Abstract
The dielectric properties of ten solid amino acids, namely, glycine, proline, cysteine, glutamine, lysine, methionine, histidine, arginine, tyrosine, and tryptophan, and the effects of amino acid concentrations (0, 1, 3, and 5%) on the dielectric properties of minced Antarctic krill (Euphausia superba) were studied within a frequency range of 300–3000 MHz at 20.0 ± 0.5 °C. The dielectric constants and dielectric loss factor of amino acids were 2.0 ± 0.4 and 0.2 ± 0.3, respectively (moisture contents, 0.05–0.33%), except those of lysine (5.5 ± 0.5 and 1.5 ± 1.0, respectively) (moisture content, 4.5%). The dielectric properties of non-polar, neutral-polar, alkaline amino acids added to Antarctic krill were affected by the frequency and moisture contents, molecular weight, and isoelectric points (pI) of amino acids. As the molecular weight and pI values increased, the critical frequencies of aliphatic amino acid added to minced Antarctic krill decreased, whereas heterocyclic amino acid added to minced Antarctic krill increased. Quantitative relationships between parameters that define both the real and the imaginary parts of the Debye relations and the weight fraction of amino acid contents in Antarctic krill were determined. Moreover, glycine (at 5%) or lysine (at 5%) significantly influenced the dielectric constants and dielectric loss factor of minced Antarctic krill (p < 0.05) but did not have significant effects on temperature history and distribution (p < 0.05) during microwave heating at 2450 MHz.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctic krill (Euphausia superba) is a key species in the Antarctic ecosystem (Farber-lorda et al. 2009). Based on its large abundant biomass of 342–356 million tons, Antarctic krill is the largest animal protein resource in the world (Wang et al. 2011). The constituent proteins contain all the essential amino acids for humans (Chen et al. 2009), and their biological values are higher than those of milk proteins and other meat products (Wang et al. 2015). Consequently, the high nutritive value of Antarctic krill has resulted in an increased demand for Antarctic krill products, such as protein derivative, krill oil, chitosan, and astaxanthin (Wang et al. 2015; Zhu et al. 2015; Wu et al. 2015). Commercial productions of these products involves various processing steps such as heating, extracting, and separating. However, the conventional processing methods of steam heating, air heating, and solvent extraction have the following disadvantages over microwave processing: high consumption of energy, time, solvent, and involvement in environmental pollution.
Microwave heating is widely used in the food industry given its relatively short heating time due to its ability to generate volumetric heating within food materials (Fu et al. 2012). However, this technique also has some drawbacks, such as non-uniform heating and dependence of the heating rate depends on the dielectric properties of the material. The dielectric properties of food materials have been considered the major factors contributing to the interactions between microwaves and food (Ahmed et al. 2008).
The methods used to determine dielectric properties of material were as follows: resonant cavity method (Afsar et al. 1999), waveguide measurement (Jarvis et al. 2001), open-ended coaxial probe (Hagl et al. 2003), impedance measurement (Halter et al. 2007; Nordbotten et al. 2011), and free-space field method (Sagnard et al. 2005). The dielectric properties of solid foods such as rice (Lagunas-Solar et al. 2007), walnut (Wang et al. 2007a, b), and legumes (Guo et al. 2010) were already determined using open-ended coaxial-line probe technology. However, the dielectric properties of solid amino acids (powder or crystal) have not been reported so far.
There are many factors that influence the dielectric properties of food, including frequency, temperature, moisture content, salts, fat/lipid, protein, and other food constituents (Okiror and Jones 2012; Tang 2005; Zhang et al. 2008; Lyng et al. 2002, 2005; Ahmed et al. 2008; Feng et al. 2015). As one of food constituents, amino acid can also affect both dielectric properties and microwave heating characteristics (heating rate, uniform heating, etc.) of food, which have not been reported so far. However, it is difficult to investigate the effect of amino acids on these parameters because there are various kinds and contents of amino acids in different foods. Therefore, the single amino acid with a certain concentration was chosen to be added into the minced Antarctic krill in this study.
In this study, we determined the dielectric properties of solid amino acids; furthermore, we studied the effects of amino acids on the dielectric properties of Antarctic krill and studied the penetration depths and temperature history and distribution. Our results can provide the fundamental basis for further studies and commercial processing of the Antarctic krill.
Theory
Normally, dielectric properties are described by the complex permittivity (ε*) (Sosa-Morales et al. 2010): ε* = ε′ − jε″, where j = \( \sqrt{-1} \), the real part ε′ and the imaginary part ε″ were the dielectric constant and dielectric loss factor, respectively. Dielectric constant and dielectric loss factor indicate the abilities of a material to store electric energy and a material to transform electromagnetic energy into thermal energy, respectively (Feng et al. 2015).
In order to understand the effect of amino acids on dielectric properties of Antarctic krill and reveal the energy exchange mechanism of microscopic particles, Debye relation was used. The permittivity of a polar material is well described by the Debye relation (Bohigas et al. 2008; Bohigas and Tejada 2009).
