH-treated proteins were able to promote MP gelation.
pH Value
Changes of pH profiles of cold-set MP gels along with incubation time and different partial substitutes of NaCl are shown in Fig. 1a. The pH values of all treatments changed slowly after day 0. No significant differences (P > 0.05) in pH values were observed between NaCl and KCl groups during the whole incubation time. The pH values ranged for each treatment were 6.73 to 6.30 (control), 6.93 to 6.45 (NaCl), 6.94 to 6.44 (KCl), 6.56 to 6.36 (MgCl2), and 6.83 to 6.30 (CaCl2), respectively. During the cold-set gelation process, the change of pH was related to the electrostatic interaction between protein molecules. On day 6, NaCl and KCl groups had slightly higher pH values (6.93 and 6.94, respectively) than other groups. Except that day, no significant differences in pH values of cold-set gelation samples with different NaCl substitutes at the same incubation time were observed (P > 0.05).
Water Holding Capacity
The gel properties of proteins in food systems depend in part on the WHC which refers to the ability of proteins to imbibe and retain water against a gravitational force within protein matrix (Koli et al. 2012). The effects of cold-set gelation incubation time and partial substitutes of NaCl (KCl, MgCl2 and CaCl2) on WHC of MP gel system are shown in Table 2. WHC was affected by the kinds of NaCl substitutes. The results showed that WHC tended to significantly increase with the addition of the neutral salts, showing water might exist inside the gel network structure by capillary action and/or H-bonding (Lee et al. 2003). The NaCl group had the highest WHC, but no significant difference was observed between NaCl and other neutral salt groups. The similar results of WHC and pH values in KCl and NaCl groups showed that KCl can partly replace NaCl without affecting the quality of gel products (Greiff et al. 2015), and the difference between NaCl and KCl groups was not significant. The presence of magnesium and calcium ions tended to stop the penetration of chloride ions into the protein molecules, thus negatively affecting WHC of proteins compared with NaCl and KCl (Baratli et al. 2013; Martinezalvarez and Gomezguillen 2005). Thus, there are different effects of Na+, K+, Ca2+, and Mg2+ ions on MP gel formation, because different ions possess different electrical fields, which could result in different solubilities of MPs. It is found that conformational changes in protein that occurred at low temperatures were analogous to those under high-temperature treatments (Koob et al. 2009). Protein denaturation induced by chemical forces usually irreversibly unfolds the complete protein structure because of covalent bonds’ breakage and molecules’ aggregation. In contrast, low temperature can leave parts of the molecules unchanged, indicating that the protein denaturation mechanisms are substantially different. There were no significant differences (P > 0.05) in WHC of all samples during the whole incubation time, which indicated that cold-set gelation is a slow process.
Gel Whiteness
The consumers use appearance factors to provide an indication of freshness and flavor quality (Beaulieu 2010). The evaluated whiteness also is a good indicator of color change during storage which was related to the degree of protein denaturation (Xia et al. 2010). The effects of NaCl and partial substitutes (KCl, MgCl2, and CaCl2) on gel whiteness characteristics of MPs during the cold-set gelation are shown in Fig. 1. The whiteness of gels from the control and the treated groups showed different change trends during 4 °C storage. The whiteness of the control group was the highest (P < 0.05) on day 4 stored at 4 °C. According to the control group gel-setting strategy, it was observed that as storage time increased, whiteness first increased and then reached a maximum on day 4 and then decreased. The increase and decrease in whiteness of protein gels depend on the structure of the protein gel matrix and the size of scattering particles (Estevez et al. 2010). No significant differences were detected in whiteness between the NaCl group and partial substituted groups with KCl, MgCl2, and CaCl2. This was expected, given that salt substitution was performed based on the same molar concentration and these treatments would have no obvious difference in appearances, so they could be used as good substitutes for sodium replacers. A similar result was found in the study by Horita et al. (2011), in which consumers could not identify a color difference in gel products of surimi by visual inspection, even though measured color characteristics determined using an instrument were shown to be significantly different.
