Abstract
Ultrafiltration was applied to diluted potato fruit juice, a side-stream from potato starch production. The aim of the study was to selectively concentrate the potato proteins in the permeate, while isolating polyphenol oxidase (PPO) in the retentate. A profound difference was found in protein retention between two 300-kDa molecular weight cutoff (MWCO) ultrafiltration membranes, of either regenerated cellulose (RC) or polyethersulfone (PES). The use of the 300-kDa MWCO RC membrane resulted in a twofold higher retentate protein content as well as total retention of all PPO activity, as compared with the PES membrane. Comparison tests with 100- and 300-kDa MWCO PES membranes indicated that concentration polarization and gel layer formation, and not MWCO definitions, were governing factors for protein retention, since proteins with a MW of 10 kDa were retained in all the experiments. PPO activity in potato fruit juice was measured in permeate and retentate to assess its selective retention by the applied ultrafiltration processes. Of the specific PPO activity, 94–100 % was retained by either 300 MWCO RC or 100 MWCO PES, while only 49 % specific activity was retained by the 300 MWCO PES. By in situ blotting experiments, the molecular weight of active PPO was found to be present at three different molecular weights, at positions of 40, 47, and 100 kDa, respectively, with the major activity present at 47 kDa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato fruit juice (PFJ) is produced in large amounts as a side-stream during industrial production of potato starch. Typically, PFJ contains 2–5 % solids; of which, 35 % is crude protein (N-containing substances) (Knorr et al. 1977), which is in fine accordance with a total true protein content of PFJ of 1.8 % (w/w) (Zwijnenberg et al. 2002). The proteins in PFJ can roughly be divided into three groups. The first group is the patatins, representing up to 40 % of the total protein. Patatins are 39–43 kDa glycoproteins (existing as 80 kDa non-covalent dimers) with pI values of 4.45 to 5.17 and varying glycosylation patterns (Barta et al. 2012). The second group of PFJ proteins is the protease inhibitors (PI), which constitutes up to 50 % of the total protein and is divided into seven sub-groups (Pouvreau et al. 2001). The molecular weight of the PI family varies widely, from 4.3 to 20.6 kDa, with pI values of 5.1–9.0 (Pouvreau et al. 2001). The third group is mainly composed of oxidative and other enzymes, like polyphenol oxidase (PPO), lipoxygenase, and enzymes associated with starch synthesis (Jorgensen et al. 2011).
Potato PPO has a molecular weight of 40–69 kDa (Hunt et al. 1993; Marri et al. 2003; Eidhin et al. 2010) and a reported native multimeric structure with a mass of 340 kDa (Marri et al. 2003). The physiological role of PPO is related to plant defense, e.g., wounding of plant tissue induces increases in PPO activity (Thipyapong et al. 1995). PPO catalyzes the hydroxylation of mono-phenols to o-diphenols and oxidation of o-diphenols to o-quinones (Ramírez et al. 2003). Maximum activity for potato PPO is found at pH 6–6.5 at 30 °C (Eidhin et al. 2010). PPO enzyme acting on the phenolic compound, chlorogenic acid, as found in potatoes is responsible for the well-known brown color formation (Narvaez-Cuenca et al. 2013). Possible subsequent reaction products from this reaction can result in covalent- and non-covalent interactions with other potato proteins, causing the final potato products, e.g., potato protein powder, to have not only a brown hue but also poorer solubility, digestibility, and content of essential amino acids (Prigent et al. 2007; Rawel et al. 2001), all leading to poorer quality. Therefore, there is a great interest in avoiding or inhibiting PPO activity during refinement of PFJ into protein products. PPO can be inhibited by heat treatment (80 °C for 15 min), low pH (pH <3.5), or by addition of antioxidants or inhibitors, like ascorbic acid or sodium metabisulfite (Eidhin et al. 2010). Acid or heat treatments are however not advisable or applicable in relation to protein extraction for food applications, due to denaturation and precipitation of some of the potato protein sub-fractions (Straetkvern and Schwarz 2012).
