Abstract
The significance of heat moisture treatment (HMT) of non-wheat teff (T), chestnut (CN), and chickpea (CP) flours on dough viscoelastic and thermal parameters and on the structural and nutritional pattern of breads was investigated in untreated (−) and HMT (+) associated wheat-based (WT) matrices (WT:T:CN:CP, 66.20:7:7, wt. basis). Suitable trends for the enhancement of the physical characteristics of breads in terms of larger specific volume, higher viscoelastic and textural profiles, with lower and slower staling kinetics on aging were achieved by the pairs T-CN+, T-CP+, CN-CP+, and CN + CP+. In addition, a fine and uniformly sized cell structure with similar cell wall thickness was achieved in crumb samples. The pair T-CN+ enhanced extracted bioaccessible polyphenols, and the pair CN + CP+ synergistically promoted the antiradical activity in breads. Blended breads can be labeled as high-fiber breads (≥ 6 g DF/100 g food), and a recommended daily consumption of 250 g of bread fulfilled from 44% (men) to 67% (women) of dietary fiber requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing demand for novel, tasty, and healthy foods has boosted a new market in which products traditionally made from wheat are partially replaced or supplemented with alternative nutrient-dense and health-promoting non-wheat ingredients. In this context, ancient crops (Angioloni and Collar 2011), minor cereals (Angioloni and Collar 2013), pseudocereals (Collar and Angioloni 2014), legumes (Angioloni and Collar 2012), and non-traditional fruit and seed flours (Paciulli et al. 2016) have received much attention over the last years as functional ingredients for potential breadmaking applications.
Chestnut flour contains high-quality proteins with essential amino acids, dietary fiber, low amount of fat, and also vitamin E, vitamin B group, potassium, phosphorous, and magnesium of nutritional interest on both wheat (Dall'Asta et al. 2013) and gluten-free (Paciulli et al. 2016) breadmaking. It has been reported that wheat breads enriched with the addition of chestnut flour presented an increased quality from both organoleptic and nutritional points of view (Dall'Asta et al. 2013).
Legumes constitute traditional, ubiquitous, and wholesome imaged foods providing nutritional (high protein, mineral and fiber contents, low digestible starch), health (protective and therapeutic effects to chronic health conditions), and functional promoting effects (body, texture, and taste enhancement) to foods (Angioloni and Collar 2012). Associated mixtures of grain (chickpea, greenpea) and oilseed (soybean) legumes replacing wheat flour at 42% provided highly nutritious breads meeting viscoelastic restrictions and sensory standards (Collar and Angioloni 2017).
Teff (Eragrostis tef) is a nutritious cereal wheat-type gluten-free grain indigenous to Ethiopia, rich in carbohydrates and fiber, microelements, and phytochemicals with superior amounts of iron, calcium, and zinc than wheat, barley, and sorghum (Abebe et al. 2007). Teff was successfully applied in breadmaking matrices up to 40% of wheat replacement (Ronda et al. 2015).
Despite partial wheat flour replacement by nutrient-dense flours constitutes a plausible simple strategy to create value-added baked goods, making highly replaced wheat flour breads to assure nutritional and health-related benefits often encompasses a fall in techno-functional bread quality, particularly loaf volume and texture. Technological treatments have been applied with variable success to palliate the adverse effects associated to gluten and starch dilution in wheat matrices. High hydrostatic pressure treatment has been recently used as an alternative to hydrocolloids/gluten addition for the structure rearrangement of legume batters and consequently for their incorporation, in high amount, in breadmaking systems (Collar and Angioloni 2017). Heat moisture treatment (HMT) constitutes a clean label alternative to chemical modification for altering the gelatinization and retrogradation properties of starches (Gunaratne and Hoover 2002), flours, and doughs (Collar and Armero 2018), and the aggregation/disaggregation equilibrium of proteins (Mann et al. 2013). Microscopic observations by confocal laser scanning microscopy and light microscopy revealed that HMT caused the clumping of starch granules and the aggregation of denatured protein (Chen et al. 2015). HMT of flours has successfully been applied to wheat (Cetiner et al. 2017), sorghum (Marston et al. 2016), composite oat-wheat (Verdú et al. 2017), and barley-wheat (Collar and Armero 2018) flours to improve dough functionality, and both volume and textural profile of the resulting breads. Improvement was ascribed mainly to the increase of disulfide cross-linkages of amino acids, and to changes on starch granules conformation, principally in physical reorganization in their structure resulting in higher dough viscosities (Ovando-Martínez et al. 2013).
Significance of HMT on viscoelasticity and functional performance of blended doughs—hydrated wheat/non-wheat flours—has been recently addressed in diluted wheat: barley binary systems (Collar and Armero 2018). Results pointed out the importance of both the water availability and the heat treatment of compositional flours to obtain a reinforced dough structure with partially restored viscoelasticity.
Although the use of clean label treatments is regaining interest among consumers due to an increasing commitment with the environment, the evaluation of the application of HMT in breadmaking in associated wheat/non-wheat flours is little explored in the literature. This paper is aimed at investigating the potential of HMT to restore and/or improve bread viscoelasticity and functional performance keeping nutritional value of diluted wheat matrices with incorporation of nutrient-dense value-added non-gluten forming flours (teff, chestnut, chickpea), by studying the changes induced in treated blended bread matrices at both functional and nutritional levels.
Materials and Methods
Materials
Commercial flours from refined common wheat Triticum aestivum (WT), teff E. tef (T), chestnut Castanea sativa (CN), and whole decorticated chickpea Cicer arietinum (CP) were obtained from the Spanish market (Navarro, Valencia, Spain). Refined WT (70% extraction rate) of 195 × 10−4 J energy of deformation W, 0.57 curve configuration ratio P/L, and 58.8% water absorption in Brabender Farinograph was used. Carboxymethylcellulose Aquasorb® A-500 (CMC) was acquired from Copenhagen Pectin (Denmark), and commercial wheat sour dough Pie was kindly supplied by Ireks (Spain).
