Abstract
In an effort to purify potato proteins of superior food grade quality, a new procedure involving anion exchange (ion exchange (IEX)) and hydrophobic interaction chromatography (HIC) was established. Liquid potato fruit juice (PFJ) or re-suspended spray-dried protein was separated by IEX yielding two fractions: a protease inhibitor (PI)-rich fraction and a patatin-rich fraction. Each of these fractions was re-chromatographed on HIC, resulting in two new sub-fractions which were characterised by electrophoresis and mass spectrometry. A high-quality powder should have high lightness, low polyphenol oxidase (PPO) activity and glycoalkaloid content below 150 μg/g. The PI fraction from spray-dried powder had high lightness (L* = 83) compared to patatin (L* = 50), whereas IEX purification from PFJ resulted in a PI fraction with decreased lightness (L* = 66) and a patatin fraction with increased lightness (L* = 68). HIC fractionation led to increased lightness for patatin fraction, but decreased lightness for the PFJ PI fractions. HIC purification significantly lowered PPO activity in the fractions due to its selective affinity. The lowest total glycoalkaloid content was generally found in HIC fractions from spray-dried powder (150 μg/g), while high levels (>1000 μg/g) were found in PI fractions from PFJ. In conclusion, it was possible to obtain a PI-rich protein isolate from powder with good-quality attributes after both IEX and HIC, while for patatin, the best quality was obtained after the HIC only, and there, the colour or PPO activity may still be a problem depending on source material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the world population growing rapidly and an ever-increasing demand for high-quality and sustainable food protein, new alternative sources like insects, algae and plants have attention. One potential source is the potato proteins, which are present in potato fruit juice (PFJ), which is produced in large amounts as a side stream of potato starch manufacture. PFJ contains 2–5% solid material, hereof 35% crude protein (N-containing substances) (Knorr et al. 1977).
The potato proteins can be divided roughly into three overall groups, with the first group being the patatins, which make up to 40% of the total protein. Patatin monomers have a molecular weight of 39–43 kDa, depending on N-glycosylation pattern and have pI values of 4.45 to 5.17 (Barta et al. 2012). The second group of potato proteins is the protease inhibitors (PIs), which can be divided into seven sub-groups: PI 1, PI 2, potato cysteine PI, potato aspartate PI, potato Kunitz-type PI, potato carboxypeptidase inhibitor and “other serine PIs” classified according to inhibitory activity, pI and molecular weight (Pouvreau et al. 2001). The PI accounts for up to 50% of the total protein in PFJ and has molecular masses in a range of 4.3–20.6 kDa and with pI values of 5.1–9.0 (Pouvreau et al. 2001). The third group of potato proteins is composed of oxidative enzymes, including polyphenol oxidase (PPO), lipoxygenase and enzymes functioning in starch synthesis (Jorgensen et al. 2011). Potato PPO is, in food contexts, an unwanted enzyme catalysing the hydroxylation of mono-phenols to o-diphenols and further oxidation of formed o-diphenols into o-quinones (Ramírez et al. 2003). The presence in potatoes of PPO combined with presence of the major phenolic compound, chlorogenic acid, is responsible for formation of undesired brown colour (Narvaez-Cuenca et al. 2013). Possible covalent- and non-covalent interactions between phenolic compounds and potato proteins may cause the final potato protein powder to have a brown appearance, but also decreased solubility and digestibility (Prigent et al. 2007; Rawel et al. 2001), thus resulting in poor quality. It is therefore of interest to inhibit PPO activity in the PFJ used for protein isolation or separate the quality decreasing enzyme from the other proteins during the processing steps.
Heat treatment (80 °C for 15 min), low pH (pH <3.5), addition of ascorbic acid or sodium metabisulphite can each inhibit PPO (Eidhin et al. 2010). Low pH and heat treatments of PFJ can, however, also result in denaturation and precipitation resulting in protein not suitable for human consumption (Straetkvern and Schwarz 2012). Additionally, in relation to its use as a source of food protein, potatoes contain the unwanted metabolites including the steroidal glycoalkaloids, α-solanine and α-chaconine. These compounds confer a bitter taste and possible toxicological reactions, like gastrointestinal disturbance, neurological disorders or even death if consumed in large amounts (Alt et al. 2005). The EU Commission has therefore set a limit of 150 μg/g total glycoalkaloids (TGAs) in the final protein powder (Byrne 2002) Apart from the possible presence of glycoalkaloids does potato protein show excellent nutritional value with a high biological value and a good amino acid profile (Alting et al. 2011). Digestion of patatin and PI in vivo shows stable and slow postprandial plasma amino acid levels and no increase of postprandial insulin in contrast to whey proteins that produced a fast increase in amino acids and an increase in insulin (He et al. 2013).