In Eqs. (1) and (2), ε′ ∞ is the real permittivity at a very high frequency, ε′ s is the real static permittivity at low frequency, ω is the angular frequency of the radiation, and τ is relaxation time and described by 1/(2πf R). The relaxation frequency (f R) is the value at which the dipole displacement lags behind that of the driving field by π/2. At this frequency, the imaginary part of the complex permittivity reaches a maximum (Bohigas et al. 2008).
In addition to dipolar molecules, Antarctic krill also contains free ions. The ionic conduction can cause microwave energy loss. At microwave frequencies, the dielectric loss factor due to ionic conduction varies with the reciprocal of the frequency (García et al. 2001; Nunes et al. 2006). This dielectric loss factor can be well described by the following term:
In Eq. (3), the adjustable constant, σ, is proportional to the direct-current conductivity of the Antarctic krill, ε 0 is the free space permittivity. Equation (3) is non-physical at very low frequencies, where the dielectric loss factor, σ/ω, approaches infinity (Bohigas and Tejada 2009).
Materials and Methods
Materials
Frozen Antarctic krill (July–August 2016) was purchased from DA KANG SHANG PIN Trade Co., Ltd., Qingdao, Shandong, China. All amino acids (Table 1) were of analytical grade and were purchased from MEI LUN Biotechnology Co., Ltd., Dalian Liaoning, China. Parafilm (PARAFLIM) was purchased from Polysciences, Inc., Warrington, Pennsylvania, USA. Toluene, sulfosalicylic acid, and other chemicals used were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. Shanghai, China.
Sample Preparation
Frozen Antarctic krill brick (−18 °C) was thawed using still tap water for 1 h; the heads, tails, and shells of these shrimps were removed, and the resulting meat of Antarctic krill was homogenated intermittently using a homogenizer (JT-B homogenizer, Jintian Experimental Facilities Research Laboratory, Luohe, Henan, China) at a speed of 23,000 rpm for 3 min. The homogenated sample was kept in ice until used for analysis. The main composition of the used Antarctic krill was as follows: 76.83 ± 1.07% moisture (wet basis (w.b.)), 18.32 ± 0.15% crude protein, and 1.48 ± 0.50% crude fat.
Amino Acid Contents
After removing the heads, tails, and shells of the thawed Antarctic krill, the Antarctic krill muscle sample (2.0 ± 0.1 g) was weighed using BS224S analytical balance (Sartorius Co., Ltd., Gottingen, Germany), pulverized, and homogenized in 20 mL of 0.02 mol/L HCl, and the homogenate was centrifuged at 5000 rpm for 10 min at 4 °C. The supernatant was collected and transferred to a 50 mL volumetric flask and filled to the mark with deionized water. Then, 2 mL of the supernatant and 2 mL of sulfosalicylic acid (5%, w/v) were mixed and centrifuged at 10,000 rpm for 10 min, and the resulting solution was filtered through a membrane of 0.22 μm pore size. The filtered sample was injected into an L-8800 amino acid analyzer (Hitachi, Tokyo, Japan). The operating conditions were as follows: analytical column (4.6 mm × 60 mm) filled with cation exchange resin, temperature of column oven at 57 °C, UV wavelengths of 440 nm for proline and 570 nm for others, injection volume of 20 μL, and flow rate of buffer at 0.35 mL/min, ninhydrin reagent (flow rate, 0.35 mL/min; cell temperature, 135 °C) as reaction solution. The regression coefficients of calibration curve were above 0.99 (Table 2).
Amino Acid Bulk Density
The bulk densities of amino acids were determined to determine the dielectric properties of solid amino acids (powder or crystal). The liquid displacement method was used to determine the bulk density of amino acids (Guo et al. 2008). Toluene (C7H8) was used as the immersion liquid, instead of water, to avoid absorption by the samples. Bulk density was determined by dividing the weight of samples (2.0 ± 0.1 g) by the volume occupied by those powder or crystal particles as measured with toluene in 10 mL measuring cylinder. For each amino acid, experiments were replicated three times, and the mean density values were calculated from the replicated results.
Sample Preparation for Analysis of Dielectric Properties
A pre-weighed amount of Antarctic krill meat was mixed with predefined quantities of amino acids and deionized water, such that final amino acid content was 1, 3, and 5%, followed by adequate stirring, and degassing for 1–2 min using vacuum drying using DZF-6021 oven (Jingmai Instrument and Equipment, Shanghai, China). The pH values of the amino acids added to Antarctic krill were measured using a pH meter (MP522, Sanxin Instrument Factory, Shanghai, China). Minced Antarctic krill (5 ± 0.1 g) was placed in the cylindrical nylon container (height, 1.5 cm; inner diameter, 1.0 cm), sealed by parafilm, and incubated in a SH-241 temperature and humidity chamber (ESPEC, Kyoto, Japan) at 4 °C, for at least 1 h.
