Abstract
Obtaining native protein from potato fruit water (PFW) acceptable for food consumption was attempted by comparing expanded bed adsorption (EBA) and ultrafiltration (UF).The methods were assessed on their process performance and the product quality. Extractable tuber proteins were recovered from lab-prepared PFW either by adsorption to an EBA column using a mixed mode resin (0.31 L) or by batch concentration in an UF (10 kDa MWCO, 0.093 m2) unit and then freeze dried. The yields on protein and esterase activity were higher (p < 0.05 and p < 0.01; Mann–Whitney U-test) in UF (3.2 g l−1 PFW and 3.17 kU l−1 PFW) than in EBA (1.8 and 1.21). The performance difference was also reflected in process productivity for esterase activity which was fivefold better (p < 0.01) in UF (4.30 kU h−1) than with EBA (0.84) due to the higher enzyme retention; protein productivities were the same. The content of solanidine glycoalkaloids was depleted to moderate levels but came out unaffected by the processing method: EBA 286 ppm, UF 213 ppm. The low levels of chlorogenic acid in all EBA preparations were negatively correlated to high brightness score (L* = 73.8%), a favorable attribute in food-quality proteins. Both methodologies produced native preparations of comparable protein content (75%). EBA processing, however, increased the fraction of the patatin protein which may offer advantages in food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato tuber proteins are nutritious and can have promising applications in human food systems, provided that they are isolated with methods conserving their functionality and separating the proteins from extract components that reduce palatability. The major soluble potato proteins are found to be around 19–22 kDa (protease inhibitor group, PI) and at 41 kDa (patatin group). These fractions are also associated with interesting functional properties (Giuseppin et al. 2008; Løkra et al. 2008; Løkra et al. 2009; van Koningsveld et al. 2006). Patatin is the protein component with highest nutritional value with an EAAI value of 86.1% (Bartova and Barta 2009). However, these benefits of potato proteins can only be fully utilized with native preparations. Conventional protein recovery in the milling industry includes deleterious steps like steam pressure coagulation and acid and salt precipitation. This results in denatured proteins of only animal feed grade. Over the last decades, several alternative recovery methods have been attempted (see review by Løkra and Strætkvern 2009). Membrane separation and expanded adsorption chromatography are both process-scale technologies with the potential for producing native protein isolates from potato fruit water (PFW).
Membrane Processes Applied to PFW
Membrane separation is an established unit operation in the food industries and is widely applied in the starch processing industry (Rausch 2002). Reverse osmosis (RO) has, over several years, been used for the treatment of voluminous amounts of potato fruit water (Bidinger 1992; Rüffer et al. 1997). PFW is concentrated by RO after starch separation before protein coagulation by steam. The benefits of the twofold concentration of solids are increased protein yield and reduced energy input. The permeate water recovered during the RO process is reused in upstream washing steps. In obtaining a refined protein preparation, RO is unsuitable since minerals and low molecular weight molecules are retained as well. RO is typically carried out in hollow-fiber format with cellulose acetate membranes rejecting over 95% of NaCl.
Ultrafiltration (UF), based on retention by molecular size, has the capability of dewatering the feed stream and separating low molecular weight species from macromolecules by choosing the appropriate membrane cutoff range. Low molecular weight species like salts, sugars, plant pigments, organic acids, and free amino acids are removed through the permeate.
UF processes have seen only limited large-scale application, and only few reports have been cited in the literature (Eriksson and Sivik 1976; Oosten 1976; Rüffer et al. 1997; Wojnowska et al. 1981). Protein extracts from jojoba (Nabetani et al. 1995) and from alfalfa (D’Alvise et al. 2000) are examples of other plant-derived feedstock treated with UF to remove low molecular weight materials like sugars, toxicants, and polyphenols. The major obstacle with the UF treatment of PFW is the severe fouling, caused by foaming and the presence of plant microfiber, and cleaning problems of the membranes (Eriksson and Sivik 1976). Thus, both permeate flux and the membrane lifetime are affected and limit the extent that UF can be used in protein recovery. Internal fouling, pore blocking, and cake formation were all causes of flux decline with UF treatment of complex olive mill waste water containing high amounts of solids and polyphenols (Tsagaraki and Lazarides 2010).
A few studies on membrane processing of potato fruit water have been published, and the industry-related applications are typically redeemed proprietary. Wojnowska et al. (1981) compared ultrafiltration to polyelectrolyte coagulation and cryoconcentration and the functional properties after drying. Of these methods, best results were obtained with ultrafiltration. In a study by Zwijnenberg et al. (2002), comparing different pore sizes and membrane configurations, a fourfold volume concentration was followed by diafiltration. The separation was largely independent of membrane cutoff (5–100 kDa), membrane type, and geometry. The dewatered protein was reported to have very good functional properties.