Dynamic Rheological Analysis
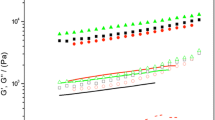
Temperature sweeps state the temperature dependence of dynamic rheological parameters. Elastic (storage) modulus (G’) was determined as a function of temperature from 20 to 90 °C at 0.1 Hz frequency. The effects of NaCl, KCl, MgCl2, and CaCl2 addition on the rheological properties of the MP gels are shown in Fig. 3. The G’ values were found to be significantly affected by the kinds of partial substitutes of NaCl on the cold-set gelation. The highest G’ was observed in control group, which demonstrated that neutral salt addition resulted in less elastic gels in cold-set gelation process and the result was consistent with that of gel strength. Different neutral salts have a crucial effect on the gel ability of proteins as a result of their effects on the solubility of MPs. NaCl and KCl groups had better gel network structures formed with more rigid and fine-stranded gels than those partially substituted by CaCl2 and MgCl2. Moreover, gel was considered as a three-dimensional network in which MPs are linked through weak or strong interactions by different chemical forces. G’ values, which are helpful to explain the texture of semi-solid gels, showed an increase with the increase of incubation time in cold-set gelation process, which reflected the interactions between protein molecular aggregates. The results were consistent with the previous study, and neutral salts had a significant effect on porcine MP gels in the presence of MTGase (Hong and Koobok 2010).
As shown in Fig. 2, the curves overall showed a gradual increase tendency until the end temperature, at which the increase of G’ values indicating transition was from a liquid-like state to a solid-like state. And the transition temperature is usually taken as gelling temperature corresponding to that G’ values significantly increased. The detailed G’ profiles are as follows: the control MPs displayed a characteristic rheological peak at 45 °C, a G’ trough at 49 °C, and a rapidly increase of G’ thereafter. As the temperature increased to 75 °C, a gradual increase in G’ was observed in the control gel samples. The G’ of low sodium gel samples started to increase to reach the first peak when the temperature increased from 20 to 45 °C. Above 45 °C, G’ increased rapidly to reach the peak up to 66 °C then slightly increased. This indicated MPs-MPs interacted and networks were developed in the range of 45–66 °C when the neutral salts existed, which was probably due to the fact that a longer incubation time created a network with a higher density of protein matrix structure, which then resulted in a higher elasticity.
In conclusion, the incubation time significantly influenced the rheological properties of cold-set gels. This can be attributed to cross-linking reactions occurring among the MPs resulting in the formation of a network structure. As suggested in the research that strong disulfide bindings formed at 40–70 °C, which protein-protein interactions through disulfide interchange reactions and electrostatic interaction would begin to provide an increasingly highly cross-linked structure, resulting in high G’ values (Mahawanich et al. 2010). Therefore, the rapid increase in G’ was probably due to stronger interactions between denatured myosin molecules and implied the formation of irreversible gel network.
DSC
Figure 3 shows the differential scanning calorimeter (DSC) thermograms of MPs alone or in combination with chlorinated salts during cold-set gelation process. Two endothermic peaks can be observed at transition temperatures corresponding to the denature points of the myosin (between 54 and 66 °C) and actin (between 66 and 86 °C) as described by other studies (Barbut and Findlay 2010). In addition, Table 3 shows the detailed peak maximum temperatures (Tmax) and denaturation enthalpy (△HD) for the main proteins of MP with chlorinated salts induced by MTGase during cold-set gelation.
The neutral salt treatment led to some shifts in transition temperatures and the decrease in peak area (Fig. 3). The control exhibited two endothermic peaks at 43.48 and 70.48 °C, which represented myosin and actin, respectively. The transition peaks of other samples became broader and more separable compared to the control. The addition of NaCl, KCl, MgCl2, or CaCl2 decreased the denatured temperature of the myosin and actin (Table 3). The reason was that actin might be involved in the cross-links of protein-protein by covalent bonding (Nam et al. 2017). These results suggested that the interactions between chlorinated salts and MPs influenced the patterns of protein denaturation during the cold-set gelation process. The addition of the salts resulted in lower peak temperatures of myosin and actin, caused a severe denaturation of MPs, and created aggregated MP particulates (Thorarinsdottir et al. 2002), indicating protein unfolding, which is the initial process in protein gelation. The results were consisted with the former research that protein unfolding is an essential process in protein gel formation. The decrease in △HD of actin suggested protein denaturation, and a partially unfolded polypeptide would require less heat energy (lower △HD) to denature completely.