Determination of Dielectric Properties
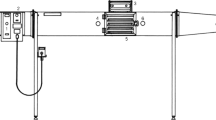
The dielectric properties of food samples were determined using an open-ended coaxial probe (85070E, Agilent Technologies, CA, USA) which was coupled to PNA-L Network Analyzer (N5230C, Agilent Technologies, CA, USA). The schematic used for measuring the dielectric properties is shown in Fig. 1. To obtain uniform readings, the measurement system was turned on and kept in a standby condition for at least 1 h before calibration and measurement. The open-ended coaxial-line probe was calibrated with air, short-circuit, and deionized water at 25 °C. Once the calibration was performed, deionized water was measured again to check its validity. The dielectric properties were determined over a frequency range of 300–3000 MHz for temperatures ranging from 20 to 80 °C with 10 °C increments. A temperature and humidity chamber was used to heat the samples, and THERMIC MODEL 2100A thermocouple thermometer (ETO DENKI Co., Ltd. Tokyo, Japan) was used to measure the temperature change in the sample center. When the center of sample reached the desired temperature, measurements were performed quickly. However, the dielectric properties of solid amino acids were determined using a compressed sample (about 2.0 ± 0.1 g) placed in a nylon tube sample cell. Solid amino acids (0.30 ± 0.01 g) were placed on top of the compressed sample to ensure good contact with the coaxial probe. The cell was raised to bring the coaxial probe into firm contact with the sample.
Determination of the Power Penetration Depth
An important concept in dielectric heating is the power penetration depth, which is defined as the depth at which the microwave power decreases to 1/e (e = 2.718) or 36.8% of the initial power entering the surface of a sample (Zhang et al. 2015). After determining the dielectric properties, the penetration depth of the electromagnetic energy in minced Antarctic krill could be calculated with the following equation (Sosa-Morales et al. 2010):
where dp is the penetration depth (m), c is the speed of light in free space (3 × 108 m/s), and f is the temporal frequency (Hz). This property is generally used to select an appropriate thickness of the food package to ensure relatively uniform heating along the depth of the food package (Wang et al. 2003).
Determination of the Output Power of Microwave Oven
The rated output power of the Panasonic NN-GD568M microwave oven (Xinshilian Science and Technology Ltd., Shenzhen, Guangdong, China) used in the experiments was 1000 W; width, depth, and height were 359 mm × 352 mm × 217 mm, respectively; oven volume was 27 L; diameter of turn plate was 340 mm; and frequency was 2450 MHz. Microwave heating real power of 250 W was used in this experiment, and the rated output power was tested using a standard test method (Stanford). The calculation formula of real output power was as follows:
P is output power (W), Q is quantity heat (J), t is heating time (60 s), C p is specific heat (the specific heat of water is 4.19 J/(g °C)), m is weight of sample (1000 g water), ΔT is temperature difference (°C), and the initial temperature of water was 20 ± 0.1 °C. All the results were obtained from at least triplicate measurements and each deviation was below 5%. The results showed that 194 W was the real output power in this experiment.
Determination of Temperature History and Distribution
Microwave station (FISO Technologies Inc., Quebec City, Canada) was used to heat and monitor the temperature change in the sample. The experimental set-up for measuring temperature is shown in Fig. 2. Microwave heating condition was set at 250 W and 60 s, and the initial temperature was 4 °C. Sample (20.0 ± 0.1 g) was placed in a nylon cylinder container (height, 2.5 cm; inner diameter, 3.0 cm), covered by polyethylene foam (thickness, 2.0 cm), and placed in the center of microwave oven turntable. Optical fiber was inserted into sample center to measure change in temperature, heating time ranging from 0 to 60 s with 5 s increments and recording the temperature. After heating, the sample was cut into two parts quickly at cross-section using filament, and Thermo Tracer TH7700 thermal infrared imager (NEC San-Ei Instruments, Ltd. Tachikawa, Japan) was used to measure the temperature distribution. All procedures were finished within 30 s. After the microwave oven recovered to its cold state, the next sample was measured.
Statistical Analyses
All experimental data were calculated as mean ± standard deviation (n ≥ 3). Origin8.0 (Originlab Co. Northampton, Massachusetts, USA) and SPSS 16.0 (SPSS Co. Chicago, USA) were used to compare the differences between data and plot them.
Results and Discussion
Free Amino Acid Contents of Antarctic Krill
The contents of free amino acid in Antarctic krill are shown in Table 3. A total of 19 amino acids were determined, including all essential amino acids, except tryptophan. However, the total essential amino acid content was 95.9 mg/g protein, which was lower than 277 mg/g (292.6) protein, specified by the WHO/FAO/UNU for adults (infants) (Wang et al. 2015). Moreover, the contents of essential amino acid were 11.13 ± 0.77%, which were lesser than those of non-essential amino acids (82.08 ± 0.51%). The contents of aliphatic, aromatic, and sulfur amino acids were 7.78 ± 0.29, 0.27 ± 0.02, and 0.15 ± 0.01 mg/g, respectively. Among them, Pro (3.77 ± 0.12 mg/g), Arg (3.41 ± 0.10 mg/g), and Gly (1.63 ± 0.05 mg/g) were present in higher levels than other amino acids (≤1.00 mg/g), together comprising nearly 70% of the total amino acid content. These results were different from those reported previously (Chen et al. 2009; Wang et al. 2015), possibly due to the different capturing times, storage times, treatment methods, and other factors. In this study, we evaluated the dielectric properties of ten amino acids including four essential amino acids (Met, Lys, His, and Try), four non-essential amino acids (Gly, Pro, Cys, and Arg), and two others (Gln and Tyr), which are not found in Antarctic krill. The dielectric properties of these amino acids (solid) were studied. Moreover, the effects of those amino acids added to minced Antarctic krill on dielectric properties were also studied.