Methods
Chemical and Nutritional Composition of Flours
Chemical and nutritional composition of native WT, T, CN, and CP flours (Table 1) was determined following the ICC methods (ICC 2014) regarding moisture, protein, and fat contents. Total, soluble, and insoluble dietary fiber contents were determined according to the AOAC method 991.43 (AOAC 1991). Three replicates were made for each analysis. Digestible carbohydrates were calculated by indirect determination as 100 − [moisture + protein + fat + dietary fiber] (FAO/WHO 2003).
HMT
HMT conditions (15% moisture content, 1 h, and 120 °C) were selected based on previous experiments (Collar and Armero 2018), in which maximization of viscometric profile and minimization of loss of hydration properties of flour samples were applied as criteria. Single T, CN, and CP flour samples were placed into screw-capped cylindrical glass containers (150 mm Ɵ, 250-mm height). Small amount of distilled water was added slowly with frequent stirring until moisture levels (w/w) of the total mixture reached 15%, and equilibrated for 24 h at room temperature. Hydrated samples that occupied 13-mm height in containers were kept for 1 h at 120 °C in a convection oven (P-Selecta, Barcelona, Spain). Samples took 20 min to reach the preset temperature, and 30 min to reach the room temperature after heating. Untreated native flours were used as controls. Untreated (−) and HMT (+) single flours were used in quaternary blends (T:CN:CP:WT) in presence of WT for doughmaking.
Bread Making of Wheat-Based Blended Flours
Specific flour composition was set after a prospective study on the compositional and functional characteristics of non-wheat flours (native and HMT) was performed. Results pointed out that besides the superior nutritional value as compared to wheat, teff, chestnut, and chickpea, individual flours were sensitive to HMT in terms of increased water absorption, viscosity after heating-cooling cycles, increased consistency (forward-extrusion test), and acceptable dough handling ability during processing. This behavior made flours interesting candidates to be integrated in wheat-diluted systems with good prediction as dough strengtheners. Percentages of replacement resulted from experimental studies aimed at knowing maximum amount of each flour without significant deleterious effect on dough machinability.
Doughs and breads were prepared from wheat-based blended flours (T, CP, CN) by WT replacement at 34%, and incorporation of ternary blends of T (20%, flour basis), CN (7%, flour basis), and CP (7%, flour basis) flours according to a multilevel factorial design with the following attributes: three experimental factors (T, CP, and CN flours) at two levels, coded 0 (untreated) and 1 (HMT), and five error degrees of freedom. The model resulted in eight randomized runs in one block. A three-digit bread sample code was set, referring to no HMT (0) and HMT (1) T (first digit), CN (second digit), and CP (third digit) flours in sample formulation, as it follows: 110, 101,100, 000, 001, 111, 010, and 011. Blended flours (100 g), water (100%, flour basis), commercial compressed yeast (3%, flour basis), salt (2%, flour basis), commercial sour dough Pie (5%, flour basis), CMC (3%, flour basis), and calcium propionate (0.5%, flour basis) were mixed in a 10-kg mixer at 60 rpm−1 for 10–13 min up to optimum dough development. Preliminary tests were performed to know the amount of water necessary to avoid stickiness and deleterious effects on dough machinability, and 100% of water absorption was enough for all the formulations to assure dough handling ability during processing. Fermented doughs were obtained after bulk fermentation (10 min at 28 °C), dividing (300 g), rounding, molding, panning, and proofing up to maximum volume increment (50 min at 28 °C), and were baked at 225 °C for 25 min to make blended breads. Two baking trials were conducted per formulation. Bread samples were packaged in co-extruded polypropylene bags, and stored for 1, 3, 6, and 8 days at room temperature and 56% relative humidity to describe firming kinetics.
Dough Measurements
Texture Profile Analysis
Texture profile analyses (TPA) of quaternary doughs were performed in absence of yeast by applying a double-compression cycle in a TA-XT2 Texture Analyzer (Stable Micro Systems, Surrey, UK) equipped with a 30-kg load cell and operating at 10-mm/s head speed, by using a 1-cm-diameter probe, 75-s waiting period, and 60% compression. The primary textural properties (hardness, cohesiveness, springiness, and resilience) were measured in absence of dough adhesiveness by using a plastic film on the dough surface to avoid the distortion induced by the negative peak of adhesiveness, while dough adhesiveness was measured separately by running a second TPA without the plastic film and disregarding the other parameters. Runs were performed in triplicate per sample.
Stress Relaxation Test
Doughs without yeast were submitted to uniaxial compression in the Texture Analyzer using an acrylic probe (37-mm diameter) to a 10% strain, and the change in force with time was measured for 300 s. A pretest speed of 5 mm/s and test speed of 0.5 mm/s were used. The obtained stress relaxation curves were normalized and linearized according to the Peleg model: F0t/(F0 − F(t)) = k1 + k2 t, where F0 is the initial force, F(t) is the momentary force at time (t), and k1(s), k2 are the constants related to stress decay rate and to residual stress at the end of the experiment, respectively. In addition, percent stress relaxation (%SR = (F0 − F300) × 100/F0), 1/k1 (initial rate of relaxation), 1/k2 (extent of relaxation), and relaxation time (RT) as the time required for F0 to drop to 36.8% of its values, respectively, were compared for the different samples. Tests were performed in duplicate per sample.
Thermal Analysis
The thermal properties of native and HMT flours were determined by using a differential scanning calorimeter (DSC-7, Perkin-Elmer, Norwalk, CT). Dough samples were prepared by mixing flour blends with excess water (1:3) to avoid partial gelatinization of samples due to water restrictions. For DSC analysis, 50–70 mg of dough samples was weighed in large volume preweighed, sealed stainless-steel pans. An empty pan was used as a reference. Simulation of the temperature profile in the center of the bread crumb during baking was performed in the calorimeter under the following scanning conditions: samples were kept at 30 °C for 2 min, then heated from 30 to 110 °C at a rate of 11.7 °C/min, kept at 110 °C for 5 min, and finally cooled from 110 to 30 °C at a rate of 50 °C/min. Thermal transitions of samples for gelatinization were characterized by T o (onset temperature), T p (peak temperature), T e (end temperature), and ∆H g (enthalpy of gelatinization). The enthalpy calculations were based on a dry-flour weight. The samples were analyzed three times, and the data were calculated with a Pyris software (Perkin-Elmer, Norwarlk, CT).
