Abstract
Purpose of Review
The aim of this review is to describe the preoperative evaluation, surgical techniques, and postoperative management of patients with renal cell carcinoma (RCC) undergoing radical nephrectomy (RN) and inferior vena cava (IVC) thrombectomy.
Recent Findings
RN and IVC thrombectomy remains the standard management option in non-metastatic RCC patients with IVC thrombus. A comprehensive preoperative workup, including high-quality imaging, blood works, and appropriate consultations are required for all patients. The aim of the surgery is complete resection of all tumor burden, which requires a skillful surgical team for such a challenging procedure and is inherently associated with a high rate of perioperative morbidity and mortality.
Summary
Preoperative CT or MRI is essential for surgical planning. The surgical approach is mainly determined by the level of the tumor thrombus. The open approach has been the standard, though minimally invasive and robotic techniques are emerging in selected cases by experienced surgeons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Involvement of renal vein or inferior vena cava (IVC) is seen in up to 10% of the renal cell carcinoma (RCC) cases, of which 1% extends to the right atrium [1]. Tumor thrombus is a significant adverse prognostic factor in these patients, and the thrombus level is an independent predictor of survival [2]. The 1-year disease-specific survival of untreated RCC with venous tumor thrombus is 29% that improves significantly following radical nephrectomy (RN) and venous thrombectomy [3, 4]. Ciancio et al. reported a 5-year disease-free survival of 64% in RCC patients with any level of IVC thrombus following surgery [5]. The survival rate has been improving in recent years with the introduction of novel therapeutic agents. However, aggressive surgical resection remains the default management option in non-metastatic RCC patients with IVC thrombus, irrespective of the thrombus level [6, 7]. It is a challenging procedure with high difficulty and potential mortality. Surgical approach for the IVC thrombectomy varies mainly based on the thrombus level and the surgeon’s experience. These complex surgeries require an excellent experience of a multi-disciplinary team including urologists and anesthesiologists, as well as cardiothoracic and vascular surgeons in selected cases. In this report, we describe the preoperative evaluation, surgical techniques, and postoperative considerations of patients with RCC undergoing RN and IVC thrombectomy.
Preoperative Evaluation
The most important part of the preoperative workup is to determine the level of the tumor thrombus, which is essential for the surgical planning. Several classifications have been proposed to describe the cephalad extent of the IVC thrombus (Table 1) [8,9,10,11]. The Mayo staging system is the most common classification that was originally defined by Neves and Zincke in 1987 (Table 1-B). Ciancio et al. proposed a modified staging system and subdivided the level III thrombi into 4 groups (Table 1-C). In addition, Blute et al. characterized different patterns of the bland thrombus inferior to the tumor thrombus in RCC cases to help with the preoperative surgical planning (Table 1-D).
Imaging
Historically, vena cavography was the gold standard for evaluation of IVC thrombus. However, the use of this modality is now limited due to its invasive nature, need for high contrast loads, and risks of complications [12]. Ultrasonography (US) is a non-invasive, though largely operator-dependent imaging modality that can be used for the evaluation of thrombus. The sensitivity of US in detecting tumor thrombus is dependent to the thrombus level and is reported as low as 68% below the level of hepatic vein insertion. Furthermore, the renal vein and IVC are not completely visualized by US in 12.5% and 43.5% of cases, respectively [13]. Contrast-enhanced ultrasound (CEUS) is emerging as a valuable imaging modality that can enhance the accuracy of conventional US. Li et al. recently evaluated the accuracy of CEUS in detecting bland from tumor thrombus in patients with RCC and reported the sensitivity and specificity of 87.5% and 100%, respectively [14•].
Nowadays, the two most commonly used imaging modalities for the evaluation of IVC thrombus as well as metastatic workup in patients with RCC are computed tomography (CT) scan and magnetic resonance imaging (MRI). Multi-phasic contrast-enhanced CT is the standard imaging technique for the diagnosis and staging of RCC. It has a sensitivity and specificity of 93% and 97% in detecting tumor thrombus, respectively [12, 15, 16]. MRI has a higher sensitivity (up to 100%) in detecting IVC thrombus and may provide additional information on venous involvement if the extent of thrombus is poorly defined on CT. It can distinctly show the relationship of the thrombus to other vital structures including the liver and heart and differentiate specifically between bland and tumor thrombus [12]. The European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) guidelines recommend MRI to better evaluate venous involvement in patients with RCC and IVC thrombus (https://uroweb.org/guideline/renal-cell-carcinoma/, https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf). Timing of the imaging is of utmost importance for the surgical planning; it is recommended that CT/MRI to be done within 30 days, and ideally ≤ 14 days before surgery [17].