Potato proteins have been purified from PFJ by a vast number of techniques. In laboratory scale, this includes precipitation with acids (hydrochloric acid, sulfuric acid, acetic acid, citric acid), organic solvents (methanol, ethanol, 2-propanol, acetone), metal salts (FeSO4, FeCl3, ZnCl2) (Barta et al. 2008), separation by ion exchange (van Koningsveld et al. 2001), affinity or hydrophobic chromatography (Racusen 1989), expanded bed adsorption, or by ultrafiltration (UF) (Straetkvern and Schwarz 2012). UF is a powerful technique for protein concentration and fractionation; problems however exist with membrane fouling due to plant fibers and proteins forming a layer on the surface or constricting the pores of the UF membranes (Eriksson and Sivik 1976; Zwijnenberg et al. 2002; Haberkamp et al. 2008). The method of purification and the conditions used will affect the functionality of the final powder, e.g., expanded bed adsorption will result in native protein with high solubility compared to that of acid-precipitated proteins, being denatured (Lokra et al. 2008).
The aim of the present study was to understand the governing factors responsible for retention of proteins from potato fruit juice during ultrafiltration. Specific focus was on testing different membrane types and molecular weight cutoff (MWCO), 100 and 300 kDa, applied to two differently processed industrial potato fruit juices. The hypothesis was that a 100-kDa filter would selectively retain the majority of the 340-kDa PPO, while permitting passage of the protease inhibitor fraction with molecular weight <21 kDa or even letting the combined fraction of patatin plus protease inhibitors with molecular weight <80 kDa pass.
Materials and Methods
Materials
Two different kinds of PFJ were obtained from a local starch-processing plant (KMC, Karup, Denmark), a feed liquid containing residual fiber and cell wall material and a clarified fraction obtained after filtration on a ceramic filter. Both PFJs were from the same year of production campaign, but from different batches. The visual appearance of both PFJs was a brownish color with the feed PFJ being more turbid. The samples were kept at −18 °C until use (max 8 months). The PFJs were centrifuged at 3945g for 10 min at 4 °C in a Heraeus Multifuge 3 s-r (Kendro, Osterode, Germany) and mixed with an equal volume of 20 mM Bis-Tris buffer, pH 6.0.
Ultrafiltration Equipment
Three different ultrafiltration membranes (Millipore Pellicon XL 50) were tested: (1) 100-kDa MWCO Biomax® polyethersulfone (PES) (Millipore, Jaffrey, USA), (2) the same type as (1) but with MWCO of 300 kDa, and (3) 300-kDa Ultracel® regenerated cellulose (RC) (Millipore, Jaffrey, USA). The three membranes had a water cross flow at a pressure drop of 138 kPa at 50–80, 60–100, and 40–70 ml/min, respectively, as reported by the manufacturer. Two different systems were used for the experiments: a small-scale system with a 100-ml reservoir equipped with a peristaltic pump, operated to achieve a pressure drop over the membrane of 103.5 kPa and a transmembrane pressure of 134.5–138 kPa. This system was used for testing all the three membrane types and both types of the PFJ. The resulting fractions of permeate and retentate were used for protein determinations, PPO in situ blotting assay, and SDS-PAGE analysis. The second system was a commercial Millipore 29751 Labscale TFF System with 500-ml reservoir (Millipore, Jaffrey, USA), operated at the recommended pressure drop of 138 kPa and transmembrane pressure of 138 kPa. This system was used for reconfirmation of the findings with clarified juice tested with small system. The resulting fractions were used for protein determination and SDS-PAGE.
Calculation of membrane flux (J) (l m−2 h−1) was carried out by Eq. 1.
where Qp is permeate flow rate (ml min−1) and Area refers to the cross flow area (m2) of the membrane. Transmembrane pressure (TMP) was calculated by Eq. 2.
Protein Content
The bicinchoninic acid assay (BCA, Thermo Scientific™ Pierce™), with bovine serum albumin (2 mg/ml) as reference protein, was used for protein determination (Smith et al. 1985). Measurements were conducted in triplicates.
SDS-PAGE Analysis
SDS-PAGE using Criterion™ TGX™ 8–16 % precast gels (Bio-Rad, Richmond, CA, USA) was performed according to (Laemmli 1970). Samples were mixed 1:1 with sample buffer (20 mM Tris, 2 % SDS, 20 % glycerol, pyronin Y) and reduced with 1/10 vol 0.2 M dithioerythritol (DTE) and boiled for 3 min; 30-μl sample of 2 mg/ml were loaded onto the gel. Samples with a higher protein concentration than 2 mg/ml were diluted prior to loading. Gels were stained with Coomassie Brilliant Blue G-250. Molecular mass was estimated by a prestained broad range molecular weight marker (Thermo Scientific™ Spectra™ Multicolor Broad Range Protein Ladders).