Concentration and separation of the proteins in PFJ have been done in a number of ways. This includes precipitation with acids (citric acid, acetic acid, hydrochloric acid, sulphuric acid), organic solvents (methanol, ethanol, 2-propanol, acetone) and metal salts (FeSO4, FeCl3, ZnCl2) (Barta et al. 2008). Separation techniques like ion exchange (van Koningsveld et al. 2001), hydrophobic interaction chromatography (Racusen 1989), expanded bed adsorption or ultrafiltration (Straetkvern and Schwarz 2012) and direct capture membrane adsorption (Schoenbeck et al. 2013) have been employed. It has been found that ultrafiltration with different membrane materials and molecular cut-off values cannot separate the unwanted PPO from the other potato proteins (Schmidt et al. 2016). Expanded bed adsorption and direct capture membrane adsorption are both promising techniques since the used equipment can handle crude feed liquids that would potentially clog conventional ion exchange columns or membranes (Lokra and Straetkvern 2009; Schoenbeck et al. 2013). Ion exchange and expanded bed adsorption result in one or two fractions: i.e. a total protein isolate or a separated patatin and PI fraction. Further purification of the patatin and the PI protein isolates has been reported only scarcely in the literature (Pots et al. 1999; Pouvreau et al. 2001). The aim of the present study was therefore to explore the potential benefits of combining ion exchange (IEX) and hydrophobic interaction chromatography (HIC) on protein fractionation of major potato proteins and characterise quality parameters like colour, glycoalkaloids and PPO activity in the final protein fractions. The aim was further to test the potential for use in large scale and the robustness of the method by using either liquid PFJ or suspensions of spray-dried potato protein as source of starting material.
Materials and Methods
Materials
Liquid PFJ (stored at −18 °C) and spray-dried potato protein isolate powders from the harvest 2014 were provided by KMC (Brande, Denmark) and used for the studies. Furthermore, a powder from 2015 was also analysed; this new powder was made with the inclusion of sodium bisulphite to inhibit PPO activity during manufacturing at the plant.
Chromatographic Protein Purification
The purifications were carried out using liquid PFJ or re-solubilised spray-dried protein powder. A two-step procedure was developed consisting of first an anion exchange column followed by HIC.
IEX purification was performed with a ÄKTA purifier 100 (GE Healthcare, Uppsala, Sweden) by loading samples at a flow of 17 ml/min onto a XK 50 column (GE Healthcare, Uppsala, Sweden) with a 980 ml packed bed volume of DEAE Sepharose Fast Flow medium (GE Healthcare, Uppsala, Sweden). The ÄKTA purifier 100 was equipped with a multi-wavelength UV-Vis monitor, and the wavelengths 280, 320 and 400 nm were used in all runs.
Ion Exchange: Potato Fruit Juice
Liquid PFJ was adjusted to pH 8 with 6 M NaOH and centrifuged (45 min, 5000×g, 4 °C) to remove insoluble material. One and a half litre was loaded equilibrated with a 25 mM Tris-HCl buffer, pH 8 (buffer A). The unbound fraction (PI-rich) was washed out with 1.2 column volumes (CV) of buffer A. Elution of the bound fraction (patatin rich) was conducted with 25 mM Tris-HCl, pH 8.0, with 1 M NaCl (buffer B) in two steps, with 28% (0.9 CV) and 100% (1.5 CV) buffer B.
Ion Exchange: Powder
Spray-dried powder suspension of 30 g/l (w/v) in 25 mM Tris-HCl buffer, pH 8, adjusted to a conductivity of 10 mS/cm by addition of NaCl was stirred for 1 h. Suspensions were centrifuged (25 min, 25,000×g, 4 °C) and filtered to remove undissolved material. The column was equilibrated with 9% buffer B and 1150 ml loaded. Elution gradient and conditions are summarised in Table 1.
HIC purification was performed with A XK 26 column with a 175 ml packed bed volume of Butyl Sepharose High Performance beads (GE Healthcare, Uppsala, Sweden), and samples were loaded at a flow of 7 ml/min.
Hydrophobic Interaction Chromatography: Potato Fruit Juice Fractions
For liquid PFJ, the PI fraction was adjusted to 145 mS/cm with NaCl centrifuged (25 min, 25,000×g, 4 °C) and 550 ml of fraction solution loaded. The patatin-rich fraction was adjusted to a conductivity of 110 mS/cm and 200 ml loaded. Details on buffers and elution conditions are summarised in Table 1.
Hydrophobic Interaction Chromatography: Powder Fractions
The PI fraction from spray-dried powder was adjusted to 145 mS/cm centrifuged (25 min, 25,000×g, 4 °C) and 550 ml of fraction solution loaded. The patatin-rich fraction was adjusted to a conductivity of 110 mS/cm and 200 ml loaded. Details on buffers and elution conditions are summarised in Table 1.
Ultra- and Diafiltration
Ultrafiltration and diafiltration were performed with a PALL Ultralab™ system equipped with a 10-kDa molecular weight cut-off Minimate membrane (PALL, New York, USA). All patatin fractions were concentrated at pH 8 and diafiltrated with demineralised water to a final conductivity of 1.2–1.7 mS/cm. All PI fractions were adjusted to pH 3 with 6 M HCl following concentration and diafiltration to a conductivity of 1.2–1.7 mS/cm. After diafiltration, the samples were freeze dried and stored at −18 °C.
pH and Conductivity Measurements
pH of the solutions was analysed by a PHM 92 pH meter (Radiometer, Copenhagen, Denmark) and conductivity measured at 25 °C by a CDM 210 Conductivity Meter (Radiometer, Copenhagen, Denmark).
Protein Concentration Determination
Protein concentration was determined by the bicinchoninic acid assay (BCA, Thermo Scientific™ Pierce™), with bovine serum albumin (2 mg/ml) as reference protein for protein determination (Smith et al. 1985). Measurements were conducted in triplicates.