Concentration factor, permeate yield, and membrane retention should favor time-efficient protein enrichment and at the same time reduce undesirables like plant pigments, protease inhibitor activity, and glycoalkaloids (Eriksson and Sivik 1976; Wojnowska et al. 1981).
Adsorption of Protein from PFW
Adsorption chromatography is characterized by the selective capture of proteins from a feed stream, thus increasing the purity of the protein. However, mass transfer limitations due to diffusion barriers in the chromatographic support impede high protein loads. Adsorption recovery methods with potato proteins are discussed in a few studies (Andersson et al. 2008; Graf et al. 2009; Strætkvern et al. 1999; Strætkvern et al. 2002).
Expanded bed adsorption was used by Strætkvern and coworkers to isolate the patatin fraction using a mixed mode adsorbent at pH 7.5 (Strætkvern et al. 1999). Later, a modified mixed mode ligand was employed to capture all the tuber proteins at pH 4.5 (Strætkvern et al. 2002). Interestingly, patatin adsorbed also even though the pI for this protein group is between 5.2 and 4.5, and thus it has a reduced solubility. It was assumed that patatin and other protein species bind because of the combined hydrophobic and electrostatic forces of the ligand. The quality of the potato protein isolated by EBA is such that important functional qualities are conserved (like emulsification and solubility), also after drying the protein (Claussen et al. 2007). The color of the isolate at pH 4.5 was improved considerably by removing the plant pigments, and a significant reduction in glycoalkaloid content was demonstrated (Løkra et al. 2008; Løkra et al. 2009).
Simulated moving bed (SMB) technology has been used for the capture of proteins from PFW (Andersson et al. 2008). The lab scale SMB configuration captured approximately 80% of the protein from a clarified feed using an anion exchanger resin, with a minimum use of extra water. However, only process properties were reported and no details on the ion exchange resin given. By combining large surface area access with ionic exchange adsorption, tuber protein capture was carried out with membrane adsorption capsules (Graf et al. 2009). Both the patatin and the PI groups were isolated and separated on anion and cation exchangers, respectively, from a diluted feed stream.
In the present study, expanded bed adsorption (EBA) chromatography is compared to UF membrane separation to observe actual differences on potato protein recovery with the same process feed. The target product will be a native preparation consisting of the major protein fractions of functional value. Each processing method offers advantages to the product quality and process performance. Thus, an evaluation of the two methods at bench scale will help in adopting strategies for a better utilization of proteins in potato processing by-streams for food applications.
Materials and Methods
The chemicals used were of technical or analytical grade. Potatoes (US#1 red potato) were obtained at a local food market in bags of 20 lbs (9 kg).
Processing Methods
Potato Juice
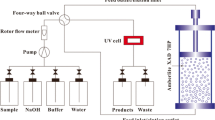
Tubers were sliced in cubes and weighed and a solution of sodium bisulfite (500 ppm) was added to the potato cuts at a ratio of 1:4 (w/v). Homogenization was carried out batch-wise in a R800 Commercial Food Processor (Robot Coupe USA, Inc., Ridgeland, MS, USA). The six-blade rotor was run at 3,000 rpm for about 20 s to obtain a fine-grained pulp. Liquid was collected from the pulp by pressing it through two layers of cheesecloth by hand, typically obtaining 60–70% liquid yield (v/w) from the weighed potato. To settle the starch, the pressed homogenate was centrifuged at low speed (3,000 g) for 10 min. The supernatant, still cloudy from suspended particles, was collected and stored cold until processing. A single juice extraction was split equally for processing either by EBA or UF. Figure 1 shows a schematic diagram for the two experimental setups.
Expanded Bed Adsorption
Chromatography was carried out based on the methodology described by Strætkvern et al. (1999), but with adaptations pertinent for the current resin (Strætkvern et al. 2002). The potato juice extract was adjusted to pH 4.5 by the addition of 6 M HCl shortly before column loading. The column was equilibrated with three to four volumes of tap water, with no further pH equilibration before loading, as the buffer capacity of the feed (pH 4.5) was sufficient to obtain starting conditions. The column wash buffer was 10 mM citric acid/citrate pH 4.5, and the eluting solution was 20 mM sodium hydroxide, pH 12. Cleaning in place (CIP) was obtained with a pulse of 1 M NaOH before re-equilibration with tap water.
The glass column, 5 cm i.d., height 65 cm (FastLine 50; UpFront Chromatography AS, Copenhagen, Denmark), was loaded with 315 or 510 ml resin which could be expanded two to three times on the column. The two different resin volumes were used to assay for the effect of sample residence time on the column. One column volume (CV) corresponds to the volume of the settled resin. The resin consisted of a wolfram carbide agarose matrix (density 3.5 g cm−3, 20–200 μm) modified with a mixed mode (MiMo) ligand developed purposely for capturing all major tuber protein fractions. The MiMo ligand technology (UpFront Chromatography AS) displays a combined hydrophobic and electrostatic interaction, with a change of pH as an effective way to desorb proteins through electrostatic repulsion (Lihme et al. 2000). The chromatographic flow arrangement involved upward pumping of liquid with a superficial velocity of 400–700 cm h−1 (corresponding to volumetric rates 7.9–13.8 l h−1) and flow distribution at the bottom inlet equipped with a magnetic stir bar. The column cycle was carried out in expanded mode during all steps.