Bread Measurements
Physico-Chemical Determinations
Loaf volume was determined using the rapeseed displacement method as in AACC (2005). Specific loaf volume was calculated dividing the loaf volume by the corresponding loaf weight.
Color determinations were carried out on bread crumbs using a Photoshop system according to the method previously described by Angioloni and Collar (2009), and results were expressed in accordance with the Hunter Lab color space. Parameters determined were L (L = 0 [black] and L = 100 [white]), a (− a = greenness and + a = redness), b (− b = blueness and + b = yellowness), ΔE = total color difference, and WI = whiteness index. All measurements were made in triplicate.
Crumb grain characteristics were assessed in bread slices using a digital image analysis system. Images were previously acquired with a ScanJet II cx flatbed scanner (Hewlett-Packard, Palo Alto, CA, USA) supported by a Deskscan II software. The analysis was performed on 40 × 40-mm squares taken from the center of the images. Data were processed using SigmaScan Pro 5 (Jandel Corporation, San Rafael, CA, USA). The crumb grain features evaluated were mean cell area, cells/cm2, cell/total area ratio, wall/total area ratio, and crumb area/total cell ratio. In addition, area distribution and cell number distribution were counted, and percentages of cell area and cell number were calculated according to preset cell size ranges: < 1, 1–5, 5–50, and > 50 mm2.
Bread mechanical characteristics (TPA in a double-compression cycle) of fresh and stored breads were recorded in a TA-XT2 Texture Analyzer using a 10-mm-diameter probe, a 30-kg load cell, 50% penetration depth, and a 30-s gap between compressions on slices of 15-mm width (Armero and Collar 1998). For textural measurements, three slices of two breads were used for each sample. The obtained firming curves during bread storage were modeled using the Avrami equation, and model factors were estimated by fitting experimental data of hardness to the non-linear regression equation \( \theta =\frac{T_{\infty }-{T}_t}{T_{\infty }-{T}_0}={e}^{-{kt}^n} \), where θ is the fraction of the recrystallization still to occur; T0, T ∞ , and T t are the crumb firmness at time 0, ∞, and time t, respectively; k is a rate constant; and n is the Avrami exponent.
The stress relaxation data were collected by applying an instantaneous strain to the sample, and the force required to maintain the formed deformation was observed as a function of time. Samples from the center of the crumb slices were cut into cylinders (27-mm diameter × 15-mm thick) and were compressed using a TA-XT2 Texture Analyzer with a load cell of 30 kg. For compression, a cylindrical upper die of 25-mm diameter was used at a cross speed of 0.5 mm/s and a pretest speed of 5 mm/s. Samples were placed in a semi-close cabinet to prevent moisture loss. The strain used was 20% and the whole relaxation experiment lasted for 10 min. The obtained stress relaxation curves were normalized and linearized according to Peleg model as described for doughs, and previously applied by Angioloni and Collar (2009) for breads. In addition, percent of stress relaxation (%SR = (F0 − F600) × 100/F0), 1/k1 (initial rate of relaxation), 1/k2 (extent of relaxation), and relaxation time (RT as the time required for F0 to drop to 60% of its values) were compared for the different bread samples. Stress relaxation tests were replicated two times.
Nutritional Parameters
Bioaccessible phenol determinations were carried out by conducting an “in vitro” digestive enzymatic mild extraction that mimics the conditions in the gastrointestinal tract according to the procedure of Glahn et al. (1998) and adapted by Angioloni and Collar (2011) for breads. Calibration curve was performed using gallic acid, and therefore, obtained amounts of phenolics were expressed as gallic acid equivalents.
The stable 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical was used to measure the radical scavenging capacity of the bioaccessible polyphenol enzymatic extracts of bread samples according to the DPPH• method modified by Sánchez-Moreno et al. (1998) and adapted by Collar et al. (2014). Readings were taken from two replicates per sample. Plots of μmol DPPH vs time (min) were drawn, and calculations were made to know the antiradical activity (AR), AR = [([DPPH]INITIAL − [DPPH]PLATEAU) × 100] / [DPPH]INITIAL.
Statistical Analysis
Multivariate analysis of variance of data and non-linear regression analysis were performed by using Statgraphics v.7.1 program (Bitstream, Cambridge, MN). Multiple range test (Fisher’s least significant differences (LSD)) for analytical variables was applied to know the difference between each pair of means.
Results and Discussion
Significance of HMT of Non-Wheat Flours on the Functional Performance of Blended Doughs
Functional performance of untreated and HMT blended doughs were characterized at macroscopic and structural levels in terms of their viscoelastic, mechanical, and thermal profiles (Table 2), and the significance of HMT of non-wheat flours on the functional parameters of blended matrices was determined (Table 3).