PPO Activity Assay
A combination of the methods by Cheng et al. (2007) and Eidhin et al. (2010) was used to analyze activity of PPO. Activity was assayed in microtiter plates with 200-μl substrate (50 mM 4-methylcatechol in 0.1 M phosphate buffer pH 6.5) and 50-μl enzyme solution. The reaction was carried out at room temperature, and an increase in absorbance at 420 nm was measured for 30 s in a Synergy 2 Microplate reader (BioTek Instruments Inc, Winooski, VT 05404, USA). One unit of activity was defined as the change in absorbance of 0.001/min/ml of enzyme solution. Specific activity was calculated by dividing the number in units with the protein concentration in milligram per milliliter. Measurements were conducted in triplicates.
PPO Blotting
In situ blotting of PPO activity was conducted essentially as described by Cheng et al. (2007). Briefly, samples were separated by SDS-PAGE as described in the above protocol, but without the addition of DTE and with lower heating (T < 50 °C). A blotting paper was prepared by soaking Whatman® 40 filter paper (Whatman, Maidstone, England) in a 10 % 4-methyl catechol solution following drying for 5 min at 37 °C. SDS-PAGE analysis was performed, and the blotting paper was pressed onto the surface of the gel, without allowing any air bubbles to be present. After a few minutes, color development occurred and a picture was taken by a digital camera. The gel was finally stained with Coomassie Brilliant Blue G-250 to visualize protein bands present in the analyzed lanes.
Statistics
Origin 2015 (OriginLab, Northampton, USA) was used in the statistical analysis. One-way ANOVA was performed, and means were compared in pairs to test for significant differences at the P value of 0.05 by a Tukey test.
Results and Discussion
Ultrafiltration of Industrially Processed Potato Fruit Juices
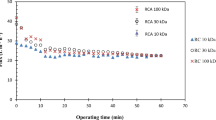
An initial 15-min stabilization of flux was performed for all the experiments before filtration was conducted, and samples were drawn from retentate and permeate. Based on it, it can be observed that the flux is decreasing over time due to formation of a secondary gel layer and blockage of membrane pores (fouling). The flux of the feed PFJ is lower than that for the clarified PFJ when using the same 100-kDa PES membrane due to the presence of various fibers and cell wall material that quickly attach to the membrane (Fig. 1). The higher protein content of the feed PFJ (15.85 mg/ml) compared to the clarified PFJ (5.79 mg/ml) may also contribute to the observed lower flux, since an increase in the protein concentration has a limiting effect on the flux (Darnon et al. 2002). The 300-kDa RC membrane has a lower flux than the 100-kDa PES, but a similar profile over time.
Example of development in flux during the gel polarization phase with 100-kDa MWCO PES membrane (closed circles) and 300-kDa RC membrane (closed triangles) with clarified PFJ (protein 5.79 mg/ml) and 100-kDa PES (open circles) with crude feed PFJ (protein 15.85 mg/ml) according to Table 1
Using clarified PFJ, the permeability of membranes was tested (Fig. 2). The 300-kDa RC and 100-kDa PES show almost similar profiles, while the 300-kDa PES displays a tenfold higher flux at the TMP used at the given concentration conditions of the samples at 134.5–138 kPa, indicating either a more open structure of the 300-kDa PES membrane and/or less fouling. The fluxes increased in parallel to the water cross flow reported by the manufacturer; however, the difference in flux between the 300- and the 100-kDa PES appeared larger when applying clarified potato fruit juice. The 100-kDa PES was also tested with feed PFJ, resulting in roughly 2.5-fold reduction in the observed flux, when compared to the clarified PFJ (Fig. 2).
Illustration of flux development as a function of transmembrane pressure (TMP) for the three membrane types: 300-kDa PES (closed triangles), 100-kDa PES (closed circles), and 300-kDa RC (open circles) as tested on clarified PFJ or 100-kDa PES (open triangles) tested with feed PFJ. The pressure drop was 103.4 kPa
This difference in performance between membranes types was also evident when comparing the protein content of retentate and permeate of the clarified PFJ (Table 1). The 300-kDa PES had a twofold lower protein content in the retentate compared with the 100-kDa PES and 300-kDa RC membranes, again indicating a more open structure, which will result in less membrane fouling.