The outlet tubing was fitted with pH (WTW, Germany) and UV-280 (GE Healthcare, Piscataway, NJ, USA) flow cells. Liquid was fed from the inlet side, through a pulse dampener, using a Masterflex (Cole Parmer, Vernon Hills, IL, USA) L/S standard drive equipped with HP pump head and LS15 silicone tubing. Samples were collected throughout the column cycle to monitor the protein level and enzyme activity.
Ultrafiltration
The Pall Centramate (East Hills, NY, USA) filtration unit was used with a polyether sulfone membrane cassette with suspended screened channels (OMEGA 0.093 m2). The nominal molecular weight cutoff (MWCO) was 10 kDa. The pump (Masterflex L/S drive) was fitted with LS25 silicone tubing, and the general tubing arrangement was laid out for batch concentration recycling retentate back to the feed reservoir. The tubing was fitted on the feeding side with a pulse dampener and on the retentate side with a restriction valve and a rotameter. In the feed reservoir, both pH and conductivity were measured. There was no adjustment of the extract pH in the UF process. Recirculation crossflow rate was maintained between 1.1 and 1.3 l min−1. The initial transmembrane pressure (TMP) was approximately 10–12 psi and ended at approximately 15–18 psi. Samples (1 ml) for protein and enzyme analysis were collected throughout the process. The concentration was stopped when the reservoir level was ca. 70 ml; the system holdup volume was approximately 250 ml, thus a total retentate volume of approximately 320 ml.
Diafiltration was carried out by adding distilled water and keeping a constant reservoir volume at approximately 250 ml. After the addition of four diafiltration volumes, the reservoir volume was reduced back to 50–70 ml and retentate was recovered from the system. The cleaning procedure was flushing with distilled water at high flow rate and then a recycle of 0.3 M NaOH for 30–45 min before rinsing with several volumes of distilled water. Water permeability was measured before processing and after cleaning. Storage solution was 0.1 M NaOH.
Freeze Drying
The procedure was carried out on shelves in the vacuum chamber of a Genesis 25EL freeze dryer (Virtis Company, Gardiner, NY, USA) using aluminum trays (20 × 20 cm) to obtain thin layers of ice. The ice condenser temperature was −80 °C and the shelf temperature was adjusted in steps from −15 to 20 °C over 24–30 h.
Analytical Procedures
Samples taken during EBA and UF processing were analyzed for protein and esterase activity. Freeze-dried preparations were, in addition, also analyzed for color, chlorogenic acid (CQA), and total glycoalkaloids (TGA).
Esterase Assay
The method of Bohac (1991) was used to assay patatin esterase activity in fractions and juice extracts. The activity was assayed using 4-nitrophenyl dodecanoate (Sigma) as substrate and the resulting color was detected at 410 nm using a Shimadzu UV2501 spectrophotometer.
Protein Assays
The Biuret assay was employed for the routine analysis of liquid fractions, with BSA (10 mg ml−1) as standard protein. For the analysis of protein in both dried and liquid samples, the total N determination by Dumas combustion method was employed using the FP-528 nitrogen analyzer (Leco Corp., St. Joseph, MI, USA). The calibration standard was glycine, 0.275 g l−1 (0.500% N), for liquid samples and powdered EDTA for dry samples. Analysis was performed in triplicate using approximately 0.200 g of dry powder or 500 μl of liquid. To convert from %N to %protein, a factor of 6.25 was applied.
Electrophoresis
SDS-PAGE analysis of pooled fractions was carried out using 3% stacking and 15% separation gels with the Laemmli buffer system under reducing conditions. Samples were mixed 1:1 with 2X sample buffer, preheated at 95 °C for 5 min, and added 2-mercaptoethanol. Electrophoresis conditions were 200 V and 25 mA/gel. Gels were visualized by staining with Coomassie solution and destained in deionized water in microwave oven (600 W, 10 min).The prestained broad-range molecular mass markers were of sizes 7.6, 21.3, 29.3, 34.7, 48,3, 79, 117, and 206 kDa (BioRad Life Science, Hercules, CA, USA).
Color Measurements
The color of freeze-dried samples was analyzed with a LabScan XE spectrophotometer (Hunter Lab, Reston, VA, USA), applying the CIE L*a*b* scale. Samples were ground and packed evenly into a sample cup to a thickness of ca. 4 mm. Readings from the sample cup were obtained in five different positions, and the measured reflectance values were averaged.
pH and Conductivity Measurements
Values were obtained from a pH/cond 340i portable unit (WTW, Weilheim, Germany).