Raman Spectra
MPs play important roles in the development of a three-dimensional gel matrix in the meat gelling process. Raman spectra of the MPs in cold-set gelation systems under different neutral salt treatments in the 400–2000 cm−1 region are shown in Fig. 4a. The Raman spectra were averaged prior to baseline correction of the spectra. The assignments of the corresponding bands of Raman modes, which are useful for interpretation of protein structure and modification, are included in Table 4, which involves mainly COO stretching, to lesser degree, C–N stretching, C–C–N bending, and N–H in-plane bending of peptide groups (Sun et al. 2011; Fan et al. 2017). The frequency and intensity changes in the Raman bands were mainly indicative of changes in the secondary structure and variations in local environments of MPs. Protein gel properties are closely related to the changes in its conformation, which is related to the intensity changes of Raman bands.
Comparison of the Raman spectra region of treated and control gels reveals slight shifting of the amide I band toward higher frequencies when added with different neutral salts during cold-set gelation process, with more marked changes after treatment of CaCl2. In the control group, the amide I band of MP gels exhibited maximum scattering at 1656 cm−1. After added with NaCl, KCl, MgCl2, and CaCl2, the maximum scatterings were shown at 1659, 1658, 1659, and 1660 cm−1, respectively, indicating that the most obvious changes occurred during cold-set gel formation. The bands became sharp through different treatments. This indicated that the structure of MP gel changed after adsorption to the neutral salt, resulting in a more ordered structure than the control. It can be observed that cold-set gelation gels have been formed by the types of conformational changes. The intensity of amide I was significantly increased and became sharper in the gel state.
Different neutral salts affected the secondary structure of MPs with the incubation time, by increasing conformational changes, aggregation and thermal denaturation of proteins, and exposure of buried hydrophobic amino acids. The strong intensities of bands generated by disulfide and S–H bond assignment in the 460–670 cm−1 region were observed in NaCl and KCl groups, but showing weak bands in other groups. The results showed that the presence of low sodium salts could alter the molecular structure of proteins and result in changes in conformation of disulfide groups.
The secondary structures of protein, such as amides, are presented by several bands using Raman Spectroscopy, among which those of amide I (1650–1680 cm−1) and amide III (1200–1350 cm−1) are the most useful (Yang et al. 2016). The quantitative estimation of the secondary structure fractions of MPs by the amide I band as affected with chlorinated salts during cold-set gelation using Alix’s method are shown in Fig. 4a. The amide I includes α-helix (1650–1660 cm−1), β-sheet (1665–1680 cm−1), β-turns (near 1680 cm−1), and random coil (1660–1665 cm−1) structures.
Both the unfolding of α-helices and the formation of β-sheets play an important role in MP gel formation. During the cold-set gelation process, a significant decrease or increase in α-helix, β-sheet, and β-turn could be observed, correspondingly. The α-helix content of control sample was 48.31%, and NaCl, KCl, and MgCl2 groups increased to 59.03, 62.60, and 51.88% respectively, while the CaCl2 group decreased to 41.16%. In cold-set gelation process, a high α-helix content indicated that the α-helix structure was the dominant pattern of secondary structure of MPs. The β-sheet content decreased from 24.75 to 22.02, 16.54, and 13.80% for MgCl2, NaCl, and KCl groups, respectively. In contrast, the β-sheet content increased to 30.23% for CaCl2 groups. The β-turn content changed from 16.28 to 14.60, 14.04, 15.72, and 17.40% for NaCl, KCl, MgCl2, and CaCl2 groups, respectively. The contents of random coil had no significant changes between five groups. The results of Raman spectra indicated specific conformational changes in the secondary structure and micro-environment of MP molecules during cold-set gelation process.
Conclusions
In summary, the sodium partial substitution and incubation time affected the gel properties of MPs through the cold-set gelation process mediated by MTGase. Gel strength and G’ analyses showed that longer incubation time could induce complete denaturation of MPs and form dense three-dimensional mesh gel structure. No significant differences in whiteness were observed between NaCl group and partial substituted groups with KCl, MgCl2, and CaCl2. MP gels added with NaCl and KCl had significantly higher WHC than the MgCl2, CaCl2, and control groups. Generally, NaCl replaced by KCl could be chosen as the best substitutes to reduce the sodium content without affecting cold-set gel properties of the product. The results of DSC thermograms and Raman spectra indicated specific conformational changes in the secondary structure and micro-environment of MP molecules during cold-set gelation process which affected the gel properties including gel strength and rheological behavior. Thus, these treatments might be a useful means of producing low sodium cold-set gel products with different edible qualities to help satisfy the diverse requirements of the consumers.