The UF fractions were tested by SDS-PAGE to visualize membrane retention of proteins of different sizes. Feed PFJ was tested with the 100- and 300-kDa PES membranes. Both membranes resulted in high retention of proteins in the retentate as seen in Table 1 and Fig. 3a. The 300-kDa membrane had a more open structure as indicated by denser bands and the presence of a 40-kDa band in the permeate (lane 4) than the 100-kDa permeate (lane 2).
SDS-PAGE of ultrafiltration fractions of feed and clarified PFJ by the small-scale 100-ml system. a Lane 1, feed PFJ; lane 2, permeate 100-kDa PES; lane 3, retentate 100-kDa PES; lane 4, permeate 300-kDa PES; and lane 5, retentate 300-kDa PES. b Lane 1, clarified PFJ; lane 2, permeate 100-kDa PES; lane 3, retentate 100-kDa PES; lane 4, permeate 300-kDa PES; lane 5, retentate 300-kDa PES; lane 6, permeate 300-kDa RC; and lane 7, retentate 300-kDa RC
For the clarified PFJ (Fig. 3b), the 100-kDa PES had a low amount of protein with a MW below 25 kDa in the permeate (lane 2) and high retention of proteins from 10–100 kDa in the retentate (lane 3). The 300-kDa PES had no apparent difference in the specific proteins between permeate (lane 4) and retentate (lane 5) as proteins from 10–100 kDa was found in both fractions. The 300-kDa RC membrane permitted passage of proteins below 40 kDa in the permeate (lane 6) and proteins of all sizes in retentate (lane 7).
The 300-kDa PES membrane showed much higher retention of proteins when using feed PFJ compared to clarified PFJ. This is probably related to enhanced degree of membrane fouling due to the feed PFJ’s content of fiber and cell wall material.
PPO Activity of Separated Fractions
PPO activity was measured in the different fractions to analyze the retention of this specific enzyme (Table 1). For feed PFJ, 99 % of the specific activity was found in retentate for the 100-kDa PES whereas 97 % of the specific activity was found in the retentate of the 300-kDa membrane. The specific PPO activity was much higher in the fractions from the feed PFJ than in those from the clarified PFJ, indicating that the clarification process could lower the content of PPO. It must be noted, however, that the two PFJs were not from the same processing day, and therefore, batch to batch variation could be expected, as has been observed by the industry (KMC, personal communication). In clarified PFJ for the 300-kDa RC and 100-kDa PES membranes, PPO was highly retained, and 94–100 % of the specific PPO activity was found in the retentate fraction. The 300-kDa PES membrane showed PPO activities in both the permeate and retentate with a distribution of specific activity of approximately 50/50.
The three membranes types were also tested with the Millipore 29751 Labscale TFF system to see how a larger system operated at a different pressure would affect the results. The protein content was higher in the different fractions, but the performance of the different membranes was generally observed in the small-scale experiment, i.e., the 100-kDa PES and 300-kDa RC membranes had a high retention of material in the retentate, while the 300-kDa PES membrane had a lower protein retention (Table 2) and SDS-PAGE gel (Fig. 4).
The nominal cutoff values of UF filters were found to have little influence in processing of potato fruit juice since proteins with a MW down to 10 kDa were found in the retentate in all the experiments, indicating that the secondary membrane layer made of proteins and cell wall material is a governing factor for actual retention. Differences were, however, found between the membranes used in this study with a 300-kDa PES being more permeable than a 100-kDa PES and the 300-kDa PES being more permeable than the 300-kDa RC membrane. Zwijnenberg et al. (2002) reported negligible differences when concentrating PFJ by ultrafiltration between PES, RC, and polyvinylidenefluoride membranes with MWCOs of 5 to 150 kDa. Zwijnenberg et al. (2002) suggested that the high protein content of 18 mg/ml in the PFJ could be the reason for fast buildup of material on the membrane surface and that this layer was responsible for the actual separation process.