Moisture Analysis
The dry substance content of potato juice extracts was obtained from a Mark 2 Standard (Omnimark Instrument Corp., Tempe, AZ, USA) moisture analyzer at 125 °C. Stable readings of 4- to 5-ml samples were typically obtained after 30–35 min.
Chlorogenic Acid Analysis
The analysis of CQA was adopted from the spectrophotometric method of Dao and Friedman (1992). Lyophilized protein (100 mg) was boiled in 80% ethanol (20 ml) and refluxed for 4 h. The sample was filtered and the volume readjusted. The clear filtrate was measured at 335 nm, and the CQA content was calculated from a standard curve (10–50 μg ml−1) of pure CQA. Extraction recovery was 65%, calculated from spiking experiments.
Total Glycoalkaloid Analysis
TGA in protein preparations included the two major trisaccharide solanidine glycoalkaloids in potato, α-solanine and α-chaconine. The HPLC method developed by Alt et al. (2005) was employed and analysis was performed by the Leibniz Universität Hannover. A freeze-dried sample (0.5 g) was homogenized and extracted in 5% acetic acid, containing 50 mM Na2SO3, followed by multiple washing steps on SPE cartridges (Oasis HLB, Waters GmbH). The purified sample, eluted in methanol, was injected (20 μl) to a Reprosil-Pur NH2 column (5 μm, 250 × 4, Trantec-Analysentechnik) and separated at 40 °C under isocratic conditions (3:1 CH3CN/20 mM KH2PO4, pH 6) and detected by UV (200 nm). Determination of glycoalkaloids [μg g−1 dry weight; ppm] was performed in triplicate for each sample; SD 3–18 ppm.
Statistical Method
The Mann–Whitney U-test was applied when comparing the sample means from EBA and UF processing. Error range is indicated as standard deviation (±SD). Statistical significance was indicated at a P-value < 0.05.
Results and Discussion
Extraction
Potato juice extract was prepared in the lab with the intention of simulating industrial PFW, with respect to pH, conductivity, enzyme activity, protein content, dry substance, and particulate matter. Ground batches of ca. 8 kg potato yielded ca. 5.5–6.0 l of raw juice where 20% of the volume came from the added sulfite solution. Table 1 shows the characteristics of the lab-made PFW from several preparations. The values are comparable to those obtained from byproduct effluent samples of a starch processing plant collected over several months. Low-speed centrifugation removed the dense starch granules, a processing step comparable to the decanters used in milling plants. Though the final potato juice extract appeared to have more particulate matter than the industrial effluent, this difference was regarded to have no effect on the column performance. Column expansion is caused mainly by the suspended solids in PFW, not due to viscosity, as the effluent is a dilute liquid (Strætkvern et al. 1999). Thus, the degree of resin expansion will reflect the content of suspended solids. The model PFW, on average, caused a bed expansion of 2.6 at 560 cm h−1 in the 5-cm column. Using industrial potato water effluent, expansion between 2.0 and 2.7 at 400–750 cm h−1 was obtained on the same resin (Strætkvern et al. 2005).
EBA Processing
Several EBA column trials were performed with the model juice adjusted to pH 4.5, using volumes of 1.0 to 3.2 l of juice, corresponding to feed loads between 3.2 and 7.6 times the settled resin volume. Protein yield remained approximately the same for loads 4.7–7.6 CV. In Fig. 2a, a high-load chromatographic profile shows the feed step and column wash, followed by a protein desorption through a step increase of pH. It is observed from the pH profile that the column is not fully equilibrated to pH 4.5 but attains this value on loading of the PFW. Column regeneration was obtained with a pulse (0.1–0.2 CV) of 1 M NaOH and several volumes of tap water for re-equilibration. Not shown in the diagram is the fraction of dark-colored material of plant pigments eluting at high pH at approximately 5–5.5 l liquid volume, indicating that a large amount of plant pigments was captured by the column and could be separated from the desired protein fraction. The eluted protein fractions were pooled and prepared for freeze drying.
a Expanded bed chromatography of 2.4 l PFW (7.6× resin volume) applied to a 5-cm EBA column containing high-density MiMo resin (315 ml). Diagram shows profiles of protein (closed symbols) and patatin esterase activity (open symbols). The broken lines are recordings of outlet pH (long dash) and bed expansion (short dash). The arrows indicate the onset of feed load (0), column wash pH 4.5 (2.4), elution pH 12 (3.3), CIP (4.4), and tap water re-equilibration (4.6 l). b Breakthrough profiles (C/C 0) from column runs using various feed load ratios (× settled resin volume, CV) of PFW applied to a 5-cm EBA column containing high-density MiMo resin (315 ml). Curves are protein (solid symbols) and esterase activity (open symbols). Volume axis is normalized to the expanded bed volume
The frontal analysis of the loading step is shown in Fig. 2b. The axes are displayed in relative units: the fraction of breakthrough of feed concentration (C/C 0) versus the normalized residence time on the expanded column, i.e., expanded column volumes (expCV). There is a significant breakthrough of non-retained protein as seen by the curve rising sharply after one column volume (expCV = 1). After the mass transfer zone eclipsing at C/C 0 = 0.85, the protein curve approaches unity after the passage of four column volumes. The breakthrough of patatin esterase activity is initially very low but then it increases constantly, ending at C/C 0 = 0.5 for the highest load run.