Determination of Glycoalkaloids
The two glycoalkaloids solanine and chaconine were determined by the HPLC method as described (Hellenas and Branzell 1997) with extraction of the protein sample in 3% acetic acid followed by Ultra-Turax treatment for 3 min and centrifugation in a tabletop centrifuge for 2 min at 2000×g. The supernatant was further processed by solid-phase extraction on a Bakerbond® C18 column (Avantor Performance Materials, PA, USA) washed with 15% acetonitrile and glycoalkaloids eluted with 60% acetonitrile. Twenty-microlitre purified supernatant was injected onto a Purospher STAR RP-18250-mm column (Merck Millipore, MA, USA) operated at 40 °C and a flow of 1.5 ml/min with isocratic elution in a mobile phase of 60% acetonitrile in 10 mM potassium potassium monohydrogen phosphate and detection at 202 nm. The total glycoalkaloid (TGA) content was calculated as additions of means of the individual glycoalkaloids. Two replicates were performed, but in case of low TGA content, four measurements were conducted.
Polyphenol Oxidase Activity Assay
A combination of the methods by Cheng et al. (2007) and Eidhin et al. (2010) was used to analyse the activity of PPO. Activity was assayed in microtiter plates with 200 μl substrate (50 mM pyrocatechol in 0.1 M phosphate buffer, pH 6.5) and 50 μl enzyme solution. The reaction was carried out at room temperature, and an increase in absorbance at 420 nm was measured for 30 s in a Synergy 2 Microplate reader (BioTek Instruments Inc., Winooski, Vermount, 05404, USA). One unit of activity was defined as the change in absorbance of 0.001 per minute per millilitre of enzyme solution. Specific activity was calculated by dividing the number in units with the protein concentration in milligram per millilitre. Measurements were conducted in triplicates.
Colour Measurement of Powder
Colour of freeze-dried powder, ground by hand, was measured with a Minolta Chroma Meter CR-400 (Konica Minolta, Osaka, Japan) using the CIE (Commision Internationale de L’Enclairage) Lab scale calibrated against standardised daylight (D65). The L*, a* and b* values correspond to lightness (0: black; 100: white), redness (−100: green; 100: red) and yellowness (−100: blue; 100: yellow), respectively. Measurements were conducted in quadroduplicates. A photo of the different freeze-dried fractions was taken with a digital camera.
One-Dimensional Gel Electrophoresis
One-dimensional SDS-polyacrylamide gel electrophoresis (1-DGE) using Criterion™ TGX™ 8–16% precast gels (Bio-Rad, Richmond, CA, USA) was carried out as described (Laemmli 1970). Samples were mixed 1:1 with sample buffer (20 mM Tris, 2% SDS, 20% glycerol, pyronin Y), reduced with 1/10 vol 0.2 M dithioerythritol (DTE) and boiled for 3 min. Thirty microlitres of sample was loaded in each well. Gels were stained with Coomassie Brilliant Blue G-250. Molecular mass was estimated by a prestained broad-range molecular weight marker (Thermo Scientific™ Spectra™ Multicolor Broad Range Protein Ladders).
Two-Dimensional Gel Electrophoresis
Two-dimensional gel electrophoresis (2-DGE) was conducted essentially as described previously (H. B. Jensen et al. 2012) with focusing of 100 μg protein on either pH 4–7 or pH 5–8 immobilised gradient strips and running on 8–16% gradient polyacrylamide gels under reducing conditions. The gels were stained by Coomassie Brilliant Blue G-250.
Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry
Intact mass analyses of HIC-purified patatins were performed by matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS). The analyses were carried out with an Ultraflextreme instrument (Bruker Daltonics) in linear mode with detection of positively charged ions. Protein samples were mixed with ferulic acid matrix solution (12.5 mg ml−1 solution in water/acetonitrile/formic acid 50:33:17, v/v) and droplets applied to a ground steel MALDI target plate. Mass spectra were calibrated externally using a Protein Mixture II standard (Bruker Daltonics). Mass spectra were acquired under FlexControl software 3.4 and processed by Flex Analysis 3.4 (Bruker Daltonics).
In-Gel Digestion and Identification of Proteins by Liquid Chromatography Mass Spectrometry
Spots from 2-DGE were cut and subjected to in-gel tryptic digestion with reduction and alkylation essentially as described by O. N. Jensen et al. (1998). Peptides were extracted from gel pieces using 50% acetonitrile solution with 2.5% formic acid and concentrated in a SpeedVac concentrator (Thermo Fischer Scientific). Peptide mixtures were subjected to LC-MS/MS analysis using the RSLCnano system (Dionex, Sunnyvale, CA) coupled to an Impact II QTOF mass spectrometer (Bruker Daltonics, Bremen, Germany). The peptide separation was based on using an Acclaim PepMap 100 C18 column (3-μm particles, 75 μm × 500 mm; Thermo Fisher Scientific, 300 nl/min) and 0.1% FA/acetonitrile gradient. MS data were acquired in a data-dependent strategy with 3-s-long cycle time. Mass range was set to 150–2200 m/z, and precursors were selected from 300 to 2000 m/z. Proteins were identified by use of in-house Mascot database search engine (version 2.5.1) using the UniProtKB Potato database (version from November 5, 2016). Peptides with a Mascot expectation value <0.01 were considered for protein identification. Mass tolerance for precursors and MS/MS fragments were 15 ppm and 0.05 Da, respectively. Oxidation of methionine, deamidation (N, Q), carbamidomethylation (C) and acetylation (Protein N-term) as variable modifications were set for all searches. From each spot analysed, several protein identifications were reported by MASCOT with varying peptide spectra matches (PSM) (total number of identified peptide spectra matched for the protein). The protein with the highest PSM, relative to the protein theoretical molecular mass, was listed in a summary table. All proteins reported had a minimum of three peptides identified. Protein theoretical molecular weight (kDa) and theoretical isoelectric point (pI) of mature protein without signal peptide were calculated using online molecular weight and pI tools from www.uniprot.org.