Reduction in flux or selectivity during ultrafiltration is a well-known phenomenon in processing industries and is a complex mechanism governed by many factors. The reduction can be ascribed to reversible concentration polarization or irreversible fouling. During concentration polarization, solutes are concentrated in a boundary layer on the retentate side of the membrane, causing an increase in the osmotic pressure difference and a resulting lower driving force for transport over the membrane (Bacchin et al. 2006). Fouling can be ascribed to three different mechanisms: (i) adsorption of solutes onto the membrane surface due to specific interactions between solutes and membrane; (ii) deposit of multiple layers of material on the membrane surface resulting in a gel or cake layer on the surface; and (iii) pore blockage, where the solutes block the pores of the membrane (Bacchin et al. 2006). A difference in flux decline and fouling mechanism exists between small (<5 nm) and large (>100 nm) macromolecules (Bacchin et al. 2002). Concentration polarization is of prime importance for small macromolecules, where concentration polarization increases with increasing TMP until a critical TMP is reached, and a gel layer is formed over the entire membrane. Concentration polarization is of less importance for large macromolecules; here, buildup of deposited material appears from the outlet to the inlet of the membrane channel when TMP is increased until a cake layer has spread over the entire membrane (Bacchin et al. 2002).
For the clarified PFJ, the observed decrease in flux may be attributed to concentration polarization, adsorption of proteins to the membrane, or pore blockage. Depending on the size of the proteins relative to the membrane pores, small proteins may enter the pores of the membrane and cause pore constriction, whereas large proteins can form a gel layer at the surface—both mechanisms result in decrease flux over the membrane (Haberkamp et al. 2008; Zhang and Ding 2015).
The lower flux of feed PFJ compared to clarified PFJ (Fig. 2) may be ascribed to additional cake formation of the larger particles present in this more crude liquid.
The observed difference between the two different 300-kDa membranes may be attributed a more open pore structure of PES membranes, but also differences in surface characteristics may contribute. Surface roughness as well as electrostatic and hydrophobic forces can affect membrane-protein interactions, e.g., adsorption of proteins to the membrane surface will be enhanced if protein and membrane have opposite charge (Haberkamp et al. 2008). The zeta potential, which gives the electric potential difference across an ionic layer around a charged colloid ion, at pH 7 has been determined for both PES and RC membranes with PES having values of −15.1 (Cheang and Zydney 2003), −6.0, and −10.0 (Kwon et al. 2008) and RC having values of −2.2 (Cheang and Zydney 2003) and −2.9 (Kwon et al. 2008). The lower zeta potential of the PES membranes has been associated with an electrostatic exclusion of negatively charged proteins during ultrafiltration (Cheang and Zydney 2003). In PFJ with a pH of 6, the patatins are negatively charged. Repulsion between negative patatin and the negative PES may result in a lower degree of gel formation and therefore higher permeability through the membrane. Further detailed studies at different pH and conductivity levels are, however, needed to establish the role of surface charge and protein retention with PFJ as source material.
Visualization of PPO in PFJ by In Situ Blotting
It was not possible to selectively retain PPO in the retentate by using UF as a method to isolate this enzyme, which for reasons of solubility and browning is unwanted in potato protein products as described earlier.
Based on the results from the 300-kDa PES membrane, where PPO activity was found in both permeate and retentate, it is assumed that the active enzyme has a lower molecular weight than 340 kDa or alternatively that high shear during filtration disrupts the tertiary and quaternary structure (Schneider et al. 2007; Ashton et al. 2009). By running an SDS-PAGE gel without prior reduction of the samples, it was possible to visualize the active enzyme in the gel at distinct bands of 40 and 47 kDa and with more faint bands from 50 to 100 kDa (Fig. 5). The 40-kDa band is in agreement with data from (Eidhin et al. 2010), who purified PPO from fresh potatoes of Irish origin. Two different temperatures (20 and 50 °C) were used during sample preparation for the SDS “boiling,” as higher temperature may lead to increased interactions between SDS and proteins and hence different separation in the gel. It was observed, however, that the position of PPO was not affected by the applied temperatures. Heating above 50 °C may denature PPO and thus limit enzyme activity and blotting intensity (results not shown).
The presence of multiple PPO bands could be due to a.o. heterogeneity of PFJ, as it is derived from potatoes of different varieties. In the case of patatin, it has been showed to differ in pI and molecular weight between varieties (Barta et al. 2012). In addition to this, Ramírez et al. (2003) find PPO at multiple locations after gel electrophoresis and explain this by enzymatic browning reactions, resulting in modifications of PPO at the ɛ-amino group of its lysyl residues with ο-benzoquinones. The used potato fruit juices had been exposed to enzymatic browning by PPO based on their visual appearance. Therefore, modifications of the potato proteins by binding of phenolic compounds or cross linking to other proteins are likely to have taken place during processing.