The simultaneous increase of breakthrough curves for protein and esterase suggests an adsorption process of loosely bound patatin in a dynamic equilibrium with the mobile phase (Yang and Etzel 2003). The protein in the breakthrough is mostly patatin as seen from SDS-PAGE analysis (Fig. 4, lane 2). The unbound patatin may be in the form of aggregated protein since the captured fraction also contains a high proportion of patatin (lane 3). Steric hindrance at the resin surface and in the pores may allow only soluble small- to medium-sized proteins to bind. Larger proteins as well as aggregates which require multipoint interaction with the ligand are thus excluded (Bolivar et al. 2010). Increasing the sample residence time by increasing the total resin volume from 315 to 510 ml did not alter the breakthrough curves (results not shown) or affect the protein yield significantly.
Ultrafiltration Processing
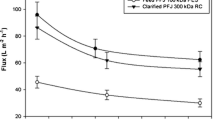
Ultrafiltration was performed without prior pH adjustment of the feed. Feed flow rate and pressure conditions were chosen to give measurable permeate flow. Figure 3 shows an experimental run of the UF/diafiltration process, and the calculated concentration and retention values are given in Table 2. Typically, in all trials on the UF unit, the flux gradually diminished as the TMP was building up due to increased solids content in retentate. Increasing recirculation flow rate only improved the flux temporarily.
Batch concentration and diafiltration of 2.4 l PFW using ultrafiltration with 10-kDa MWCO. Data points indicate the effect of volume reduction on the concentration of protein (closed circles), esterase activity (open circles), retentate volume (open squares), and change in permeate flux (small dots). Onset of diafiltration with distilled water was at 118 min
The average volume concentration factor was 5.5-fold and was achieved in 60–90 min of filtration, with a paralleled increase in enzyme activity (CF = 5.9). This is in agreement with the patatin protein (monomeric form 41 kDa/dimeric ca. 80 kDa) being efficiently retained (R app = 0.99). The retained bulk protein reached only 3.2-fold concentration, with a corresponding lower rejection coefficient (R app = 0.84). Throughout the concentration step, a steady level (3–6 g/l) of proteinaceous material was measured in the permeate fraction; however, no distinct band was visible in SDS-PAGE analysis (Fig. 4, lane 7). Thus, this material most likely consisted of low molecular weight peptides below the sieving limit of the 15% gel.
Coomassie-stained SDS-PAGE (15%) analysis of fractions from EBA and UF processing steps. Lane 1–4, EBA: 1, crude PFW; 2, flow through; 3, eluate; 4, regeneration. Lane 5, empty. Lane 6–9, UF: 6, crude PFW; 7, permeate; 8, retentate 10× dil.; 9, retentate 5× dil.; 10, molecular weight markers (BioRad Broad Range)
The amount of pigment removed by the filtration was moderate over the course of the experiment, though salts passed freely as indicated by a stable conductivity level both in the retentate and permeate. In theory, the 10-kDa membrane should allow low molecular species like pigments to pass through. The retention of pigment may be caused by the extensive concentration polarization of protein, as observed also by Zwijnenberg et al. (2002). In order to remove more of the pigments, batch concentration was followed with a constant volume diafiltration (Fig. 3). The retentate was re-diluted and washed with ca. four volumes of water before concentrating it back to a minimal volume (200–300 ml).
Only a slight depletion of pigment was observed during diafiltration, suggesting that a membrane deposit of solids ensued. Additives can assist in resolving secondary layers reducing membrane transport. In a filtration process for canola proteins, additions of NaCl (0.05 M) and SDS (0.1%) were effective in removing polyphenolic substances to obtain a product with more brightness (Xu and Diosady 2002). Diafiltration before the concentration step was another approach used with a membrane process for upgrading jojoba protein extract, to remove low molecular weight material such as sugars, non-protein nitrogen, and toxicants (Nabetani et al. 1995). In a study by Stevens and Davelaar (1996), black spot compounds in bruised potato tubers were shown to be of at least 70 kDa, possibly as a complex of pigment and protein. If present here, the pigment is likely to be retained.
Comparing Performance of Unit Operations
The process yields of protein (total N) and esterase activity which are calculated from the amounts captured or retained from the initial feed are summarized in Table 3. The yields on protein from the two methods are slightly better for the EBA method (54%) than for UF (48%), but the difference is not significant. Patatin makes up the major protein in both end products as evaluated by the high process yields of esterase activity at 80% and 91%, respectively. Again, the difference is not significant.