Statistics
Data are presented as means with standard deviations. One or two-way ANOVA analysis was calculated by GraphPad Prism 6 (GraphPad Software Inc., La Jolla, USA, version 6.01). Differences were regarded significant at minimum 95%-level (P < 0.05).
Results and Discussion
Separation Flow and Conducted Analyses
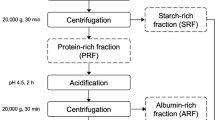
Figure 1 depicts a schematic representation of the different fractions obtained after IEX and HIC purification, with PFJ or spray-dried powder as source materials, as well as experiments performed on all the resulting fractions. IEX purification yields two fractions: the flow through rich in PI proteins and a bound fraction rich in patatin. HIC purification gives two fractions: one eluting early at high salt (HIC 1) and one eluting late at low salt (HIC 2).
Schematic representation of purification flow and the various analyses conducted on the separated protein fractions. As presented in the “Materials and Methods” section, 2014 and 2015 refer to the sample material. MALDI refers to MALDI-TOF MS of patatin fractions, and MS/MS refers to LC-MS/MS QTOF of tryptic peptides from 2-DGE spots
Elution Profiles of Ion Exchange and Hydrophobic Interaction Chromatography Separations
Figure 2a, b shows the IEX purification of 2014 PFJ and 2014 re-suspended powder, respectively. In both chromatograms, the first major peak (flow through/FT) corresponds to unbound protein, rich in protease inhibitor (PI). The second peak represents bound material rich in patatin, while the third peak is rich in phenolic compounds, especially for the PFJ sample, as indicated by high absorbance values at 320 and 400 nm (Narvaez-Cuenca et al. 2013). As shown in the 1-DGE (Fig. 3a, b), lane 4, the third fraction is low in protein, which is in contrast to the relatively high signals at 280 nm in the chromatogram. Chromatograms of powder 2015 did not differ from powder 2014 (results not shown).
1-DGE gels of purified fractions based on a PFJ or b re-solubilised 2014 powder. Dilution factors (before mixing with Laemmli sample buffer) or concentration of samples are in brackets. a Lane 1 PFJ (×10); lane 2 IEX FT (×4); lane 3 IEX patatin (×10); lane 4 IEX high salt (4500–5000 ml in Fig. 2a) (×0); lane 5 HIC FT patatin (×0); lane 6 HIC 1 patatin (×10); lane 7 HIC 2 patatin (×40); lane 8 HIC FT PI (×0); lane 9 HIC 1 PI (×10); lane 10 HIC 2 PI (×10). b Lane 1 powder (~0.5 mg/ml); lane 2 IEX FT (×4); lane 3 IEX patatin (×16); lane 4 IEX high salt (3800–4000 ml in Fig. 2b) (×0); lane 5 HIC FT patatin (×0); lane 6 HIC 1 patatin (×16); lane 7 HIC 2 patatin (×50); lane 8 HIC FT PI (×0); lane 9 HIC 1 PI (×10); lane 10 HIC 2 PI (~0.5 mg/ml)
Table 2 shows the integrated peak areas under the curve at the different wavelengths, and it is seen that IEX purification of PFJ is able to separate the majority of phenolic compounds, i.e. chlorogenic acid (320 nm) and especially the oxidised phenolics (400 nm), from the main protein peaks. Figure 2c, e displays the PI and patatin fractions from PFJ, further purified by HIC. Both chromatograms have significant signals at both 280, 320 and 400 nm in the flow through, but as seen in the 1-DGE picture (Fig. 3a, b), lanes 5 and 8, these fractions are quite low in protein, though presenting the majority of both phenolics and oxidised phenolics (Table 2). The large signal at 280 nm may be due to phenolics bound to proteins, since derivatisation of protein with phenolic acids increases absorption compared to pure protein (Rawel et al. 2002). The HIC chromatograms for powder (Fig. 2d, f) show significantly lower signals in the flow-through fraction, but relatively higher values at 320 and 400 nm, co-eluting with the bound proteins (Table 2).