Significant differences (p < 0.05) were found in the physico-chemical patterns of doughs from untreated and HMT blended flours (Table 2). Dough viscoelasticity parameters ranged from 0.89 N (000) to 1.73 N (001, 100) for the initial force F0, from 11.39 s (110) to 19.50 s (000) for k1, and from 1.36 (110) to 1.52 (000, 111.011) for k2, providing relaxation times RT that varied from 53 (110) to 367 s (000), and extent of relaxation SR ranging from 63% (000) to 71% (110). HMT of single flours in blended doughs, particularly significant for T and CN, provided a fall in the elastic-like nature of doughs. This led to a concomitant promotion of the viscous nature of doughs evidenced by the decreased values of both k1 and k2. As a consequence, increased values for the reciprocal 1/k1 and 1/k2 related to the initial stress decay rate and the asymptotic level of stress not relaxed at long times, respectively, were obtained. In accordance, effects of HMT led to shorter RT and higher extent of SR, particularly for T, which values changed from 181 to 101 s (RT) and from 66 to 68% (SR), respectively (Table 3). In the current work, simultaneous presence of thermally treated T+ and CP+ magnified the abovementioned changes, while treating the pair CN+ and CP+ flours reduced the extent of dough weakening, leading to values for dough viscoelasticity near those of untreated flours (Table 3). Observations are in agreement with previous changes of the rheological properties at small and large deformations described for doughs made of heat-treated wheat flour (Mann et al. 2013). Changes comprise dough reinforcement by increase of the dynamic moduli, an easier destruction of the dough network by increase of protein solubility, and an unequal change in loss and storage moduli, leading to irreversible changes in flour. These changes can be ascribed assuming protein aggregation (leading to a weakened protein network and possibly acting as additional filler particles) and starch surface modifications (leading to changed starch-protein and starch-starch) interactions (Mann et al. 2013). Later, it was observed that HMT favored the strengthening of arrowroot starch gels determined by oscillatory rheological tests, and gave them greater resistance, particularly to acidification stress (Pepe et al. 2015).
Dough mechanical profile of blended flours underwent little changes with HMT in terms of cohesiveness (0.788–0.858), springiness (0.822–0.884), and resilience (0.095–0.108) values (Table 2). On the contrary, hardness and adhesiveness depended on the thermal treatment of the pairs T/CN and T/CP (Table 3). Increase of dough hardness and adhesiveness by thermal treatment of individual flours was observed earlier for treated sweet potato starch with HMT (Collado et al. 1999). The observed increase was counteracted after HMT of both T+ and CP+ (4.75 vs 6.22 N) and after HMT of either T/CN (35.75 vs 42.37 N s) or T/CP (31.06 vs 43.27 N s), respectively (Table 3). It was found that modification by HMT resulted in a reduction of gel hardness of both starch and flour rice samples (Puncha-arnon and Uttapap 2013). In the case of HMT flours, a sharp decrease in gel hardness was affected by components other than starch. Protein layers formed on the starch surface, as well as lipid complexes formed during HMT, could both inhibit the swelling of starch granules. Hamaker and Griffin (1993) earlier explained that proteins with intact disulfide bonds made the swollen granules less susceptible to breakdown, either by imparting strength to the swollen granules or by reducing the degree of swelling. The flours used in this study are rich in both protein and lipids, particularly CP (Table 1), and these components may favor the formation of protein layers and starch-lipid complexes on HMT, boosting the inhibition of starch granule swelling and the dough softening.
During gelatinization, at high water concentration (> 66 wt% or water/starch ratio > 1.5), a single symmetrical endothermic transition appears in a temperature range of 60–80 °C in the DSC profiles (called endotherm G), as reported by Donovan (1979). In the present research, blended dough samples contain high water concentration (75 wt%), and only one peak was defined during the DSC scan of all samples. The corresponding thermal transitions for the peak occurred at close temperature intervals (°C) of 61.23–62.24 (T0), 69.30–70.50 (T p ), and 78.47–80.39 (T e ), and similar temperature and enthalpy (J/g; dry flour) ranges of 17.18–18.09 and 5.548–6.170, respectively (Table 2). According to the different models, the endotherm G (peak 1) was suggested to result from (a) plasticization in amorphous regions, (b) swelling-driven crystalline disruption, (c) melting of the less stable crystallites in sufficient water, and (d) associated with the smectic-nematic/isotropic transition (Wang and Copeland 2013). The differences in gelatinization temperatures among doughs can be attributed mainly to differences in size, form, and distribution of starch granules in the blended flours, and to the internal arrangement of starch fractions within the granule, as stated earlier for legume flours (Kaur and Singh 2005). In fact, starches in the flour blends are composed of granules differing in size, from small to large: pea (wrinkled) 5–34 μm, small wheat granules 2–3 μm (Zhou et al. 2004), large wheat granules 22–36 μm, teff 2–6 μm (Bultosa and Taylor 2004), and chestnut 2.9–21.4 μm (Demiate et al. 2001) that exhibit irregular oval-shaped granules of broad size range. In addition, despite HMT did not alter the size or shape of the starch granules of some cereals, roots, and legumes (Hoover et al. 2010), the thermal treatment may affect the aggregation of starch granules as observed for oat starch. Starch granules that were aggregated in the native state were less compactly packed after heat treatment (Hoover and Vasanthan 1994). Significant single effect of HMT of T flour revealed a discreet broadening of the gelatinization-temperature range (R) and a shifting of the endothermal transition towards higher temperatures (Table 3), being values 17.34 vs 17.87 °C (R), 61.54 vs 62.28 °C (T0), 69.60 vs 70.50 °C (T p ), and 78.87 vs 80.15 °C (T e ). Previous studies on tuber, legume, and cereal (normal, waxy, high amylose) starches (Hoover et al. 2010) and flours (Satmalee and Matsuki 2011) ascribed the observations to amylose-amylose, amylose-amylopectin, and amylose-lipid interactions, as well as other chemical bonding/interactions that occur during HMT (Watcharatewinkul et al. 2009). Authors stated that interactions suppress the mobility of starch chains in the amorphous regions. Consequently, the amorphous regions would require a higher temperature to incur swelling that could contribute to the disruption of the crystalline regions. Lim et al. (2001) proposed that the increase in gelatinization temperature was caused by the transformation of the inter-crystalline amorphous regions to amorphous phases, which may provide the short chains in the crystalline structure more freedom. Thus, the crystalline micelles undergo a structural transformation towards an increased thermodynamic stability. Besides, it was probably due to the restriction of water penetration into the granules by the new superficial protein layer formed by HMT, thus retarding the granule swelling (Chen et al. 2015).