Dielectric Properties of Solid Amino Acids
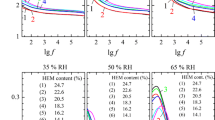
The dielectric constant and dielectric loss factor of ten solid amino acids (powder or crystal) over the frequency range of 300–3000 MHz are presented in Fig. 3. Figure 3a–d shows the dielectric properties of those ten amino acids and nine amino acids not including Lys, respectively. The dielectric constant and dielectric loss factor of amino acids were 2.0 ± 0.4 and 0.2 ± 0.3, respectively, (moisture contents, 0.05–0.33%), except those of Lys (5.5 ± 0.5 and 1.5 ± 1.0, respectively) (moisture content, 4.5%). The moisture content and dielectric value of Lys were greater than those of the other amino acids (Table 1; Fig. 3). The dielectric constant and dielectric loss factor of Lys decreased with increasing frequency (Fig. 3a, b). As shown in Fig. 3c, the dielectric constant of amino acids (except Lys) increased with the frequency below 700 MHz and remained almost unchanged between 700 and 3000 MHz. It is possible that the dipole displacement lags behind the changing driving electric field. The dielectric constant of Gly and His were lower than other amino acids; Pro, Met, Arg, Tyr, and Try have similar dielectric constant values. As shown in Fig. 3d, the dielectric loss factors of amino acids decreased with increasing frequency; dielectric loss factor of Lys decreased fastest, whereas those of Met and Cys did just the opposite. The dielectric loss factors of Lys, Met and Cys at 3000 MHz reduced by 62.73 ± 0.41%, 59.62 ± 0.38%, and 48.68 ± 0.10% than at 300 MHz, respectively. In addition, the dielectric loss factors of Gly, Pro, Gln, His, Arg, Tyr, and Try were similar and lower than those of Lys, Met, and Cys. In low-moisture foods, water is mainly bound, and the dielectric properties increased slowly with increasing moisture content (Guo et al. 2010). Thus, the above results could be explained owing to the highest moisture content of Lys, and the lowest moisture contents of His, among other amino acids. Other amino acids at higher moisture contents (4.5%) would probably produce a lower than or similar results as Lys. For instance, the dielectric constants and dielectric loss factor of Pro were 5.40 ± 0.80 and 1.30 ± 0.50, respectively, and it is similar with Lys at the same experimental conditions. Moreover, the purity and states of amino acids may also influence the dielectric properties.
Dielectric Properties of Minced Antarctic Krill
The dielectric constant and dielectric loss factor of minced Antarctic krill decreased with increasing frequency (Fig. 4). The dielectric constant of Antarctic krill decreased with increasing temperature at the experimental frequencies. Moreover, increasing temperature resulted in increased dielectric loss factor of Antarctic krill over the range of frequencies from 300 MHz to about 2.3 GHz, after which the dielectric loss factor of Antarctic krill decreased. The reduction in the dielectric constant might be caused by interruption of the ordered water molecule arrangement due to increased intermolecular vibrations at elevated temperatures (Wang et al. 2008). Similar trends have been reported for other food products with high moisture contents (Tang 2005). The temperature-dependent behavior of the dielectric loss factor of Antarctic krill should be determined by the free water and dissolved ions (Ahmed et al. 2008). At lower frequencies, the dielectric loss factor continued to increase with increasing temperature because the ionic conductivity was more influential, and it increased as the temperature increased at higher frequencies, where the dipolar rotation of free water was the dominant contributor (Tang 2005) and decreased as the temperature increased.