The recovery during the two purification steps is summarised in Table 3. In case of IEX purification of powder, patatin fraction contained higher protein amount (ca 30%) than PI fraction while PFJ purification resulted in doubled protein amount in PI fraction. For HIC purification of patatin, both derived from powder or PFJ, more than double of the protein is found in the second eluting fraction (patatin HIC 2). For PI, both derived from powder or PFJ, the first eluting peak (PI HIC 1) contained double the amount of protein than the second (PI HIC 2). The relatively low recovery during HIC purification of PFJ PIs (~32%) is believed to be caused by overestimation of protein in the injected PI fraction, due to high concentrations of phenolic substances which can interact with common protein determination methods like BCA, Bradford or Lowry (Lindeboom and Wanasundara 2007; Mattoo et al. 1987; Kamath and Pattabiraman 1988). Based on the 1-DGE gel (Fig. 3a, b), recovery of all fractions should be high with minor losses in the high-salt fraction (lane 4) and minor loss in flow through for HIC purification. In conclusion, main potato proteins, either as re-solubilised powder or as PFJ, can be separated and purified by a new combination of IEX and HIC yielding equivalent isolated protein fractions, though use of PFJ as a start material leads to better separation of phenolic compounds from protein, as based on integrated peak areas at 320 and 400 nm compared with the results obtained for re-solubilised spray-dried potato protein powder.
Two-Dimensional Gel Electrophoresis of Hydrophobic Interaction Chromatography-Purified Fractions
In an effort to expand the knowledge about protein, differences between the HIC-purified fractions were the patatin and PI fractions derived from 2015 powder separated by 2-DGE depicted in Fig. 4a–d. The patatin fraction had different patatin and PI profiles (marked by arrows) with patatin HIC 2 having proteins with a more acidic pI than the patatin HIC 1 fraction. The PI HIC 1 fraction (Fig. 4c) had a prominent spot at pH 6 and multiple spots around 10 kDa, which was absent in the PI HIC 2 gel. In contrast, PI HIC 2 presented several dense spots at pH 7. A large proportion of the proteins in the PI HIC 1 fraction had pI values above pH 8, in contrast to PI HIC 2.
Mass Spectrometry Identification of Protease Inhibitor Proteins from Two-Dimensional Gel Electrophoresis Gels
Distinctive spots from the 2-DGE gels of PI proteins were identified by LC-MS/MS analysis. The identity of numbered spots is presented in Table 4 with spots from the HIC 1 fraction having spot ID 1–9 and HIC 2 fraction spot ID 10–18. Many different proteins were identified from the PI HIC 1 fraction while only different forms of two proteins serine PI 5 and aspartic PI 11 were identified in the HIC 2 gel. The redundancy in identified proteins in the PI HIC 2 gel may be due to a high homology between the different PI proteins as well as too low separation during the 2-DE gel preparation. The difference observed between theoretical and calculated pI and molecular masses may be due to posttranslational modifications on the proteins, e.g. degree of glycosylation and phosphorylation and possible proteolytic degradation (H. B. Jensen et al. 2012) during the processing of the raw PFJ.
Analysis of Molecular Masses of Patatins by Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry
HIC purification resulted in two patatin fractions with the second eluting fraction being enriched in a high molecular weight isoform of patatin (Fig. 3a, b, lane 7). The four patatin HIC fractions were analysed by MALDI-TOF MS. The obtained masses are reported in Table 5, and an example of a representative mass spectrum is shown in Fig. 5. The masses of the different isoforms correspond to one, two or three glycosylations with previously reported masses of 40,513–40,666; 41,702–41,884; and 42,884–42,975 Da, respectively (Barta et al. 2012). The isoform containing three glycoforms has been reported not being present in all potato varieties, but it is found in relatively high proportions in the cultivar “Kuras” (Barta et al. 2012), which is commonly grown in Denmark. The mass spectra (Fig. 5) indicate additional peaks with m/z values of 40,700–40,900. These peaks may be associated with covalently bound phenolics since binding of one chlorogenic acid molecule inducing a mass shift of 353.3 (Ali et al. 2013).
Total Glycoalkaloid Content in the Separated Fractions
The content of solanine and chaconine and the total sum of the two glycoalkaloids (TGAs) are summarised in Table 6. For resolubilised spray-dried powder, the PI fractions had a 2.3-fold lower total TGA content compared with the initial spray-dried powder, whereas the patatin fraction had a 1.9-fold increase. When looking at individual glycoalkaloids, solanine was associated with the PI fraction, while chaconine was associated with the patatin fraction. Further purification by HIC led to an enrichment of TGA in PI HIC 1 (low hydrophobicity) compared to PI HIC 2 (high hydrophobicity). For patatin, HIC leads to an enrichment of TGA in the patatin HIC 2 fraction. The fractions purified from PFJ had a different enrichment of TGA with the PI fraction exceeding 1000 μg/g while patatin was 10-fold lower. Hydrophobic interaction chromatography of the PI fraction resulted in an enrichment in PI HIC 2 with a 3-fold higher content than the PI fraction. For patatin, HIC leads to an enrichment of TGA in the patatin HIC 1 fraction.