In addition, T significantly interacted with CP, modifying in small extent the values of the gelatinization enthalpies. HMT of either T or CP resulted in a small decrease of ΔH g compared to values observed for the untreated pair of flours (5.64 vs 6.12 J/g), while HMT of both flours led to intermediate enthalpy values (5.88 J/g). Enthalpy change reflects the melting of imperfect amylopectin-based crystals, with potential contributions from crystal-packing and helix-melting enthalpies (Lopez-Rubio et al. 2008). The decrease in ∆H g on HMT reflects disruption of double helices present in crystalline and non-crystalline regions of the granule (Gunaratne and Hoover 2002). In addition, most of the semi-crystalline structure and a considerable extent of the concentrated crystalline region were destroyed during HMT.
Significance of HMT of Non-Wheat Flours on the Physical and Nutritional Profiles of Blended Breads
In general, mixed HMT breads were visibly similar in color (Fig. 1), and exhibited low-medium lightness L (54.8–60.4), > 0 a (2.3–3.4), and b (10.3–12.3) values, resulting in low WI crumbs (53.6–58.6) (Table 4). Color coordinates of breads were not dependent on the thermal treatment of any compositional flour, so that all sample crumbs were characterized by a dark orange-brown color (Fig. 1).
Digitalized images of central slices of wheat-based blended breads formulated with teff (T), chestnut (CN), and chickpea (CP) flours. Three-digit code refers to untreated (0) and heat-moisture-treated (1) T:CN:CP mixed flours replacing wheat flour in sample formulation. a 110. b 101. c 100. d 000. e 001. f 111. g 010. h 011
Common crumb grain features of breads (Table 4; Fig. 1) evidenced main small cells < 1 mm2 accounting for 81–92% of total cells, intermediate medium size (1–50 mm2) cells (7–19%), and marginal big cell (> 50 mm2) proportion (0–0.2%). Mean cell area (mm2) ranged from 0.43 to 0.82, and cell area distribution covered by cells sized 1–50 mm2 varied from 63% (001) to 87% (100). Cell density (cells/cm2) ranged from 48 (111) to 107 (100), being cell/wall ratio from 38/62 (000, 111, 011) to 50/50 (110). Except for the samples 110, 101, and 100 that exhibited heterogeneous and/or highly packed crumb structure, a fine and uniformly sized cell structure with similar cell walls thickness were achieved in crumb samples (Fig. 1). Improvement of the internal grain structure with heat treatment was reported previously for substandard wheat flour breads (Gélinas et al. 2001) and for sorghum breads (Marston et al. 2016). The high fiber content of chestnut flour was reported to enhance the viscoelastic properties and to reduce dough expansibility due to the entanglement of fibers, entrap more air bubbles, and produce pores with a small cell area when added with a relatively high amount of this flour (Demirkesen et al. 2010). Positive effects of HMT of CN and/or CP on bread crumb grain have not been described so far.
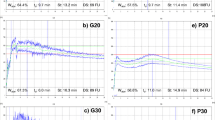
Macrostructural properties of HMT breads were characterized in terms of specific volume, mechanical/ textural behavior, stress relaxation pattern, and firming kinetics on aging (Table 4), and the significant (p < 0.05) single and interactive effects of thermal treatment of compositional flours were determined (Table 5). Compared to untreated blended breads (000), HMT breads exhibited in general from smaller to larger specific volumes (2.59–3.28 vs 3.07 mL/g), similar percent of stress relaxation (43–51%), slightly variable stress decay rate k1 (45.67–66.58 vs 57.24 s), and residual stress k2 (1.86–2.19 vs 1.98), similar or higher cohesiveness (0.460–0.568 vs 0.485) and resilience (0.186–0.245 vs 0.200), variable fresh crumb firmness (7.89–10.58 vs 9.44 N), and rate of staling kinetics n f (0.390–1.817 vs 1.097) (Table 4). HMT of single flours T, CN, or CP significantly (p < 0.05) affected bread physical characteristics, being the trend and extent of the changes dependent on the treated flour. CN+ and CP+ increased bread specific volume by 4 and 9%, respectively, while T+ led to loaves with 8% lower specific volume, compared to untreated flours. Effects of CN+ and CP+ on bread viscoelasticity were of similar extent but followed an opposite trend. CP+ decreased the rate of initial stress decay rate 1/k1 by 11% with a concomitant increase in the relaxation time of 33%, while CN+ promoted the decay rate by 12% (Table 5). HMT of T provided a fall in the elastic-like nature of doughs, leading to increased initial stress decay rate and level of stress not relaxed at long times. This encompasses higher extent of stress relaxation, and dough weakening. Resulting doughs encompassed lower viscoelasticity and gas retention ability, and reduced specific volume after fermentation and baking. Although legume and chestnut proteins are generally low in methionine, cysteine, and tryptophan, aggregation of proteins can take place on HMT, leading to a reinforced dough structure in some extent, able to retain carbon dioxide, and leading to increased specific volume, as previously observed for sorghum breads (Marston et al. 2016). Entanglement/disaggregation of fibers, particularly present in high amount in chickpea flour (Table 1), in the denatured protein network can also play a role. Impact of HMT flours on firming kinetics parameters were relevant (Fig. 2). Both initial T0 and final T ∞ bread firmness were lowered (− 7.5%) or promoted (+ 17–23%) by the respective addition of CP+ or T+ to the formulation. In addition, the rate of aging n f was drastically reduced (− 26%) or increased (+ 78%) by either CP+ or CN+, and k f decreased by 72% with T+ (Table 5). Simultaneous presence of native and treated flours provided variable impact on the physical profile of blended breads, particularly for the pairs T/CN and T/CP. Suitable trends for the enhancement of the physical characteristics of breads in terms of larger specific volume, higher viscoelastic and textural profiles, with lower and slower staling kinetics on aging (Table 5) were achieved by the pairs T-/CN+, T-/CP+, CN-/CP+, and CN+/CP+, as in 010, 011, and 001 samples. These samples also showed homogeneous and fine crumb grain and appealing color features (Fig. 1). Caution should be applied to the pair T+/CP+ because of the adverse effect on the firming of the initial texture of the crumb T0, and on the rate of staling denoted by increased n f . It is the case of samples 101 and 111. In fact, comparing staling kinetics parameters (averaged values) in samples T-CP+ vs T + CP+ (Table 5), values were 7.97 vs 10.84 N (T0), and 0.405 vs 1.269 (n f ). Experimental values (T0, n f ) for bread samples 001 (7.89 N, 0.420) and 011 (8.00 N, 0.390) vs 101 (10.54 N, 0.758) and 111 (11.25 N, 1.775) are in good accordance with the statistical trends (Fig. 2; Table 5).