These results indicated that the research of an adequate partial NaCl replacement by other chloride salts is very important for an effective control of the sodium amount during cold-set gel process and consequently to control the gel properties of the cold-set gel product. So, more studies will be necessary for a better knowledge of the blend compositions and to improve texture properties and to obtain a safer and healthier cold-set gel product with optimum texture properties. In addition, we should further research the difference of sensory properties between NaCl replacement and NaCl for reduction of the additive amount in the formulation. It was the necessary formality to reduce the NaCl content without affecting the product acceptability.
References
Ali, S., Zhang, W., Rajput, N., Khan, M. A., Li, C. B., & Zhou, G. H. (2015). Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chemistry, 173(8), 808–814.
Alix, A. J. P., Pedanou, G., & Berjot, M. (1988). Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman amide I band. Journal of Molecular Structure, 174(174), 159–164.
Alting, A. C., Hamer, R. J., Cgd, K., Pacques, M., & Visschers, R. W. (2003). Number of thiol groups rather than the size of the aggregates determines the hardness of cold set whey protein gels. Food Hydrocolloids, 17(4), 469–479.
Baratli, Y., Charles, A. L., Wolff, V., Ben, T. L., Smiri, L., Bouitbir, J., Zoll, J., Piquard, F., Tebourbi, O., & Sakly, M. (2013). Impact of iron oxide nanoparticles on brain, heart, lung, liver and kidneys mitochondrial respiratory chain complexes activities and coupling. Toxicology in Vitro, 27(8), 2142–2148.
Barbut, S., & Findlay, C. J. (2010). Influence of sodium, potassium and magnesium chloride on thermal properties of beef muscle. Journal of Food Science, 56(1), 180–182.
Beaulieu, J. C. (2010). Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Critical Reviews in Food Science and Nutrition, 50(5), 369–389.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Cai, L., Feng, J., Regenstein, J., Lv, Y., & Li, J. (2017). Confectionery gels: effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocolloids, 67, 157–165.
Dejong, G. A. H., & Koppelman, S. J. (2002). Transglutaminase catalyzed reactions: impact on food applications. Journal of Food Science, 67(8), 2798–2806.
Estevez, M., Ventanas, S., & Cava, R. (2010). Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: effect on color and texture deterioration. Journal of Food Science, 70(7), 427–432.
Fan, M., Hu, T., Zhao, S., Xiong, S., Xie, J., & Huang, Q. (2017). Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chemistry, 218, 221–230.
Greiff, K., Aursand, I. G., Erikson, U., Josefsen, K. D., & Rustad, T. (2015). Effects of type and concentration of salts on physicochemical properties in fish mince. LWT - Food Science and Technology, 64(1), 220–226.
Han, L., Zhang, L., Li, Q., & Luo, Y. (2017). Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp ( Aristichthys nobilis ) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chemistry, 223, 96–103.
Hong, G. P., & Koobok, C. (2010). Effects of microbial transglutaminase and sodium alginate on cold-set gelation of porcine myofibrillar protein with various salt levels. Food Hydrocolloids, 24(4), 444–451.
Hong, G. P., Chun, J. Y., Lee, S. K., & Choi, M. J. (2012). Effects of non-meat protein binders and acidification on the efficiency of cold-set pork restructuring by high pressure. Hangug Chugsan Sigpum Haghoeji = Korean Journal for Food Science of Animal Resources., 32(3), 301–307.
Horita, C. N., Morgano, M. A., Celeghini, R. M. S., et al. (2011). Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Science, 89(4), 426–433.
Kang, Z. L., Wang, P., Xu, X. L., Zhu, C. Z., Li, K., & Zhou, G. H. (2014). Effect of beating processing, as a means of reducing salt content in frankfurters: a physico-chemical and Raman spectroscopic study. Meat Science, 98(2), 17–177.
Koli, J. M., Basu, S., Nayak, B. B., Patange, S. B., Pagarkar, A. U., & Gudipati, V. (2012). Functional characteristics of gelatin extracted from skin and bone of Tiger-toothed croaker (Otolithes ruber) and pink perch (Nemipterus japonicus). Food and Bioproducts Processing, 90(3), 555–562.
Koob, C., Miy, G., & Xiong, Y. (2009). Effect of soy protein substitution for sodium caseinate on the transglutaminate-induced cold and thermal gelation of myofibrillar protein. Food Research International, 42(8), 941–948.
Lee, K. Y., Shim, J., Bae, I. Y., Cha, J., Park, C. S., & Lee, H. G. (2003). Characterization of gellan/gelatin mixed solutions and gels. LWT - Food Science and Technology, 36(8), 795–802.