Comparing product yield, i.e., the amount of protein isolated per volume PFW processed, the UF method performed significantly better (3.1 g l−1) compared to the column adsorption process (1.8). Assayed by esterase activity, the UF method (3.17 kU l−1) is also significantly better than EBA (1.21). The yield differences are predictable since resin binding is a process limited by mass transfer governed by the dynamic binding capacity of the column, while the membrane retention is more efficient in containing material. Based on the amount of dried product, however, the two methods did not differ significantly, and the higher values of dry substance are also in agreement with the protein content (Table 4).
The productivity measure for either processing method can be expressed as the mass of protein or esterase activity recovered per hour. In Table 3, the process time basis is the batch cycle time excluding the cleaning and diafiltration steps. Protein productivity is slightly higher with EBA processing, but the difference is not significant. UF productivity is fivefold more efficient based on esterase activity. Again, the dynamic adsorption process during loading in EBA could explain the reduced efficiency for patatin as demonstrated by the significant breakthrough of both protein and esterase (Fig. 2a).
The two preparations have the same composition of the major proteins according to SDS-PAGE analysis (Fig. 4). While the UF preparations show a general concentration effect on all major proteins, the EBA adsorption step appears to have enriched the patatin fraction as seen by the broader band between 35- and 48-kDa markers. A highly significant enrichment of patatin is also given by the specific esterase activity (Table 3). The activity approximately doubled compared to the crude juice (0.36 U mg−1; see Table 1), whereas after UF the mean specific activity remained the same. The selectivity for patatin on the MiMo type of resin over the other potato proteins most likely is due to the hydrophobic bonding to the ligand (Strætkvern et al. 1999).
A higher content of patatin can be favorable for protein functionality, like foaming (Ralet and Guéguen 2000; van Koningsveld et al. 2006), as well as for nutritive value (Bartova and Barta 2009). Functional properties were not included in this study, but previous reports have documented the technical functionality of EBA-recovered protein (Claussen et al. 2007; Løkra et al. 2008; Løkra et al. 2009). Good functionality of native protein from UF recovery has also been reported (Wojnowska et al. 1981; Zwijnenberg et al. 2002).
Comparing Product Quality After Drying
The most striking difference between the preparations obtained with the two methods was the product color as the EBA preparation appeared considerably brighter than the UF preparation. The Hunter brightness (L*) parameter quantified the difference; EBA-purified protein had a mean value of 73.8, whereas the UF preparation was down to 41.3 (Fig. 5). In the chromatographic procedure, the separation is promoted by the colored pigments eluting at a slower mobility than the protein itself (Strætkvern et al. 2002). On the redness (a*) and yellowness (b*) scale, there was no significant difference in color hue. The UF preparations contained two and a half times as much CQA than the EBA preparation (Table 4), another indication of the membrane retaining pigmented substances.
A modified and optimized ultrafiltration process may have provided a better separation of color; however, due to the complexity of PFW, there is a severe tendency to develop a dynamic secondary membrane with this feedstock. Previous reports on UF of potato juice and industrial effluent give no definitive answer to the choice of MWCO since membranes from 6,000 Da (Wojnowska et al. 1981) up to 150 kDa (Zwijnenberg et al. 2002) have retained the bulk protein efficiently.
Colored pigments in potato and fruits commonly arise from the oxidation of CQA, promoted by the enzymatic action of polyphenol oxidase. The measured brightness of the dried preparations correlated negatively (R 2 = 0.871) to the extracted CQA (Fig. 6). The methods differed significantly both in color (p < 0.01) and in CQA (p < 0.05). Samples from each processing method were grouped as of high CQA/low brightness (UF) and low CQA/high brightness (EBA). The low content of CQA was associated with high brightness value also in EBA potato protein obtained under various separation conditions (Løkra et al. 2009). Although depleting the substrate is one measure to control color development, inactivating the oxidase itself may be important as well. Potato peroxidases may also be responsible for polymeric products from CQA, but this reaction proceeds via the radical formation of o-quinones to dimeric variants in the presence of H2O2 (Osman et al. 2009).
Looking at the chemical composition (Table 4), the preparations have essentially the same protein content (75%), although the EBA protein value is 6–10% lower than for similar preparations reported by Løkra et al. (2008), assayed by micro-Kjeldahl. Glycoalkaloids and chlorogenic acid are major potato phytochemicals that can compromise the protein quality through toxicity and color instability. The current EU recommendation on TGA content in potato protein for food is limited to 150 ppm (Byrne 2002). Protein preparations obtained in this study were on average above this limit of TGA, but the difference between processing methods EBA (286 ppm) and UF (213 ppm) appeared not to be significant. However, TGA below 150 ppm in EBA-processed protein has been reported (Løkra et al. 2008).