The PFJ fractions had much higher total glycoalkaloid levels than samples from resolubilised powder. This difference is due to the fact that the spray-dried powder had undergone a number of diafiltration steps before drying in contrast to the fresh PFJ. The difference between TGA contents upon IEX and HIC purification suggests varying ionic and hydrophobic interaction mechanisms between TGA column and/or TGA protein, but no general mechanism was observed. Other researchers have also reported TGA content in potato protein after purification: In a study comparing ultrafiltration or expanded-bed chromatography, the resulting powders had 213 ± 100 and 286 ± 95 μg/g TGA, respectively (Straetkvern and Schwarz 2012). Expanded bed can, however, result in a low TGA content, below 50 μg/g (Lokra et al. 2008). Purification of PFJ by cationic clay minerals has also proven favourable results with reduction in α-solanine by >90% of initial values (Ralla et al. 2012). A novel adsorptive bubble separation technique in which glycoalkaloids from PFJ are concentrated in collapsed foam has shown a 99% recovery, indicating that hydrophobicity is an important factor to consider when removing glycoalkaloids (Backleh et al. 2004).
Polyphenol Oxidase Activity in the Purified Fractions
PPO activity was determined directly in the liquid fractions after IEX and HIC purification of 2014 re-suspended powder and PFJ (Table 7) and for the freeze-dried fractions after ultrafiltration/diafiltration of 2014 and 2015 powder and PFJ (Table 8).
Following IEX purification of re-suspended 2014 powder or PFJ, PPO was predominantly found in the PI IEX fraction with a higher specific activity in this fraction compared with the patatin IEX fraction. High specific activity was found in the HIC flow-through fraction of both PI and patatin, indicating that PPO had a lower binding affinity to the HIC column than both patatin and PI. When comparing specific activity of the “liquid” fractions with the freeze-dried fractions in (Table 8), a significant decrease in specific activity is seen for the PI fractions. This drop is attributed to pH-induced deactivation of PPO, since the PI fractions are adjusted to pH 3 prior to concentration/diafiltration. pH-induced inactivation of PPO has been reported by other authors (Duangmal and Apenten 1999; Eidhin et al. 2010). In conclusion, HIC purification resulted in significantly lower PPO activity in the freeze-dried fractions of all tested samples, due to an apparent selective binding of patatin and PI to the HIC column compared to PPO.
In two previously published papers, similar column binding characteristics of PPO have been reported. PPO was predominantly found in the non-binding fraction (PI-fraction) following DEAE Sepharose purification by Marri et al. (2003). It was found by Partington and Bolwell (1996) that PPO eluted prior to patatin upon octyl Sepharose purification.
Colour Analysis of Freeze-Dried Samples
A picture of the freeze-dried fractions is displayed in Fig. 6, and the colour expressed as L*, a* and b* is shown in Fig. 7a–c. The colour measurements were conducted directly on the freeze-dried powder as reported previously (Straetkvern and Schwarz 2012; Zeng et al. 2013) and not on resolubilised protein fractions. The purified fractions with powder as starting material resulted in PI fractions with high values of lightness, low positive a* values and b* values of ~11, indicating a yellow colour. HIC fractionation did not lead to further changes in L* for the 2015 powder, though there is a slight decrease in L* for PI HIC 2 for 2014 powder. The a* values changed to a negative (more green) for both 2015 PI HIC fractions and a higher positive a* (more red) for the 2014 PI HIC fractions of both powder and PFJ. The b* value decreased (more blue) for 2015 PI HIC fractions and increased (more yellow) for 2014 powder PI HIC fractions, while no significant change was found for 2014 PFJ PI and PI HIC fractions. The PI IEX fraction based on PFJ had a significantly lower L* and higher a* value than fractions based on powder. Lightness decreased and a* increased of the PFJ PI HIC fractions as a possible outcome of high PPO activity during purification.
The patatin fractions based on 2014 and 2015 powder had a lower lightness and a brown hue (Fig. 6). HIC fractionation resulted in higher lightness for HIC 1 for both powders. The patatin fraction based on PFJ had a significantly higher lightness and yellowness than the powder patatin fractions, and lightness was further increased upon HIC fractionation. HIC fractionation also resulted in a significant drop in the a* values for the PFJ patatin.
Preliminary studies by size exclusion chromatography on a Sephacryl S-200 HR column of the patatin fraction from spray-dried powder showed high signals at 320 and 400 nm eluting prior to the patatins, indicating that the coloured complexes have a larger size than the 80 kDa patatin dimers and that colour can be removed from this fraction. In conclusion, fractionation of powder by IEX resulted in a light PI fraction and a dark patatin fraction, while IEX fractionation of PFJ separated the dark-coloured compounds better from the patatin fraction due to a lower concentration of salt during protein elution (Table 1). High PPO activity in the PI fraction of PFJ did, however, result in low lightness. HIC fractionation could lead to whiter powders, but the additional processing time could also potentially lead to further discoloration by PPO.
Table 9 provides an overall grading from + to ++++ of the measured quality parameters for the IEX and HIC fractions obtained from 2014 PFJ and spray-dried powder, with ++++ being the most positive for use as a food ingredient. This means that a high value is associated with good-quality characteristics, i.e. high lightness, low TGA content and low PPO activity. The fractions with best overall performance were PI HIC 1 from spray-dried powder and Pat HIC 2 from PFJ. The applicability of these fractions as potential food ingredients will depend on further up-scaling of the isolation procedures, as well as on the resulting functional properties of each isolate, in combination with the obtained quality results here.
Conclusion
Potato proteins were fractionated into a PI-rich and a patatin-rich fraction by anion exchange chromatography with either PFJ or spray-dried powder as source materials. The obtained fractions were further separated by HIC into two PI and patatin sub-fractions, as confirmed by 2-DGE, MALDI-TOF MS and LC-ME/MS analyses.