Consultations
Anesthesia consult should be performed for > 50 years of age and/or high-risk patients [17, 18]. An anesthesiologist who is familiar with the rapid fluid shifts, cardio-pulmonary bypass (CPB), and transesophageal echocardiogram (TEE) is preferred for this procedure. Cardiology consult is recommended for patients if two or more risk factors for coronary artery disease exist (e.g., hypertension, hyperlipidemia, diabetes, and smoking) [17]. If CPB or veno-venous bypass (VVB) is anticipated in cases of a higher level thrombus (III or IV), coronary angiography and cardiothoracic consult should be performed [12, 17]. If the primary surgeon does not have expertise with complex vascular reconstruction, specifically in patients with preoperative findings of extensive intravascular tumor and/or higher level tumor thrombus, a vascular surgeon should also be consulted [18].
Anticoagulation
All patients with occlusive IVC tumor and/or bland thrombus and those with recent thromboembolic events may benefit from preoperative anticoagulation [17,18,19]. Low molecular weight heparin (LMWH) is preferred with the last dose be administered 24 h prior to the surgery. Warfarin can be used as an alternate with a target international normalized ratio (INR) of 2–3. It should be stopped at least 5 days prior and bridged till operation [20].
Renal Angioembolization
Preoperative renal embolization (RAE) has been studied to facilitate RN of RCC cases with venous thrombus. The aim of RAE is to decrease blood loss, allow early control and ligation of venous system, and decrease the cephalad extent of the IVC thrombus [21]. There is no prospective randomized trial available demonstrating the clinical or oncologic outcomes of RAE in this setting. Although some older studies have reported encouraging results, especially with decreased intraoperative blood loss [22], recent studies have shown no benefit of RAE before RN and IVC thrombectomy. Subramanian et al. have shown that routine preoperative RAE in RCC patients with IVC thrombus does not provide any measurable benefit in reducing blood loss or complications and maybe associated with increased major perioperative complications and mortality [23]. Chan et al. similarly reported increased operating time, blood loss, hospital stay, and perioperative mortality in patients who underwent RAE prior to RN and IVC thrombectomy [24]. Therefore, according to the available evidence, routine preoperative RAE is not recommended in patients with RCC and IVC thrombus. We endorse RAE only in patients with bulky retroperitoneal lymph nodes that get access to renal artery is difficult, selected minimally invasive IVC thrombectomies, and patients with religious belief not to accept blood (Jehovah’s witness).
IVC Filter Placement
The role of preoperative IVC filter placement remains uncertain. Blute et al. recommended to avoid preoperative IVC filters in patients with resectable tumors because it may lead to incorporation of the tumor in the filter, which increases the difficulty of complete resection [11]. However, IVC filters may be placed at the physician’s discretion in patients with continued pulmonary emboli (PE) despite anticoagulation or those with contraindication to anticoagulation. If an IVC filter is required, it is recommended to be placed within 48 h before surgery to reduce the incidence of thrombus infiltration into the filter [12, 17].
Surgical Management
The aim of the surgery in patients with RCC and IVC tumor thrombus is complete resection of all tumor burden to achieve negative margins. It includes RN, tumor thrombectomy, and possible IVC resection with or without vascular reconstruction.
Surgical Approach
The choice of surgical approach (i.e., open vs. minimally invasive and necessity of vascular bypass) is mainly determined by the level of the tumor thrombus. Other characteristics of the tumor including size, location, and collateral vessels as well as surgeons’ experience and preference may also contribute to the decision [12, 18]. Open approaches can be performed through midline, anterior subcostal/chevron or thoracoabdominal (modified), or flank incisions [25]. For supradiaphragmatic thrombi, median sternotomy or right-sided thoracoabdominal incision can provide appropriate exposure to the supra- and retro-hepatic IVC and intrathoracic access to the heart for possible CPB [18]. Although the open approach is generally favored, minimally invasive approaches especially robotic techniques have evolved considerably, and successfully been applied by experienced surgeons in high-volume centers [26].