Conclusion
In the current study, two diluted potato fruit juice fractions, a crude feed PFJ and a clarified PFJ, were subjected to ultrafiltration by two different types of membranes with MWCO of 100 and 300 kDa, respectively. The 300-kDa PES membrane was significantly more permeable for potato proteins including PPO compared with 300-kDa RC and 100-kDa PES membranes when testing clarified PFJ. In contrast, feed PFJ resulted in high protein retention with only slight differences in passage of PPO by testing the 100- and 300-kDa PES membranes. This observed difference in performance between feed and clarified PFJ was ascribed to the presence of fibers and other cell wall material in feed PFJ, thereby causing increased fouling. Retention and passage of the potato proteins was observed not to be directly related to the given MWCO of the membranes. It is suggested that constriction of pores by small proteins and formation of a gel layer of large proteins were mainly responsible for the actual separation.
In conclusion, it was not possible to selectively retain the unwanted enzyme PPO in the retentate and simultaneously let either the PI fraction with MW <21 kDa or the combination of patatin plus PI fraction with MW <80 kDa pass to the permeate. This could be due to a general fouling of the membrane or the fact that PPO was not present as a 340-kDa complex, but rather as monomers with approximate molecular weight of 40–50 kDa.
References
Ashton, L., Dusting, J., Imomoh, E., Balabani, S., & Blanch, E. W. (2009). Shear-induced unfolding of lysozyme monitored in situ. Biophysical Journal, 96(10), 4231–4236.
Bacchin, P., Si-Hassen, D., Starov, V., Clifton, M. J., & Aimar, P. (2002). A unifying model for concentration polarization, gel-layer formation and particle deposition in cross-flow membrane filtration of colloidal suspensions. Chemical Engineering Science, 57(1), 77–91.
Bacchin, P., Aimar, P., & Field, R. W. (2006). Critical and sustainable fluxes: theory, experiments and applications. Journal of Membrane Science, 281(1-2), 42–69.
Barta, J., Hermanova, V., & Divis, J. (2008). Effect of low-molecular additives on precipitation of potato fruit juice proteins under different temperature regimes. Journal of Food Process Engineering, 31(4), 533–547.
Barta, J., Bartova, V., Zdrahal, Z., & Sedo, O. (2012). Cultivar variability of patatin biochemical characteristics: table versus processing potatoes (Solanum tuberosum L.). Journal of Agricultural and Food Chemistry, 60(17), 4369–4378.
Cheang, B., & Zydney, A. L. (2003). Separation of alpha-lactalbumin and beta-lactoglobulin using membrane ultrafiltration. Biotechnology and Bioengineering, 83(2), 201–209.
Cheng, T. M., Huang, P. C., Pan, J. P., Lin, K. Y., & Mao, S. J. (2007). Gel electrophoresis of polyphenol oxidase with instant identification by in situ blotting. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 849(1-2), 331–336.
Darnon, E., Belleville, M. P., & Rios, G. M. (2002). Modeling ultrafiltration of complex biological solutions. AICHE Journal, 48(8), 1727–1736.
Eidhin, D. N., Degn, P., & O’Beirne, D. (2010). Characterization of polyphenol oxidase from rooster potato (Solanum tuberosum cv Rooster). Journal of Food Biochemistry, 34(1), 13–30.
Eriksson, G., & Sivik, B. (1976). Ultrafiltration of potato process water—influence of processing variables. Potato Research, 19(4), 279–287.
Haberkamp, J., Ernst, M., Makdissy, G., Huck, P. M., & Jekel, M. (2008). Protein fouling of ultrafiltration membranes—investigation of several factors relevant for tertiary wastewater treatment. Journal of Environmental Engineering and Science, 7(6), 651–660.
Hunt, M. D., Eannetta, N. T., Yu, H. F., Newman, S. M., & Steffens, J. C. (1993). cDNA cloning and expression of potato polyphenol oxidase. Plant Molecular Biology, 21(1), 59–68.