Dielectric Properties of Amino Acid Added to Minced Antarctic Krill
The effects of amino acid contents on the dielectric properties of Antarctic krill were investigated in the frequency range between 300 and 3000 MHz at 20.0 ± 0.5 °C, and those at 915 and 2450 MHz are presented in Table 4. Here, ten amino acids were added to the Antarctic krill at 1, 3, or 5%. The dielectric constants of Antarctic krill added with Gly, Pro, Arg, Cys, Gln, Lys, and His increased with the increasing amino acid contents, whereas addition of Met, Tyr, and Try did not significantly change the dielectric constants. The net amino acid charge and electrostatic repulsive forces increased as the pH shifted from the pI, and the dipolar properties increased. The pI of Lys was 9.74, and pH of minced Antarctic krill was 6.54 ± 0.03; the pH values of 1, 3, and 5% Lys added to minced Antarctic krill were 7.55 ± 0.04, 8.74 ± 0.03, and 9.74 ± 0.04, respectively. When 5% Lys was added to Antarctic krill, the pH value was similar to the pI of Lys. Other amino acids also showed similar trends. The change in pH value resulted in rearrange/rupture of intermolecular hydrophobic interactions and electrostatic repulsive forces, resulting in a change in the dielectric behavior (Sun et al. 2004). Moreover, as the molecular weight increased, molecular rotation suffered more resistance, making it harder to reach polarization. As usual, the frequencies of 915 and 2450 MHz were used in commerce and family separately. From Table 4, these trends were the same with the two microwave frequencies. At 2450 MHz, the microwave heating was characterized, which could contribute to microwave heating study at 915 MHz and a wide application in industry. The dielectric properties assay at 915 and 2450 MHz could provide fundamental basis for further studies and industrial-scale microwave process of the minced shrimp products. The difference between dielectric constants of different amino acids became smaller with the increasing frequency. The dielectric loss factors of Antarctic krill-added Gly, Pro, Arg, and Cys decreased with increasing the amino acid contents below 1 GHz, followed by an increase above 1 GHz. However, the dielectric loss factors of Antarctic krill-added Gln, Met, and His decreased with increasing amino acid contents below the inflection point (1–2 GHz) and remained unchanged above the inflection point. At low frequencies, in the range of 300 MHz to approximately 1–2 GHz, the dielectric loss factor was influenced by the ionic conduction mechanism (Lu et al. 2015; Kang et al. 2015; Song et al. 2016). For frequencies between 1 and 2 GHz, the dielectric mechanism shifts from ionic conduction to dipole polarization, and for frequencies above 2 GHz, dipolar relaxation mechanism dominates the dielectric loss behavior (Lu et al. 2015; Kang et al. 2015; Song et al. 2016). The dielectric constants of Tyr and Try-added Antarctic krill did not change with increasing contents of amino acids, and the dielectric loss factors decreased. The dielectric constant and dielectric loss factor of Lys-added Antarctic krill increased with the increasing amino acid contents. The amino acids were classified by polarity, and as the concentration of amino acids increased, the difference of dielectric properties increased which was between the amino acids with the same polarities. In order to determine the difference of different amino acids on dielectric properties of Antarctic krill, 5% amino acid added to minced Antarctic krill were used in the study.
The dielectric constants and dielectric loss factors of Antarctic krill on adding 5% of ten amino acids decreased with increasing frequency, and all inflection points were at 1–2 GHz (Fig. 5). The pI values of Pro, Met, Try, Gly, Cys, Gln, and Tyr were similar, but their molecular weights (MWs) were different (Table 1). The dielectric constants of Antarctic krill upon adding 5% non-polarity amino acids (Pro, Met, Try) decreased; whereas, the MW of amino acids increased at the same frequency, but their differences became smaller with the increasing frequency (Fig. 5a). The dielectric loss factors of Antarctic krill on adding 5% non-polarity amino acids did not change significantly with the increased in MW below 1 GHz and decreased with the increase in MW of amino acids above 1 GHz (Fig. 5b). The dielectric properties of Antarctic krill to which neutral-polar amino acids (Gly, Cys, Gln, and Tyr) were added showed similar trends as those on addition of non-polarity amino acids (Fig. 5c, d). It is possible that the molecular rotation suffered more resistance with the increasing molecular weight, which finally influenced the dielectric properties of amino acid-added Antarctic krill.
The dielectric constants of Antarctic krill added with Lys (27.88 ± 0.98) and Arg (23.37 ± 1.00) decreased faster than those on addition of Gly (21.70 ± 1.00) and Cys (20.25 ± 1.51) between 300 and 3000 MHz. The dielectric constant of Antarctic krill added with Lys was the highest, whereas that of His-added Antarctic krill was the lowest below about 0.9 GHz followed by a decrease with increasing MW at frequencies higher than 0.9 GHz (Fig. 5e). Figure 5f shows the dielectric loss factor of Antarctic krill mixed with alkaline amino acids (Lys > Arg > His) at the same frequency, which may be due to the combined effect of MW and pI.
Debye Relation
The critical frequencies of amino acid added to minced Antarctic krill were in a range of 1–2 GHz. As the MW and pI values increased, the critical frequencies of aliphatic amino acid added to minced Antarctic krill decreased, whereas heterocyclic amino acid added to minced Antarctic krill increased (Fig. 6).
In this study, the expressions for ε′(ω) and ε″(ω) were fitted to their respective data independently. However, Eq. (3) has only three adjustable parameters: σ, τ, and (ε′ s − ε′ ∞ ), as the difference ε′ s − ε′ ∞ , and not the individual values of each quantity, can be determined by fitting Eq. (3) to a data set. It was found that ε′ s − ε′ ∞ , which was determined from the dielectric loss factor, was often close to, but significantly different from the values determined from the ε′(ω) data. Similarly, τ values obtained independently from ε′(ω) and ε″(ω) were not in agreement within the error of the fit. We believe that these results may be due to the shortcomings of Eqs. (1) and (2) in describing a complex, real-world system.