Transesophageal Echocardiography
Real-time transesophageal echocardiography (TEE) is necessary to assess the thrombus features (i.e., shape, mobility, and size), and to delineate the level of the thrombus at the time of surgery. It is also helpful to evaluate cardiac function, any potential thrombus dislodgment (e.g., during the vascular intervention), and embolization of associated bland thrombus [27, 28].
Level I
Level I thrombectomy can be performed via open or minimally invasive approaches. In both approaches, early ligation of the renal artery is recommended in order to reduce the intraoperative blood loss [29]. The small, well-rounded thrombi can often be milked back to renal vein and a Satinsky vascular clamp be used on vena cava around the ostium. However, milking might be challenging with rather fat or filamentous thrombi, since it may shatter the thrombus and/or left it outside of the vascular clamp into the bloodstream. For larger volume thrombi, proper vascular control above and below the thrombus on IVC as well as contralateral renal vein will be necessary. Cavotomy and thrombectomy is then performed under direct vision. After removal of the thrombus en bloc with the nephrectomy specimen, IVC should be flushed with heparinized saline solution and inspected for any residual thrombus and be closed with a 4-0 Prolene running suture. Advanced multi-disciplinary planning with the anesthesiologist and whole surgical team is of utmost importance to succeed in these steps.
Both laparoscopic [30, 31] and robotic [32, 33] approaches have also been reported for the management of RCC with level I IVC thrombus. In the robotic setting, the patient is secured in a 60-degree lateral decubitus position and trans-peritoneal ports are inserted per standard configuration for renal surgery. Surgical steps mostly mirror open approach. Intraoperative laparoscopic ultrasonography can be used to confirm the proximal extent of the thrombus. The Endo-GIA stapler is used to transect the renal artery as well as renal vein/vena cava wall, proximal to the thrombus, when feasible [26]. If not, similar to open approach, IVC and contralateral renal vein control can be secured and applied for thrombus extraction.
Level II
Patients with level II thrombus may require more extensive vascular dissection to mobilize the IVC and contralateral renal vein. After early ligation of the renal artery, ligation of the surrounding lumbar branches (as well as adrenal and gonadal veins for the left-sided tumors) is required in order to achieve appropriate vascular control. Ligation of the minor hepatic veins is helpful, if more infrahepatic IVC exposure is needed. Before the cavotomy, communication with the anesthesia team and a “test clamp” with monitoring the patient’s hemodynamic parameters is essential. If significant hypotension happens with the test clamp, additional fluid replacement and adrenergic stimulants with or without blood transfusion may be considered before cavotomy [12]. Vascular clamps or umbilical tapes with Rummels are then placed on the infrarenal IVC, contralateral renal vein, and suprarenal IVC (above thrombus), respectively. Once the vascular control is obtained, a cavotomy is performed starting circumferentially around the ostium of the renal vein, and then is extended superiorly on the anterior surface of the IVC. If the tumor thrombus does not invade into IVC wall, thrombectomy can be completed safely; however, any suspicious areas could be biopsied and/or resected. Vena cavoscopy using a flexible cystoscope can be utilized intraoperatively to ensure clearance of residual thrombus [34•]. After complete removal of the tumor thrombus, the IVC is flushed with heparinized saline solution and cavotomy incision is closed with a 4-0 Prolene suture. At least 50% of the IVC circumference should be maintained for proper closure [35]. Following thrombectomy and caval repair, Rummel tourniquet of the suprarenal IVC, contralateral renal vein, and infrarenal IVC is released in sequence [26].
The feasibility and safety of minimally invasive approach for the management of selected cases with RCC and level II IVC thrombus have been shown in recent series [36••, 37••]. Surgical steps duplicate open surgery. Laparoscopic ultrasonography is helpful to confirm the thrombus extent. Favorable outcomes have been reported with the robotic approach. Nevertheless, it should be performed in high-volume centers with extensive experience.
Level III
Level III IVC thrombectomy is usually performed through open approach, given the complexity of the procedure and the need for getting access to the major hepatic veins. However, there are certain case reports/series of level III done in minimally invasive fashion, too. In open fashion, we prefer the anterior midline incision but thoracoabdominal approach is required in some cases, especially those with large renal upper pole tumors, challenging anatomy or body habitus. Although CPB is usually performed for the IVC thrombi involving the right atrium, it may also be necessary in selected patients with high level III thrombus. Given the fact that the decision regarding the particular surgical technique can only be indorsed intraoperatively, the operating room may be set up for possible CPB. Intraoperative TEE should be available to confirm the cranial extent of the thrombus during the surgery.