Jorgensen, M., Stensballe, A., & Welinder, K. G. (2011). Extensive post-translational processing of potato tuber storage proteins and vacuolar targeting. The FEBS Journal, 278(21), 4070–4087.
Knorr, D., Kohler, G. O., & Betschart, A. A. (1977). Potato protein concentrates: the influence of various methods of recovery upon yield, compositional and functional characteristics. Journal of Food Processing and Preservation, 1(3), 235–247.
Kwon, B., Molek, J., & Zydney, A. L. (2008). Ultrafiltration of PEGylated proteins: fouling and concentration polarization effects. Journal of Membrane Science, 319(1-2), 206–213.
Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature, 227(5259), 680–685.
Lokra, S., Helland, M. H., Claussen, I. C., Straetkvern, K. O., & Egelandsdal, B. (2008). Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT--Food Science and Technology, 41(6), 1089–1099.
Marri, C., Frazzoli, A., Hochkoeppler, A., & Poggi, V. (2003). Purification of a polyphenol oxidase isoform from potato (Solanum tuberosum) tubers. Phytochemistry, 63(7), 745–752.
Narvaez-Cuenca, C. E., Vincken, J. P., & Gruppen, H. (2013). Quantitative fate of chlorogenic acid during enzymatic browning of potato juice. Journal of Agricultural and Food Chemistry, 61(7), 1563–1572.
Pouvreau, L., Gruppen, H., Piersma, S. R., van den Broek, L. A. M., van Koningsveld, G. A., & Voragen, A. G. J. (2001). Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. Journal of Agricultural and Food Chemistry, 49(6), 2864–2874.
Prigent, S. V. E., Voragen, A. G. J., Visser, A., van Koningsveld, G. A., & Gruppen, H. (2007). Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). Journal of the Science of Food and Agriculture, 87(13), 2502–2510.
Racusen, D. (1989). Patatin purification by hydrophobic interaction chromatography. Journal of Food Biochemistry, 13(6), 453–456.
Ramírez, E. C., Whitaker, J. R., Virador, V, M. (2003). Polyphenol Oxidase. In J. R. Whitaker., A. G. J. Voragen., D. W. S. Wong (Eds.), Handbook of Food Enzymology (pp. 509–537). New York: Marcel Dekker, Inc.
Rawel, H. M., Kroll, J., & Rohn, S. (2001). Reactions of phenolic substances with lysozyme—physicochemical characterisation and proteolytic digestion of the derivatives. Food Chemistry, 72(1), 59–71.
Schneider, S. W., Nuschele, S., Wixforth, A., Gorzelanny, C., Alexander-Katz, A., Netz, R. R., et al. (2007). Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proceedings of the National Academy of Sciences of the United States of America, 104(19), 7899–7903.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., et al. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150(1), 76–85.
Straetkvern, K. O., & Schwarz, J. G. (2012). Recovery of native potato protein comparing expanded bed adsorption and ultrafiltration. Food and Bioprocess Technology, 5(5), 1939–1949.
Thipyapong, P., Hunt, M. D., & Steffens, J. C. (1995). Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry, 40(3), 673–676.
van Koningsveld, G. A., Gruppen, H., de Jongh, H. H. J., Wijngaards, G., van Boekel, M., Walstra, P., et al. (2001). Effects of pH and heat treatments on the structure and solubility of potato proteins in different preparations. Journal of Agricultural and Food Chemistry, 49(10), 4889–4897.
Zhang, W. X., & Ding, L. H. (2015). Investigation of membrane fouling mechanisms using blocking models in the case of shear-enhanced ultrafiltration. Separation and Purification Technology, 141, 160–169.
Zwijnenberg, H. J., Kemperman, A. J. B., Boerrigter, M. E., Lotz, M., Dijksterhuis, J. F., Poulsen, P. E., et al. (2002). Native protein recovery from potato fruit juice by ultrafiltration. Desalination, 144(1-3), 331–334.
Acknowledgments
We thank Future Food Innovation of Mid-Jutland region, KMC, AVK Langholt, and Aarhus University for the financial support of the studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, J.M., Greve-Poulsen, M., Damgaard, H. et al. Effect of Membrane Material on the Separation of Proteins and Polyphenol Oxidase in Ultrafiltration of Potato Fruit Juice. Food Bioprocess Technol 9, 822–829 (2016). https://doi.org/10.1007/s11947-015-1670-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1670-1