Evolution of crumb firming during aging (adjusted mean data and error bars) of wheat-based blended breads formulated with teff (T), chestnut (CN), and chickpea (CP) flours. Three-digit code refers to untreated (0) and heat-moisture-treated (1) T:CN:CP flours replacing wheat flour in sample formulation
Blended bread samples (100 g) contain 6.71 g of dietary fiber DF, while regular white breads account for 1.67 g DF, so that blended breads can be labeled as high-fiber breads (6 g DF/100 g food), according to Nutritional Claims for DF foods (Regulation EC 2006). Formulations based on WT:T:CN:CP flours, 66.20:7:7, fulfilled from 44% (men) to 67% (women) of dietary fiber daily requirements (Otten et al. 2006), when a daily consumption of 250 g of bread is accomplished, following the WHO bread intake recommendation.
Chestnut flour is an important source of antioxidant compounds, mainly containing ellagic acid (Dall'Asta et al. 2013). Teff is generally assumed to contain substantial amounts of phenolics (Dykes and Rooney 2007), particularly that ferulic acid and chickpeas exhibited total polyphenol content ranging from 0.78 to 2.3 mg/g−1 (Bravo 1998). Bioaccessible polyphenol content (mg gallic acid/100 g flour, as is) of blended breads in the current work varied from 417 to 494 mg (Fig. 3). Amount of bioaccessible polyphenols is dependent on several factors. Mechanical input during mixing and thermal treatment of flours (HMT) and doughs (baking) may induce depolymerization of constituents, mainly fiber, and hence may favor bread accessibility to solvents, acids, and enzymes and the subsequent release and extraction of fiber-associated polyphenols. Concomitantly, dietary fiber and other compounds of proven resistance to the action of digestive enzymes, such as resistant starch, resistant protein, Maillard compounds, and other associated compounds, may reduce the bread phenol bioaccessibility (Saura-Calixto et al. 2000). Extensive depolymerization of fiber constituents can be applied to the increase of bioaccessible polyphenols determined in HMT blended breads compared to the untreated sample 000, since compositional non-wheat flours accounted for > 10% dietary fiber content (Table 1). Despite HMT may favor the formation of polymeric aggregates that hinder enzyme accessibility and further attack (Mann et al. 2013; Watcharatewinkul et al. 2009), it is apparent that HMT makes bioaccessible polyphenol extraction available, in some cases. In fact, T+ decreased the amount of bioaccessible polyphenols by 4%, but the pair T/CN modulated polyphenol extractability (Table 5). T−/CN− and T+/CN+ gave similar amount of extracted polyphenols at about 425 mg, while T+/CN− led to intermediate amount of 456 mg, and the pair T-CN+ maximized extracted bioaccessible polyphenols (491 mg). In accordance, samples 010 and 011 exhibited the highest amount of bioaccessible polyphenols (≈490 mg) while samples 110, 111, 000, and 001 explicited the lowest values (420–450 mg), and samples 101 and 100 showed intermediate mean values (450–460 mg) (Fig. 3).
Antiradical activity was determined by the extent of the reduction of the stable DPPH• radical. Results expressed correspond to the remaining unreacted DPPH• amount when 0.494 μmol of the free radical is initially available to react with pepsin/pancreatin extracts from 2.4–2.6-mg freeze-dried breads. Despite different kinetics of the reduction of DPPH• were shown among untreated and treated samples (Fig. 4), discreet differences in antiradical activity of breads (38–45%) were observed. T+ and CN+, respectively, induced a depletion (− 7%) and an increase (+ 3%) of the antiradical activity, while the simultaneous presence of the pair CN+/CP+ synergistically promoted the reduction of DPPH• (Table 5). In good accordance, samples 011, 010, and 111 gave the higher values and the more rapid kinetics in terms of antiradical activity (Fig. 4).
Correlations Between Dough Physical Parameters and Bread Physical and Nutritional Parameters of Blended Matrices
Using Pearson correlation analysis, a range of correlation coefficients (r) (from − 0.4760 to 0.7352) were obtained for the relationships between viscoelastic, mechanical, and starch gelatinization transition parameters of doughs, and physical and nutritional parameters of mixed breads from untreated and HMT matrices (Table 6). Despite r values were discreet in most correlations, significant (0.01 < p < 0.05) interdependencies between the extent of stress relaxation (1/k2), starch gelatinization transition (T p , T e , ∆H g ) of doughs, and physical (specific volume, n f ) and nutritional (%ARA) parameters of breads were found (Table 6). In accordance, larger specific volume in breads exhibiting higher antiradical activity corresponded to higher elastic-like doughs with smaller initial decay rate 1/k1 and lower extent of stress relaxation 1/k2, %SR. Clumping of starch granules and aggregation of denatured protein on HMT were reported (Chen et al. 2015). Crosslinking by oxidizing the free sulfhydryl groups on HMT result in stronger doughs with a greater resistance to mechanical shock, improved oven spring, and larger loaf volume, as described for sorghum breads (Marston et al. 2016). In addition, lower temperatures for starch gelatinization T0, T p , and T e in hydrated flours led to breads with higher specific volume (r = − 0.6132, − 0.6293, − 0.6299), while smaller energy for the thermal transition ∆H g was connected with softer fresh breads (r = 0.5696) and slower crumb firming rate on aging (r = 0.718). Modifications of starch surface interactions take place on HMT (Mann et al. 2013). Most of the semi-crystalline structure and a considerable extent of the concentrated crystalline region were destroyed during the thermal treatment (Gunaratne and Hoover 2002), leading to less compact and rigid structures (Hoover and Vasanthan 1994). Resulting doughs are able to expand during fermentation, and provide breads with more aerated structure and softer crumb, undergoing slower staling kinetics.