IVC needs to be freed up completely and following ligation of the renal artery, thrombus attempted to be trapped between the infra, supra, and contralateral renal vein controls, when feasible. For left-sided tumors, we may need to control right renal artery as well, unless right renal vein is excluded by re-routing to infrarenal cava. IVC clamping below hepatic veins is preferable, if the tumor thrombus can be milked down safely. This should be performed under TEE guidance to assess the level of the clamp and the potential dislodging of the thrombus. For the small level IIIa thrombi, ligation of the short hepatic veins and intrahepatic IVC release can help with vascular control above the thrombus. The larger IIIa and IIIb thrombi require caudal hepatic mobilization to get control of the suprahepatic-infradiaphragmatic IVC. Vascular clamp is then placed above the thrombus via anterior (or lateral) approach. If this is unattainable or in cases with higher stage thrombi, complete mobilization of the liver, as previously described by Ciancio et al. [38], and IVC clamping with the lateral approach are recommended. Occasionally, thoracoabdominal incision is needed to get control of the cavoatrial junction. “Pringle maneuver” (temporarily clamping portal vein and hepatic artery) is required to prevent massive blood loss, if proximal IVC clamp is superior to hepatic veins [39,40,41]. Intermittent and continuous Pringle maneuver can both be tolerated up to 120 min, though continuous occlusion of < 20 min is recommended to minimize the risk of ischemia-reperfusion injury [38, 42]. Patients with completely occlusive thrombi who have developed various venous collaterals can safely tolerate the suprahepatic clamping. Nevertheless, in cases with partially occlusive thrombi, clamping may not be tolerated well, and bypass should be considered. Once the IVC is clamped, cavotomy and thrombectomy is performed similarly to what described for level II thrombi. In selected cases, IVC resection with or without reconstruction is necessary.
The initial series of robotic level III IVC thrombectomy has been reported by Gill et al. in 2015 [43, 44]. The feasibility of this approach then confirmed in other studies [45, 46••, 47•, 48]. Most of the surgical steps are similar to open approach. The challenging step of suprahepatic IVC control is usually set by cardiothoracic team through minimally invasive thoracic approach. Alternatively, trans-jugular or trans-vena cava balloon control has been shown to help with down-staging the thrombus or facilitate with Pringle maneuver [49].
Level IV
Level IV IVC thrombi are generally managed with a combined intraabdominal and intrathoracic approach. For the small, non-adherent thrombi, confirmed with TEE, control of the cavoatrial junction above thrombus using Rummel tourniquet can be completed with the thoracoabdominal (or midline) approach. If this technique is not successful, CPB may be required [50, 51]. For true level IV cases, we prefer sternotomy and CPB. Small volume thrombi can be managed with CPB alone, though larger thrombi with high chance of shattering may require cardiac arrest as well, to succeed complete tumor extirpation. To avoid opening the chest, complete abdominal approach with or without veno-venous bypass (VVBP) can be used in selected patients with free-floating thrombus that is able to be completely milked back into the IVC [50, 52].
Few case reports of minimally invasive level IV IVC thrombectomy have been reported in the literature [46••, 47•, 53]. The feasibility and safety of the robotic approach is yet to be confirmed in larger studies, though it is restricted to high-volume centers and experienced multi-disciplinary teams.
IVC Resection
IVC resection is indicated in patients with densely adherent intracaval tumor and/or direct caval wall invasion [54]. To obtain negative vascular margins, en bloc resection of the IVC may be necessary in selected cases where the IVC is totally occluded by either tumor or bland thrombus [18]. Real-time intraoperative assessment of the IVC by the operating surgeon is the main decision driver for IVC resection. However, some preoperative findings can help with the appropriate surgical planning. Adams et al. reported that preoperative MRI can reliably assess IVC wall invasion with positive and negative predictive values of about 90% [55]. Furthermore, it has been shown that presence of a right-sided tumor, an IVC anteroposterior diameter of ≥ 24.0 mm, complete occlusion of the IVC at the renal vein ostium, and presence of bland thrombus inferior to the tumor thrombus can predict the need for cavectomy [11, 56].