Conclusions
The production of a refined, native protein preparation from PFW is possible both by EBA and UF processing. The chemical composition of the dried protein showed that, although the same proteins are present, the EBA method resulted in an enrichment of the patatin esterase fraction which may offer advantages for functionality. On process productivity, the methods performed similarly on protein, but UF is fivefold more efficient for the patatin esterase activity due to the higher retention. Ultrafiltration, in general, is simpler in operation, but the anticipated color separation is greatly reduced when the membrane was exposed to crude PFW. The two processing methods tested differ significantly in obtaining an attractive product color; for this purpose, EBA is better in separating pigment and protein due to the chromatographic separation capability. Interestingly, TGA content appears unaffected by the processing method. Although depleted to a moderate level, the glycoalkaloids are easily retained with the protein fraction. Thus, knowledge on controlling TGA is crucial for the production of native protein from potato starch by-streams.
Abbreviations
- C :
-
Concentration outlet
- C 0 :
-
Concentration inlet
- CF:
-
Concentration factor
- CV:
-
Column volume of settled resin
- CQA:
-
Chlorogenic acid
- EAAI:
-
Essential amino acid index
- EBA:
-
Expanded bed adsorption
- expCV:
-
Column volume of the expanded resin
- L*:
-
Brightness
- MiMo:
-
Mixed mode
- MWCO:
-
Molecular weight cutoff
- PFW:
-
Potato fruit water
- PI:
-
Protease inhibitor
- R app :
-
Apparent rejection coefficient
- RO:
-
Reverse osmosis
- TGA:
-
Total glycoalkaloids
- TMP:
-
Transmembrane pressure
- UF:
-
Ultrafiltration
References
Alt, V., Steinhof, R., Lotz, M., Ulber, R., Kasper, C., & Scheper, T. (2005). Optimization of glycoalkaloid analysis for use in industrial potato fruit juice downstreaming. Engineering in Life Sciences, 5(6), 562–567.
Andersson, J., Sahoo, D., & Mattiasson, B. (2008). Isolation of potato proteins using simulated moving bed technology. Biotechnology and Bioengineering, 101(6), 1256–1263.
Bartova, V., & Barta, J. (2009). Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride. Journal of Agricultural and Food Chemistry, 57(19), 9028–9034.
Bidinger, S. C. (1992). Wertstoffgewinnung aus Abwässern/Recovery of valuable substances from waste water. Internationale Zeitschrift für Lebensmittel-Technologie (ZFL), 43(5), 222–228.
Bohac, J. R. (1991). A modified method to purify patatin from potato-tubers. Journal of Agricultural and Food Chemistry, 39(8), 1411–1415.
Bolivar, J. M., Batalla, P., Mateo, C., Carrascosa, A. V., Pessela, B. C., & Guisána, J. M. (2010). Selective adsorption of small proteins on large-pore anion exchangers coated with medium size proteins. Colloids and Surfaces, B: Biointerfaces, 78, 140–145.
Byrne, D. (2002). Commission decision of 15 February 2002 authorizing the placing on the market of coagulated potato proteins and hydrolysates thereof as novel food ingredients under Regulation (EC) No 258/97 of the European Parliament and of the Council. Official Journal of the European Communities, L, 50, 92–93.
Claussen, I.C., Strømmen, I., Egelandsdal, B., Strætkvern, K.O. (2007). Effects of drying methods on functionality of a native potato protein concentrate drying technology, 25(6), 1091–1098.
D’Alvise, N., Lesueur-Lambert, C., Fertin, B., Dhulster, P., & Guillochon, D. (2000). Removal of polyphenols and recovery of proteins from alfalfa white protein concentrate by ultrafiltration and adsorbent resin separations. Separation Science and Technology, 35(15), 2453–2472.
Dao, L., & Friedman, M. (1992). Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. Journal of Agricultural and Food Chemistry, 40(11), 2152–2156.
Eriksson, G., & Sivik, B. (1976). Ultrafiltration of potato process water—influence of processing variables. Potato Research, 19, 279–287.
Giuseppin, M.L.F., van der Sluis, C., Laus, M.C. (2008). Native potato protein isolates. Patent No WO 2008/069650.
Graf, A. M., Steinhof, R., Lotz, M., Tippkotter, N., Kasper, C., Beutel, S., et al. (2009). Downstream-processing with membrane adsorbant on the isolation of native protein fractions from potato amniotic liquid. Chemie Ingenieur Technik, 81(3), 267–274.
Lihme, A., Hansen, M., Olander, M., & Zafirakos, E. (2000). Expanded bed adsorption in the purification of biomolecules. In Desai (Ed.), Methods in biotechnology (pp. 121–141). Totowa: Humana.
Løkra, S., & Strætkvern, K. O. (2009). Industrial proteins from potato juice. A review. Food, 3(Special Issue 1), 88–95.