Every parameter can be fitted to a linear function, with a higher regression coefficient, against the concentrations of amino acids in the sample, from concentrations below 5% in weight. The models of all obtained parameters can provide a deeper understand of the Debye relation, revealing the relation between the dielectric properties and parameters. Moreover, we can use those models to calculate the dielectric properties from knowing the contents of amino acids and vice versa. The corresponding equations for τ of amino acids in Antarctic krill are τ re = (4.5 ± 0.5) × 10−11 + (6.0 ± 6.0) × 10−12C and τ im = 8.0 × 10−11 + (4.0 ± 2.0) × 10−12C. Here, C is the concentration of amino acids added to the Antarctic krill (%), and other parameters are shown in Table 5. In order to validate this model, the dielectric properties of 2% Gly and 4% Lys added to minced Antarctic krill were studied (Fig. 7). The calculated values were in good agreement with the measured values at high frequency (>1.5 GHz). For instance, the measured dielectric constant and dielectric loss factor were 60.60 ± 0.51 and 23.13 ± 0.17, and the calculated values were 61.73 ± 1.21 and 22.82 ± 1.25 for 2% Gly added to minced Antarctic krill at 2450 MHz, respectively; The measured dielectric constant and dielectric loss factor were 57.90 ± 0.20 and 27.01 ± 0.51, and the calculated values were 58.14 ± 1.08 and 26.74 ± 1.78 for 4% Lys added to minced Antarctic krill at 2450 MHz, respectively.
Penetration Depths
Two frequency bands are used for microwave heating applications: the 915 MHz band for industrial use and the 2450 MHz band for both industrial and domestic use. The penetration depth provides information related to the effective depth of microwave power disposition. All Antarctic krill samples added with amino acids exhibited a higher penetration depth at 915 MHz than their corresponding values at 2450 MHz (p ≤ 0.05). The penetration depth is calculated by Eq. (4). For example, the penetration depths for Antarctic krill added with 5% Gly was approximately 15.29 ± 0.09 mm at 915 MHz and 5.95 ± 0.02 mm at 2450 MHz (Table 6). A higher penetration depth at 915 MHz than at 2450 MHz has also been reported by other researchers (Wang et al. 2008). The penetration depths of Gly, Pro, Gln, Met, and His added to minced Antarctic krill increased at 915 MHz and decreased at 2450 MHz with increasing contents of amino acids. However, penetration depths decreased on adding Cys, Lys, and Arg in Antarctic krill and those on adding Tyr and Try in Antarctic krill increased at 915 or 2450 MHz.
As shown in Fig. 8, the amino acids have different effects on the dielectric properties of Antarctic krill when added at a 5% concentration, at a frequency of 915 or 2450 MHz. The dielectric loss factors of Gly and Lys added to minced Antarctic krill were higher than that of pure Antarctic krill at 2450 MHz. The temperature history and distribution of these two amino acid added to minced Antarctic krill were also studied.
Temperature History and Distribution
The dielectric properties of Antarctic krill added with 5% Lys and 5% Gly changed significantly, as compared with those of Antarctic krill, respectively. However, Fig. 9 shows that the temperatures of Antarctic krill samples added with 5% Lys and 5% Gly remained notably unchanged on microwave heating (2450 MHz) for 60 s. The energy absorption possibly changed due to the fluctuation of dielectric properties and penetration depths with changing temperature. Moreover, the sample and container showed different dielectric properties, and a shell was formed between the contact surfaces (Chen et al. 2016).
Figure 10 shows that in non-uniform microwave heating of sample cross-section, temperature decreased from center to periphery. The temperature at the center of each Antarctic krill sample added with 5% Lys (b) and 5% Gly (c) after microwave heating for 60 s was about 52.0 ± 2.0 °C. This is due to the non-uniform electromagnetic field distribution of microwave oven. The temperatures in the temperature distribution were lower than temperature history, possibly due to the heat loss during measurements.
Conclusions
In this study, the dielectric properties of ten solid amino acids were investigated at a frequency range of 300–3000 MHz, and so did the effects of ten amino acids on the dielectric properties of minced Antarctic krill at 20 ± 0.5 °C. Solid amino acids (moisture content, 0.05–0.33%) had small dielectric property values (lower than Lys with a moisture content of 4.0%) and similar trends between 300 and 3000 MHz. The effects of amino acids on dielectric properties of the minced Antarctic krill decreased with increasing frequency. The dielectric properties and penetration depth values of minced Antarctic krill at 915 MHz were higher than at 2450 MHz when amino acids were in. The dielectric properties of non-polar (Met, Try, and Pro) and neutral-polar (Gly, Cys, Gln, and Tyr) amino acids added to minced Antarctic krill all decreased with frequency and MW increase. However, the combined effect of MW and pI played roles for alkaline amino acids (Lys, His, and Arg). The critical frequency changed between 1 and 2 GHz with different types and contents of amino acids added to minced Antarctic krill. The concentration of amino acids was dependent on the parameters obtained from the fits of the Debye relation, followed by a linear behavior in a concentration range of 0–5%. Adding 5% Lys and 5% Gly to the minced Antarctic krill could improve the dielectric properties but not the microwave heating rate. This study provides new information related to the dependencies of the dielectric properties on the frequencies and contents of amino acids, which may be useful for commercial processing for Antarctic krill and related aquatic products.