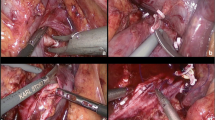
In general, resection of the < 50% of the IVC circumference can be managed by either primary closure or venous patch repair [57]. In cases that en bloc IVC resection is necessary, cavectomy can be performed with or without reconstruction. Infrarenal IVC resection without reconstruction is performed routinely [11], though a graft or prosthesis is often used in suprarenal IVC resection to re-establish venous flow [58]. Nevertheless, it has been shown that suprarenal cavectomy without reconstruction is feasible in selected cases with preexisting various abdominopelvic collaterals [59••]. If suprarenal cavectomy is indicated, left renal vein should be ligated distal to its venous branches (i.e., gonadal, lumbar, and adrenal veins) in right-sided tumors. For the left-sided tumors, right renal vein drainage can be maintained through inferior or superior IVC (with or without a flap). While IVC resection is generally performed using open approach, the feasibility of robotic suprarenal cavectomy has also been reported recently (Fig. 1) [60•].
Step by step robotic suprarenal IVC resection for right-sided renal tumor. a Securing control of the infrarenal IVC (blue arrow) and left renal vein (green arrow) with Rummel tourniquets. b Securing control of the suprarenal IVC (white arrow) using intraoperative ultrasound. c Inspection of the inner wall of the IVC following thrombectomy showing multiple remnants of adherent tumor to IVC endothelium (black arrows). d Suprarenal IVC resection completed using Endo-GIA stapler
Management of Patients with Budd-Chiari Syndrome
Patients with RCC and IVC thrombus may rarely present with Budd-Chiari syndrome (BCS) (hepatic venous obstruction) that can result in liver failure and is associated with very poor prognosis. Surgery is usually not offered for these cases unless in early stages. If severe hepatic dysfunction is not present, BCS is not independently associated with an increased risk for perioperative complications and mortality [61]. Patients with severe coagulopathy will have an increased risk of intra- and postoperative bleeding. Shirodkar and colleagues reported a series of 10 patients with BCS associated with RCC. They showed an increased rate of estimated blood loss (EBL) (mean 4.2 l), length of hospital stay (LOS) (mean 13.25 days), and high complications. In a mean follow-up of 28 months, the mortality rate was 50%, of whom one passed away during the index hospitalization [62].
Perioperative Outcomes
RN with IVC tumor thrombectomy is a complex surgery with high perioperative morbidity and mortality. Major complications include air embolism, acute PE, massive hemorrhage, hepatic dysfunction, and organ ischemia [50]. Air embolism can happen during vascular bypass and/or IVC closure. Releasing the caudal clamp before cranial clamp at the time of cavorrhaphy can help with flushing out the air and decreasing the risk of air embolism. The risk of intraoperative thrombus embolization is reported in 1.5% of cases [63]. Patients with preoperative PE are at increased risk of this complication and may benefit from preoperative IVC filter placement. Careful mobilization of the IVC and kidney before vascular control can minimize the risk of embolization. Prompt recognition of this complication with TEE and emergent embolectomy is necessary, though the mortality rate is as high as 75% [63].
Boorjian et al. reported the Mayo clinic experience of patients who underwent RN and IVC thrombectomy with a 30-day complication rate of 15% and a perioperative mortality of 2–3% [40]. Nevertheless, a Canadian population-based study reported in-hospital mortality and complication rate of 7% and 78%, respectively. In this study, in-hospital mortality was associated with age (odds ratio [OR] 1.05, P < 0.001), comorbidity (OR 4.98, P < 0.001 for Charlson comorbidity score of 3), and the intraoperative use of cardiac bypass (OR 4.12, P = 0.002). In addition, median LOS was 10 days that was associated with age, Charlson comorbidity score, and the lowest surgeon volume quartile [64]. Freidfeld et al. showed both an overall survival benefit and a trend towards better short-term outcome for patients with T3c RCC undergoing surgery in high-volume hospitals, highlighting the impact of surgical quality on the outcomes and the importance of care centralization for these patients [65•].
The higher level of IVC thrombus is associated with increased perioperative complications [40, 61, 66]. Boorjian et al. reported increased intraoperative EBL, transfusion rates, and 30-day complications with the higher levels of IVC thrombus. In their study, the 30-day complication rates of level IV versus 0 IVC thrombectomy were 46.9% and 12.4%, respectively [40]. In a multicenter study among patients with levels III and IV tumor thrombi, Abel et al. reported a 90-day major complication (≥ 3A Calvien-Dindo) rate of 34%, with the most common being respiratory followed by cardiac and hematologic. The authors reported 30- and 90-day mortality rates of 5.6% and 10.5%, respectively. Preoperative Eastern Cooperative Oncology Group (ECOG) performance status > 1 and low serum albumin were independently associated with the increased risk of 90-day mortality [61].