IEX purification of re-suspended powder yielded a white PI fraction and a brown patatin fraction, while IEX purification of PFJ could separate the majority of coloured compounds from the patatin fraction. HIC fractionation improved the colour further, but the increased processing time could also lead to increased discoloration if the fractions were rich in PPO. HIC fractionation significantly lowered the PPO activity due to low affinity between PPO and the HIC column. TGA content was below the food limit of 150 μg/g in three of four HIC fractions derived from spray-dried powder and therefore has potential as vegetable-based food protein ingredients. The TGA content did, however, greatly exceed the limit in some of the PI fractions from PFJ. Future work will focus on functional properties of the purified fractions derived from spray-dried powder.
References
Ali, M., Homann, T., Khalil, M., Kruse, H. P., & Rawel, H. (2013). Milk whey protein modification by coffee-specific phenolics: effect on structural and functional properties. Journal of Agricultural and Food Chemistry, 61(28), 6911–6920.
Alt, V., Steinhof, R., Lotz, M., Ulber, R., Kasper, C., & Scheper, T. (2005). Optimization of glycoalkaloid analysis for use in industrial potato fruit juice downstreaming. Engineering in Life Sciences, 5(6), 562–567.
Alting, A. C., Pouvreau, L., Giuseppin K. L. F., & van Nieuwenhuijzen, N. H. (2011). Potato proteins. In G. O. Phillips, & P. A. Williamn (Eds.), Handbook of food proteins (pp. 316–334). Cambridge: Woodhead Publishing.
Backleh, M., Ekici, P., Leupold, G., Coelhan, M., & Parlar, H. (2004). Enrichment of the glycoalkaloids alpha-solanine and alpha-chaconine from potato juice by adsorptive bubble separation using a pH gradient. Journal of Separation Science, 27(12), 1042–1044.
Barta, J., Hermanova, V., & Divis, J. (2008). Effect of low-molecular additives on precipitation of potato fruit juice proteins under different temperature regimes. Journal of Food Process Engineering, 31(4), 533–547.
Barta, J., Bartova, V., Zdrahal, Z., & Sedo, O. (2012). Cultivar variability of patatin biochemical characteristics: table versus processing potatoes (Solanum tuberosum L.) Journal of Agricultural and Food Chemistry, 60(17), 4369–4378.
Byrne, D. (2002). Commission decision. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002D0150. Accessed 27 July 2017.
Cheng, T. M., Huang, P. C., Pan, J. P., Lin, K. Y., & Mao, S. J. (2007). Gel electrophoresis of polyphenol oxidase with instant identification by in situ blotting. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 849(1–2), 331–336.
Duangmal, K., & Apenten, R. K. O. (1999). A comparative study of polyphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food Chemistry, 64(3), 351–359.
Eidhin, D. N., Degn, P., & O’Beirne, D. (2010). Characterization of polyphenol oxidase from rooster potato (Solanum tuberosum cv rooster). Journal of Food Biochemistry, 34(1), 13–30.
He, T., Spelbrink, R. E. J., Witteman, B. J., & Giuseppin, M. L. F. (2013). Digestion kinetics of potato protein isolates in vitro and in vivo. International Journal of Food Sciences and Nutrition, 64(7), 787–793.
Hellenas, K. E., & Branzell, C. (1997). Liquid chromatographic determination of the glycoalkaloids alpha-solanine and alpha-chaconine in potato tubers: NMKL interlaboratory study. Journal of AOAC International, 80(3), 549–554.
Jensen, O. N., Larsen, M. R., & Roepstorff, P. (1998). Mass spectrometric identification and microcharacterization of proteins from electrophoretic gels: strategies and applications. Proteins-Structure Function and Genetics, 74–89.
Jensen, H. B., Poulsen, N. A., Moller, H. S., Stensballe, A., & Larsen, L. B. (2012). Comparative proteomic analysis of casein and whey as prepared by chymosin-induced separation, isoelectric precipitation or ultracentrifugation. Journal of Dairy Research, 79(4), 451–458.
Jorgensen, M., Stensballe, A., & Welinder, K. G. (2011). Extensive post-translational processing of potato tuber storage proteins and vacuolar targeting. The FEBS Journal, 278(21), 4070–4087.
Kamath, P., & Pattabiraman, T. N. (1988). Phenols interfere in protein estimation by the bicinchoninic acid assay-method. Biochemical Archives, 4(1), 17–23.
Knorr, D., Kohler, G. O., & Betschart, A. A. (1977). Potato protein concentrates: the influence of various methods of recovery upon yield, compositional and functional characteristics. Journal of Food Processing and Preservation, 1(3), 235–247.
Laemmli, U. K. (1970). Clevage of structural proteins during assembly of head of bacteriophage-T4. Nature, 227(5259), 680–685.
Lindeboom, N., & Wanasundara, P. (2007). Interference of phenolic compounds in Brassica napus, Brassica rapa and Sinapis alba seed extracts with the Lowry protein assay. Food Chemistry, 104(1), 30–38.
Lokra, S., & Straetkvern, K. O. (2009). Industrial proteins from potato juice. A review. In N. Yee & W. T. Bussell (Eds.), Potato III, Food 3 (Vol. 1, pp. 88–95). New Jersey: Global Science Books.