Conclusions
HMT of flours modified viscoelasticity and thermal transitions of doughs and techno-functional and nutritional profiles of breads from diluted breadmaking wheat matrices made at 34% of wheat flour replacement by teff (20%), chestnut (7%), and chickpea (7%) flours. The trend and extent of the changes are mainly dependent on the simultaneous presence of specific untreated and thermally treated flours, particularly for the pairs T/CN and T/CP. Suitable trends for the enhancement of the physical characteristics of breads in terms of larger specific volume, higher viscoelastic and textural profiles, with lower and slower staling kinetics on aging were achieved by the pairs T-CN+, T-CP+, CN-CP+, and CN + CP+. Resulting breads showed all homogeneous and fine crumb grain and appealing color features. The pair T-CN+ maximized extracted bioaccessible polyphenols, and the pair CN + CP+ synergistically promoted the antiradical activity in breads. Caution should be applied to the pair T + CP+ because of the adverse effect on the firming of the fresh crumb and on the rate of staling.
HMT of associated non-wheat flours appears as a clean label simple strategy to create added value to breads from highly diluted wheat flour matrices, provided that single and interactive effects of the thermal treatment of blended flours on the structural features of breads are known.
References
AACC (Ed.). (2005). Approved methods of the American Association of Cereal Chemists (10th ed.). AACC St. Paul, MN: The Association.
Abebe, Y., Bogale, A., Hambidge, K. M., Stoecker, B., Bailey, J. K., & Gibson, R. S. (2007). Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, southern Ethiopia, and implications for bioavailability. Journal of Food and Composition Analysis, 20(3-4), 161–168.
Angioloni, A., & Collar, C. (2009). Bread crumb quality assessment: a plural physical approach. European Food Research and Technology, 229(1), 21–30.
Angioloni, A., & Collar, C. (2011). Nutritional and functional added value of oat, Kamut, spelt, rye and buckwheat versus common wheat in breadmaking. Journal of the Science of Food and Agriculture, 91(7), 1283–1292.
Angioloni, A., & Collar, C. (2012). High legume-wheat matrices: an alternative to promote bread nutritional value meeting dough viscoelastic restrictions. European Food Research and Technology, 234(2), 273–284.
Angioloni, A., & Collar, C. (2013). Suitability of oat, millet and Sorghum in Breadmaking. Food and Bioprocess Technology, 6(6), 1486–1493.
AOAC. (1991). Total, soluble, and insoluble dietary fiber in foods. Gaithersburg: Association of Official Analytical Chemists.
Armero, E., & Collar, C. (1998). Crumb firming kinetics of wheat breads with antistaling additives. Journal of Cereal Science, 28(2), 165–174.
Bravo, L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews, 56(11), 317–333.
Bultosa, G., & Taylor, J. R. N. (2004). Teff. In C. Wrigley, H. Corke, & C. E. Walker (Eds.), Encyclopedia of grain science. Oxford: Elsevier Ltd.
Cetiner, B., Acar, O., Kahraman, K., Sanal, T., & Koksel, H. (2017). An investigation on the effect of heat-moisture treatment on baking quality of wheat by using response surface methodology. Journal of Cereal Science, 74, 103–111.
Chen, X., He, X., Fu, X., & Huang, Q. (2015). In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture treatment. Journal of Cereal Science, 63, 109–115.
Collado, L. S., Mabesa, R. C., Corke, H. 1999. Genetic variation in the physical properties of sweet potato starch. J Agric Food Chem, 47(10), 4195-201.
Collar, C., & Angioloni, A. (2014). Pseudocereals and teff in complex breadmaking matrices: impact on lipid dynamics. Journal of Cereal Science, 59(2), 145–154.
Collar, C., & Angioloni, A. (2017). High-legume wheat-based matrices: impact of high pressure on starch hydrolysis and firming kinetics of composite breads. Food and Bioprocess Technology, 10(6), 1103–1112.
Collar, C., & Armero, E. (2018). Impact of heat moisture treatment and hydration level on physico-chemical and viscoelastic properties of doughs from wheat-barley composite flours. European Food Research and Technology, 244(2), 355–366.
Collar, C., Jiménez, T., Conte, P., & Fadda, C. (2014). Impact of ancient cereals, pseudocereals and legumes on starch hydrolysis and antiradical activity of technologically viable blended breads. Carbohydrate Polymers, 113, 149–158.
Dall'Asta, C., Cirlini, M., Morini, E., Rinaldi, M., Ganino, T., & Chiavaro, E. (2013). Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread-making. LWT-Food Science and Technology, 53(1), 233–239.
Demiate, I. M., Oetterer, M., & Wosiacki, G. (2001). Characterization of chestnut (Castanea sativa Mill) starch for industrial utilization. Brazilian Archives of Biology and Technology, 44(1), 69–78.
Demirkesen, I., Mert, B., Sumnu, G., & Sahin, S. (2010). Utilization of chestnut flour in gluten-free bread formulations. Journal of Food Engineering, 101(3), 329–336.
Donovan, J. W. (1979). Phase transitions of starch–water system. Biopolymers, 18(2), 263–275.
Dykes, L., & Rooney, L. W. (2007). Phenolic compounds in cereal grains and their health benefits. Cereal Foods World, 52, 105–111.