References
Afsar, M. N., Ding, H., & Tourshan, K. (1999). A new 60 GHz open-resonator technique for precision permittivity and loss-tangent measurement. IEEE Transactions on Instrumentation and Measurement, 48, 626–630.
Ahmed, J., Ramaswamy, H. S., & Raghavan, G. S. V. (2008). Dielectric properties of soybean protein isolate dispersions as a function of concentration, temperature and pH. LWT-Food Science and Technology, 41, 71–81.
Bohigas, X., Amigó, R., & Tejada, J. (2008). Characterisation of sugar content in yoghurt by means of microwave spectroscopy. Food Research International, 41, 104–109.
Bohigas, X., & Tejada, J. (2009). Dielectric properties of acetic acid and vinegar in the microwave frequencies range 1–20 GHz. Journal of Food Engineering, 94, 46–51.
Chen, F., Warning, A. D., Datta, A. K., & Chen, X. (2016). Thawing in a microwave cavity: Comprehensive understanding of inverter and cycled heating. Journal of Food Engineering, 180, 87–100.
Chen, Y., Tou, J., & Aczynskia, J. (2009). Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. Journal of Food Science, 74, H31–H39.
Farber-lorda, J., Gaudy, R., & Mayzaud, P. (2009). Elemental composition, biochemical composition and caloric value of Antarctic krill. Implications in energetics and carbon balances. Journal of Marine Systems, 78, 518–524.
Feng, D., Xue, Y., Li, Z., Wang, Y., Yang, W., & Xue, C. (2015). Dielectric properties of myofibrillar protein dispersions from Alaska Pollock (Theragra chalcogramma) as a function of concentration, temperature, and NaCl concentration. Journal of Food Engineering, 166, 342–348.
Fu, X., Hayat, K., Li, Z., Lin, Q., Xu, S., & Wang, S. (2012). Effect of microwave heating on the low-salt gel from silver carp (Hypophthalmichthys molitrix) surimi. Food Hydrocolloids, 27, 301–308.
García, A., Torres, J. L., Prieto, E., & Blas, M. D. (2001). Dielectric properties of grape juice at 0.2 and 3 GHz. Journal of Food Engineering, 48, 203–211.
Guo, W., Wang, S., Tiwari, G., Johnson, J., & Tang, J. (2010). Temperature and moisture dependent dielectric properties of legume flour associated with dielectric heating. LWT-Food Science and Technology, 43, 193–201.
Guo, W., Tiwari, G., Tang, J., & Wang, S. (2008). Frequency, moisture and temperature dependent dielectric properties of chickpea flour. Biosystems Engineering, 101, 217–224.
Hagl, D., Popovic, D., & Hagness, S. (2003). Sensing volume of open-ended coaxial probes for dielectric characterization of breast tissue at microwave frequencies. IEEE Transactions on Microwave Theory and Techniques, 51, 1194–1206.
Halter, R., Hartov, A., & Heaney, J. (2007). Electrical impedance spectroscopy of the human prostate. IEEE Transactions on Biomedical Engineering, 54, 1321–1327.
Jarvis, J. B., Janezic, M. D., Riddle, B., Holloway, C. L., Paulter, N. G., & Blendell, J. E. (2001). Dielectric and conductor-loss characterization and measurements on electronic packaging materials. NIST Technical Note, 156, 17–27.
Kang, W., Lu, J., Cheng, Y., & Jin, Y. (2015). Determination of the concentration of alum additive in deep-fried dough sticks using dielectric spectroscopy. Journal of Food and Drug Analysis, 23, 472–479.
Lagunas-Solar, M. C., Pan, Z., Zeng, N. X., Truong, T. D., Khir, R., & Amaratunga, K. S. P. (2007). Application of radio frequency power for non-chemical disinfestation of rough rice with full retention of quality attributes. Applied Engineering in Agriculture, 23, 647–654.
Lu, J., Qi, L., Guo, W., Song, Y., Jung, Y. A., Cheng, Y., & Jin, Y. (2015). Determination of fluoride concentration in Antarctic krill (Euphausia superba) using dielectric spectroscopy. Bulletin of the Korean Chemical Society, 36, 1557–1562.
Lyng, J. G., Scully, M., & McKenna, B. M. (2002). The influence of compositional changes in beef burgers on their temperatures during microwave heating and their thermal and dielectric properties. Journal of Muscle Foods, 13, 123–142.