Løkra, S., Helland, M. H., Claussen, I. C., Strætkvern, K. O., & Egelandsdal, B. (2008). Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT Food Science and Technology, 41(6), 1089–1099.
Løkra, S., Schüller, R. B., Egelandsdal, B., Engebretsen, B., & Strætkvern, K. O. (2009). Comparison of composition, enzyme activity and selected functional properties of potato proteins isolated from potato juice with two different expanded bed resins. LWT Food Science and Technology, 42(4), 906–913.
Nabetani, H., Abbott, T. P., & Kleiman, R. (1995). Optimal separation of jojoba protein using membrane processes. Industrial and Engineering Chemistry Research, 34(5), 1779–1788.
Oosten, B. J. (1976). Ultrafiltration of potato juice results in high yield of protein. Starch/Stärke, 28(4), 135–137.
Osman, A., El Agha, A., Makris, D. P., & Kefalas, P. (2009). Chlorogenic acid oxidation by a crude peroxidase preparation: biocatalytic characteristics and oxidation products. Food and Bioprocess Technology. doi:10.1007/s11947-009-0241-8.
Ralet, M. C., & Guéguen, J. (2000). Fractionation of potato proteins: solubility, thermal coagulation and emulsifying properties. LWT Food Science and Technology, 33, 380–387.
Rausch, K. D. (2002). Front end to backpipe: membrane technology in the starch processing industry. Starch/Stärke, 54, 273–284.
Rüffer, H., Kremser, U., & Seekamp, M. (1997). Experiences with reverse osmosis pilot plant for the concentration of potato fruit water in the potato starch industry. Starch/Stärke, 49(9), 354–359.
Stevens, L. H., & Davelaar, E. (1996). Isolation and characterization of blackspot pigments from potato tubers. Phytochemistry, 42(4), 941–947.
Strætkvern, K. O., Schwarz, J. G., Wiesenborn, D. P., Zafirakos, E., & Lihme, A. (1999). Expanded bed adsorption for recovery of patatin from crude potato juice. Bioseparation, 7(6), 333–345.
Strætkvern, K.O., Aae Olander, M., Lihme, A. (2002). EBA processing of potato fruit water on mixed mode adsorbent for functional protein recovery: A difficult separation task made possible. In: DOWNSTREAM—Proceedings 4th International Conference on Expanded Bed Adsorption; EBA 02 St. Petersburg Beach, Florida, USA, pp 51–52. Amersham Biosciences, Uppsala, Sweden
Strætkvern, K. O., Løkra, S., Olander, M. A., & Lihme, A. (2005). Food-grade protein from industrial potato starch effluent recovered by an expanded bed adsorption process. Journal of Biotechnology, 118, S33–S33.
Tsagaraki, E. V., & Lazarides, H. N. (2010). Fouling analysis and performance of tubular ultrafiltration on pretreated olive mill waste water. Food and Bioprocess Technology. doi:10.1007/s11947-010-0326-4.
van Koningsveld, G. A., Walstra, P., Voragen, A. G. J., Kuijpers, I. J., Van Boekel, M., & Gruppen, H. (2006). Effects of protein composition and enzymatic activity on formation and properties of potato protein stabilized emulsions. Journal of Agricultural and Food Chemistry, 54(17), 6419–6427.
Wojnowska, I., Poznanski, S., & Bednarski, W. (1981). Processing of potato protein concentrates and their properties. Journal of Food Science, 47, 167–172.
Xu, L., & Diosady, L. L. (2002). Removal of phenolic compounds in the production of high-quality canola protein isolates. Food Research International, 35, 23–30.
Yang, H., & Etzel, M. R. (2003). Evaluation of three kinetic equations in models of protein purification using ion-exchange membranes. Industrial and Engineering Chemistry Research, 42, 890–896.
Zwijnenberg, H. J., Kemperman, A. J. B., Boerrigter, M. E., Lotz, M., Dijksterhuis, J. F., Poulsen, P. E., et al. (2002). Native protein recovery from potato fruit juice by ultrafiltration. Desalination, 144, 331–334.
Acknowledgements
We are grateful for the technical assistance of Mr. Matthew Whittiker, UMES food science technician, on potato juice extraction and various analytical procedures and of Mrs. Else Berit Stenseth, HUC senior lab technician, for performing the CQA analysis. Dr. Arne Linløkken, HUC, is acknowledged for the valuable advice on statistical analysis. We are especially indebted to Dr. Sacha Beutel, at the Institut für Technische Chemie, Gottfried Wilhelm Leibniz Universität Hannover, for providing the HPLC analysis of glycoalkaloids.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strætkvern, K.O., Schwarz, J.G. Recovery of Native Potato Protein Comparing Expanded Bed Adsorption and Ultrafiltration. Food Bioprocess Technol 5, 1939–1949 (2012). https://doi.org/10.1007/s11947-010-0494-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0494-2