FAO/WHO. (2003). Food Energy – Methods of Analysis and Conversion Factors. FAO Food and Nutrition Paper 77, Rome.
Gélinas, P., McKinnon, C. M., Rodrigue, N., & Montpetit, D. (2001). Heating conditions and bread-making potential of substandard flour. Journal of Food Science, 66(4), 627–632.
Glahn, R. P., Lee, O. A., Yeung, A., Goldman, M. I., & Miller, D. D. (1998). Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. The Journal of Nutrition, 128(9), 1555–1561.
Gunaratne, A., & Hoover, R. (2002). Effect of heat-moisture on the structure and physicochemical properties of tuber and root starches. Carbohydrate Polymers, 49(4), 425–437.
Hamaker, B. R., & Griffin, V. K. (1993). Effect of disulfide bond-containing protein on rice starch gelatinization and pasting. Cereal Chemistry, 70, 377–380.
Hoover, R., & Vasanthan, T. (1994). The effect of annealing on the physicochemical properties of wheat, oat, potato and lentil starches. Journal of Food Biochemistry, 17, 303–325.
Hoover, R., Hughes, T., Chung, H. J., & Liu, Q. (2010). Composition, molecular structure, properties, and modification of pulse starches: a review. Food Research International, 43(2), 399–413.
ICC. (2014). ICC Standard Methods of the International Association for Cereal Science and Technology 104/1, 105/2, 110/1, 115/1, 136, 162, 166. Vienna: The Association.
Kaur, M., & Singh, N. (2005). Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry, 91(3), 403–411.
Lim, S.T., Chang, E.H., Chung, H.J. (2001). Thermal transition characteristics of heat moisture treated corn and potato starches. Carbohydrate Polymers, 46(2), 107–115.
Lopez-Rubio, A., Flanagan, B. M., Gilbert, E. P., & Gidley, M. J. (2008). A novel approach for calculating starch crystallinity and its correlation with double helix content: a combined XRD and NMR study. Biopolymers, 89(9), 761–768.
Mann, J., Schiedt, B., Baumann, A., Conde-Petit, B., & Vilgis, T. A. (2013). Effect of heat treatment on wheat dough rheology and wheat protein solubility. Food Science and Technology International, 20, 341–351.
Marston, K., Khouryieh, H., & Aramouni, F. (2016). Effect of heat treatment of sorghum flour on the functional properties of gluten-free bread and cake. LWT - Food Science and Technology, 65, 637–644.
Otten, J. J., Hellwig, J. P., & Meyers, L. D. (2006). Dietary reference intakes DRI. The essential guide to nutrient requirements. Washington, DC: The National Academies Press www.nap.edu.
Ovando-Martínez, M., Whitney, K., Reuhs, B. L., Doehlert, D. C., & Simsek, S. (2013). Effect of hydrothermal treatment on physicochemical and digestibility properties of oat starch. Food Research International, 52(1), 17–25.
Paciulli, M., Rinaldi, M., Cirlini, M., Scazzina, F., & Chiavaro, E. (2016). Chestnut flour addition in commercial gluten-free bread: a shelf-life study. LWT - Food Science and Technology, 70, 88–95.
Pepe, L. S., Moraes, J., Albano, K. M., Telis, V. R. N., & Franco, C. M. L. (2015). Effect of heat-moisture treatment on the structural, physicochemical, and rheological characteristics of arrowroot starch. Food Science and Technology International, 22, 256–265.
Puncha-arnon, S., & Uttapap, D. (2013). Rice starch vs. rice flour: differences in their properties when modified by heat-moisture treatment. Carbohydrate Polymers, 91(1), 85–91.
Regulation (EC) Nr (1924/2006). Regulation (EC) Nr. 1924/2006 of the European Parliament and of the Council on nutrition and health claims made on foods. Official Journal of the European Union, 24, L 404/9.
Ronda, F., Abebe, W., Pérez-Quirce, S., & Collar, C. (2015). Suitability of tef varieties in mixed wheat flour bread matrices: a physico-chemical and nutritional approach. Journal of Cereal Science, 64, 139–146.
Sánchez-Moreno, C., Larrauri, J. A., & Saura-Calixto, F. (1998). A procedure to mea-sure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture, 76(2), 270–276.
Satmalee, P., & Matsuki, J. (2011). Effect of debranching and heat-moisture treatment on the properties of Thai rice flours, rice flour. International Journal of Food Science and Technology, 12, 2628–2633.
Saura-Calixto, F., García-Alonso, A., Goñii, I., & Bravo, L. (2000). In vitro determination of the indigestible fraction in foods: an alternative to dietary fiber analysis. Journal of Agricultural and Food Chemistry, 48(8), 3342–3347.
Verdú, S., Vásquez, F., Ivorra, E., Sánchez, A. J., Barat, J. M., & Grau, R. (2017). Hyperspectral image control of the heat-treatment process of oat flour to model composite bread properties. Journal of Food Engineering, 192, 45–52.
Wang, S., & Copeland, L. (2013). Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: a review. Food & Function, 4(11), 1564–1580.
Watcharatewinkul, Y., Puttanlek, C., Rungsardthong, V., & Uttapap, D. (2009). Pasting properties of a heat-moisture treated canna starch in relation to its structural characteristics. Carbohydrate Polymers, 75(3), 505–511.
Zhou, Y., Hoover, R., & Liu, Q. (2004). Relationship between α-amylase degradation and the structure and physicochemical properties of legume starches. Carbohydrate Polymers, 57(3), 299–317.
Funding
The authors acknowledge the Institutions Ministerio de Economía y Competitividad (MINECO) and Federación Europea de Desarrollo Regional (FEDER) for funding the Project AGL2015-63849-C2-1-R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collar, C., Armero, E. Value-Added of Heat Moisture Treated Mixed Flours in Wheat-Based Matrices: a Functional and Nutritional Approach. Food Bioprocess Technol 11, 1536–1551 (2018). https://doi.org/10.1007/s11947-018-2125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2125-2