Lyng, J. G., Zhang, L., & Brunton, N. P. (2005). A survey of the dielectric properties of meats and ingredients used in meat product manufacture. Meat Science, 69, 589–602.
Nordbotten, B. J., Tronstad, C., Martinsen, Ø. G., & Grimnes, S. (2011). Evaluation of algorithms for calculating bioimpedance phase angle values from measured whole-body impedance modulus. Physiological Measurement, 32, 755–765.
Nunes, A. C., Bohigas, X., & Tejada, J. (2006). Dielectric study of milk for frequencies between 1 and 20 GHz. Journal of Food Engineering, 76, 250–255.
Okiror, G. P., & Jones, C. L. (2012). Effect of temperature on the dielectric properties of low acyl gellan gel. Journal of Food Engineering, 113, 151–155.
Sagnard, F., Bentabet, F., & Vignat, C. (2005). Insitumeasurements of the complex permittivity of materials using reflection ellipsometry in the microwave band: experiments (part 1). IEEE Transactions on Instrumentation and Measurement, 54, 1266–1273.
Song, Y., Lu, J., Cheng, Y., & Jin, Y. (2016). Determination of acrylamide content in refined wheat starch (RWS) based on dielectric property (DP) during deep-frying process. Food Analytical Methods, 9, 1068–1078.
Sosa-Morales, M. E., Valerio-Junco, L., López-Malo, A., & García, H. S. (2010). Dielectric properties of foods: reported data in the 21st century and their potential applications. LWT - Food Science and Technology, 43, 1169–1179.
Sun, Y., Ishida, T., & Hayakawa, S. (2004). Dielectric study of heat denatured ovalbumin in aqueous solution by time domain reflectrometry method. Journal of Agricultural and Food Chemistry, 52, 2351–2357.
Tang, J. (2005). 2-Dielectric properties of foods. In H. Schubert & M. Regier (Eds.), The microwave processing of foods (pp. 22–40). Woodhead Publishing.
Wang, L., Xue, C., Wang, Y., & Yang, B. (2011). Extraction of proteins with low fluoride level from Antarctic krill (Euphausia superba) and their composition analysis. Journal of Agricultural and Food Chemistry, 59, 6108–6112.
Wang, L., Xue, C., Xue, Y., Wang, Y., & Li, Z. (2015). Optimization and evaluation of a novel technique for hydrolyzing Antarctic krill (Euphausia superba) proteins. Food and Bioproducts Processing, 94, 629–636.
Wang, S., Monzon, M., Johnson, J. A., Mitcham, E. J., & Tang, J. (2007b). Industrial-scale radio frequency treatments for insect control in walnuts: II. Insect mortality and product quality. Postharvest Biology and Technology, 45, 247–253.
Wang, S., Monzon, M., Johnson, J. A., Mitcham, E. J., & Tang, J. (2007a). Industrial-scale radio frequency treatments for insect control in walnuts: I. Heating uniformity and energy efficiency. Postharvest Biology and Technology, 45, 240–246.
Wang, Y., Tang, J., Rasco, B., Kong, F., & Wang, S. (2008). Dielectric properties of salmon fillets as a function of temperature and composition. Journal of Food Engineering, 87, 236–246.
Wang, Y., Wig, T. D., Tang, J., & Hallberg, L. M. (2003). Dielectric properties of foods relevant to RF and microwave pasteurization and sterilization. Journal of Food Engineering, 57, 257–268.
Wu, S., Huang, Z., Yue, J., Liu, D., Wang, T., Ezanno, P., Ruan, C., Zhao, X., Lu, W., & Pan, H. (2015). The efficient hemostatic effect of Antarctic krill chitosan is related to its hydration property. Carbohydrate Polymers, 132, 295–303.
Zhang, L., Lyng, J. G., & Brunton, N. P. (2008). The effect of fat, water and salt on the thermal and dielectric properties of meat batter and its temperature following microwave or radio frequency heating. Journal of Food Engineering, 80, 142–151.
Zhang, W., Luan, D., Tang, J., Sablani, S. S., Rasco, B., Lin, H., & Liu, F. (2015). Dielectric properties and other physical properties of low-acyl gellan gel as relevant to microwave assisted pasteurization process. Journal of Food Engineering, 149, 195–203.
Zhu, J., Zhuang, P., Luan, L., Sun, Q., & Cao, F. (2015). Preparation and characterization of novel nanocarriers containing krill oil for food application. Journal of Functional Foods, 19, 902–912.
Acknowledgments
This work was supported by the Shanghai University Knowledge Service Platform and Shanghai Ocean University Aquatic Animal Breeding Center (ZFI206), Shanghai Science and Technology Development Project (A2-0203-00-1000208), and the Doctoral Starting up Foundation (A2-0203-00-100340).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, M., Qiu, W., Zhang, R. et al. Effect of Amino Acids on Microwave Dielectric Properties of Minced Antarctic Krill (Euphausia superba). Food Bioprocess Technol 10, 1809–1823 (2017). https://doi.org/10.1007/s11947-017-1952-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1952-x