Lokra, S., Helland, M. H., Claussen, I. C., Straetkvern, K. O., & Egelandsdal, B. (2008). Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT-Food Science and Technology, 41(6), 1089–1099.
Marri, C., Frazzoli, A., Hochkoeppler, A., & Poggi, V. (2003). Purification of a polyphenol oxidase isoform from potato (Solanum tuberosum) tubers. Phytochemistry, 63(7), 745–752.
Mattoo, R. L., Ishaq, M., & Saleemuddin, M. (1987). Protein assay by Coomassie brilliant blue G-250-binding method is unsuitable for plant tissues rich in phenols and phenolases. Analytical Biochemistry, 163(2), 376–384.
Narvaez-Cuenca, C. E., Vincken, J. P., & Gruppen, H. (2013). Quantitative fate of chlorogenic acid during enzymatic browning of potato juice. Journal of Agricultural and Food Chemistry, 61(7), 1563–1572.
Partington, J. C., & Bolwell, G. P. (1996). Purification of polyphenol oxidase free of the storage protein patatin from potato tuber. Phytochemistry, 42(6), 1499–1502.
Pots, A. M., Gruppen, H., Hessing, M., van Boekel, M., & Voragen, A. G. J. (1999). Isolation and characterization of patatin isoforms. Journal of Agricultural and Food Chemistry, 47(11), 4587–4592.
Pouvreau, L., Gruppen, H., Piersma, S. R., van den Broek, L. A. M., van Koningsveld, G. A., & Voragen, A. G. J. (2001). Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. Journal of Agricultural and Food Chemistry, 49(6), 2864–2874.
Prigent, S. V. E., Voragen, A. G. J., Visser, A., van Koningsveld, G. A., & Gruppen, H. (2007). Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). Journal of the Science of Food and Agriculture, 87(13), 2502–2510.
Racusen, D. (1989). Patatin purification by hydrophobic interaction chromatography. Journal of Food Biochemistry, 13(6), 453–456.
Ralla, K., Sohling, U., Suck, K., Kasper, C., Ruf, F., & Scheper, T. (2012). Separation of patatins and protease inhibitors from potato fruit juice with clay minerals as cation exchangers. Journal of Separation Science, 35(13), 1596–1602.
Ramírez, E. C., Whitaker, J. R., Virador, V, M. (2003). Polyphenol oxidase. In J. R. Whitaker., A. G. J. Voragen., D. W. S. Wong (Eds.), Handbook of Food Enzymology (pp. 509–537). New York: Marcel Dekker, Inc.
Rawel, H. M., Kroll, J., & Rohn, S. (2001). Reactions of phenolic substances with lysozyme—physicochemical characterisation and proteolytic digestion of the derivatives. Food Chemistry, 72(1), 59–71.
Rawel, H. M., Czajka, D., Rohn, S., & Kroll, J. (2002). Interactions of different phenolic acids and flavonoids with soy proteins. International Journal of Biological Macromolecules, 30(3–4), 137–150.
Schmidt, J. M., Greve-Poulsen, M., Damgaard, H., Hammershoj, M., & Larsen, L. B. (2016). Effect of membrane material on the separation of proteins and polyphenol oxidase in ultrafiltration of potato fruit juice. Food and Bioprocess Technology, 9(5), 822–829.
Schoenbeck, I., Graf, A. M., Leuthold, M., Pastor, A., Beutel, S., & Scheper, T. (2013). Purification of high value proteins from particle containing potato fruit juice via direct capture membrane adsorption chromatography. Journal of Biotechnology, 168(4), 693–700.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., et al. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150(1), 76–85.
Straetkvern, K. O., & Schwarz, J. G. (2012). Recovery of native potato protein comparing expanded bed adsorption and ultrafiltration. Food and Bioprocess Technology, 5(5), 1939–1949.
van Koningsveld, G. A., Gruppen, H., de Jongh, H. H. J., Wijngaards, G., van Boekel, M., Walstra, P., et al. (2001). Effects of pH and heat treatments on the structure and solubility of potato proteins in different preparations. Journal of Agricultural and Food Chemistry, 49(10), 4889–4897.
Zeng, F. K., Liu, H., Ma, P. J., & Liu, G. (2013). Recovery of native protein from potato root water by expanded bed adsorption with Amberlite XAD7HP. Biotechnology and Bioprocess Engineering, 18(5), 981–988.
Acknowledgements
The authors thank the Future Food Innovation (FFI) of region Mid-Jutland, Denmark, KMC (Kartoffelmelcentralen), AKV Langholt (Andelskartoffelmelsfabrikken Vendsyssel) and Aarhus University for financial support of the present work. Furthermore, we thank Ulla Marquardt, laboratory technician at KMC, for analysis of total glycoalkaloids. MALDI-MS and LC-MS/MS analyses were carried out with the support of Proteomics Core Facility under CIISB project (LM2015043) and the project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, J.M., Greve-Poulsen, M., Damgaard, H. et al. A New Two-Step Chromatographic Procedure for Fractionation of Potato Proteins with Potato Fruit Juice and Spray-Dried Protein as Source Materials. Food Bioprocess Technol 10, 1946–1958 (2017). https://doi.org/10.1007/s11947-017-1966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1966-4