Liu, R., Zhao, S. M., Xie, B. J., & Xiong, S. B. (2011). Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocolloids, 25(5), 898–906.
Lorenzo, J. M., Cittadini, A., Bermúdez, R., Munekata, P. E., & Domínguez, R. (2015). Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control, 55, 90–96.
Mahawanich, T., Lekhavichitr, J., & Duangmal, K. (2010). Original article: gel properties of red tilapia surimi: effects of setting condition, fish freshness and frozen storage. International Journal of Food Science and Technology, 45(9), 1777–1786.
Martinezalvarez, O., & Gomezguillen, M. C. (2005). The effect of brine composition and pH on the yield and nature of water-soluble proteins extractable from brined muscle of cod (Gadus morhua). Food Chemistry, 92(1), 71–77.
Nam, M. K., Lee, H. C., Hong, Y. J., Jang, J. Y., Choi, E. H., Chung, C. W., Jeon, S., Kim, J. M., Kang, S., & Rhim, H. (2017). The new approach for establishing the cellular response guideline for medical applications of argon-plasma jet: mitochondria and colorimetric polydiacetylene as innovative parameters. Journal of Biomedical Nanotechnology, 13(1), 77–83.
Peipei, Z., Tan, H., Shaolong, F., Qi, X., Ting, Z., Moxi, Z., Xueqi, C., Xingjian, H., Xiaonan, L., & Siyi, P. (2016). Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrasonics Sonochemistry, 29, 380–389.
Sun, X. D., & Holley, R. A. (2011). Factors influencing gel formation by myofibrillar proteins in muscle foods. Comprehensive Reviews in Food Science and Food Safety, 10(1), 33–51.
Sun, W., Zhao, Q., Zhao, M., Yang, B., Cui, C., & Ren, J. (2011). Structural evaluation of myofibrillar proteins during processing of Cantonese sausage by Raman spectroscopy. Journal of Agricultural and Food Chemistry, 59(20), 11070–11077.
Sun, L., Sun, J., Thavaraj, P., Yang, X., & Guo, Y. (2017). Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chemistry, 224, 372–381.
Thorarinsdottir, K. A., Arason, S., Geirsdottir, M., Bogason, S. G., & Kristbergsson, K. (2002). Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chemistry, 77(3), 377–385.
Tornberg, E. (2005). Effects of heat on meat proteins—implications on structure and quality of meat products. Meat Science, 70(3), 493–508.
Xia, X., Kong, B., Xiong, Y., & Ren, Y. (2010). Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Science, 85(3), 481–489.
Xiong, Y. L., & Brekke, C. J. (1989). Changes in protein solubility and gelation properties of chicken myofibrils during storage. Journal of Food Science, 54(5), 1141–1146.
Yang, H., Zhang, W., Li, T., Zheng, H., Khan, M. A., Xu, X., Sun, J., & Zhou, G. (2016). Effect of protein structure on water and fat distribution during meat gelling. Food Chemistry, 204, 239–246.
Yi, Z., He, S., & Simpson, B. K. (2017). A cold active transglutaminase from Antarctic krill (Euphausia superba): purification, characterization and application in the modification of cold-set gelatin gel. Food Chemistry, 232, 155–162.
Zhao, Y. Y., Wang, P., Zou, Y. F., Li, K., Kang, Z. L., Xu, X. L., & Zhou, G. H. (2014). Effect of pre-emulsification of plant lipid treated by pulsed ultrasound on the functional properties of chicken breast myofibrillar protein composite gel. Food Research International, 58(4), 98–104.
Funding
This study was supported by the National Natural Science Foundation of China (31401478), the National Postdoctoral Science Foundation of China (2015M570760), the Postdoctoral Special Funding of Chongqing City (Xm2015021), the Natural Science Foundation of Liaoning Province of China (20170540006), the Open Fund by Beijing Advanced Innovation Center for Food Nutrition and Human Health (20171003), and the Graduate Innovation Fund of Bohai University of Liaoning Province of China (YJC20170027).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cai, L., Feng, J., Cao, A. et al. Effect of Partial Substitutes of NaCl on the Cold-Set Gelation of Grass Carp Myofibrillar Protein Mediated by Microbial Transglutaminase. Food Bioprocess Technol 11, 1876–1886 (2018). https://doi.org/10.1007/s11947-018-2149-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2149-7