The perioperative outcomes of patients undergoing robotic vs. open IVC thrombectomy have been addressed in recent studies. Using National Cancer Database, Beksac et al. analyzed 872 patients who underwent open (n = 838) or robotic (n = 34) RN with IVC thrombectomy for cT3b RCC. In this study, robotic approach was associated with 26% reduction in LOS but no difference in readmissions or 30-day mortality. The authors also performed a multi-institutional study of 20 patients (9 open and 11 robotic) undergoing RN with level II IVC thrombectomy. In this subgroup analysis, robotic group had significantly lower EBL (100 vs. 600 mL, P = 0.02) and shorter LOS (1 vs. 5 days, P = 0.02), though no difference was seen in terms of operative time and postoperative complications [67•]. In a recent study, Rose et al. compared 27 open and 24 robotic RN and level I/II IVC thrombectomy cases. Patients in the robotic group demonstrated shorter LOS (3 vs. 7 nights, P = 0.03), lower EBL (450 vs. 1800 mL, P < 0.01), and lower transfusion rate (21% vs. 82%, P < 0.01). The robotic group had 26% fewer complications compared to open cohort (17% vs. 43%, P < 0.01) [68••].
Postoperative Considerations
Following RN and IVC thrombectomy, the patients require close monitoring. Most cases are extubated if surgery has been uneventful, though complex overload patients may need ventilation support for at least 12–24 h following the procedure. Enforced mobilization and breathing exercises should be started as soon as possible. Routine use of abdominal drainage and nasogastric tubes is not recommended. In alert patients, urinary catheters should be removed on the first postoperative day [27].
Anticoagulation with prophylactic dose should be started 24–48 h after the surgery and continued for 4 weeks in patients whose tumor is completely resected. Patients with residual tumor thrombus and/or continued IVC bland thrombus require therapeutic anticoagulation for 6 months. In patients undergoing cavectomy, we recommend warfarin with a target INR of 1.5–2. A retrievable filter should be placed during the surgery or immediately post-operatively in patients with residual thrombus. Filter should be removed after 6 months if there is no further evidence of thrombus or PE [17].
Conclusions
Radical nephrectomy with IVC thrombectomy is a complex procedure that requires the commitment of a multi-disciplinary team, particularly for higher level tumor thrombi. The aim of this surgery is complete resection of all tumor burden to achieve negative margins. Preoperative imaging (either CT or MRI) is the most crucial part of the workup that can delineate the thrombus level and surgical planning. The surgical approach is mainly determined by the level of the tumor thrombus as well as the tumor features and surgeons’ experience. Although open technique has been the standard of care, robotic approach can be applied in selected cases by experienced surgeons.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33–41. https://doi.org/10.1111/j.1464-410X.2004.04897.x.
Martínez-Salamanca JI, Linares E, González J, et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Curr Urol Rep. 2014;15:404. https://doi.org/10.1007/s11934-014-0404-7.
Reese AC, Whitson JM, Meng MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol. 2013;31:1305–9. https://doi.org/10.1016/j.urolonc.2011.12.006.
Whitson JM, Reese AC, Meng MV. Population based analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol Oncol. 2013;31:259–63. https://doi.org/10.1016/j.urolonc.2010.11.017.
Ciancio G, Manoharan M, Katkoori D, De Los Santos R, Soloway MS. Long-term survival in patients undergoing radical nephrectomy and inferior vena cava thrombectomy: single-center experience. Eur Urol. 2010;57:667–72. https://doi.org/10.1016/j.eururo.2009.06.009.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. https://doi.org/10.1016/j.eururo.2019.02.011.
Hevia V, Ciancio G, Gómez V, Álvarez S, Díez-Nicolás V, Burgos FJ. Surgical technique for the treatment of renal cell carcinoma with inferior vena cava tumor thrombus: tips, tricks and oncological results. Springerplus. 2016;5:132. https://doi.org/10.1186/s40064-016-1825-1.
Swami U, Nussenzveig RH, Haaland B, Agarwal N. Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med. 2019;7(Suppl 1):S18. https://doi.org/10.21037/atm.2019.01.50.
Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59:390–5. https://doi.org/10.1111/j.1464-410x.1987.tb04832.x.
Ciancio G, Vaidya A, Savoie M, Soloway M. Management of renal cell carcinoma with level III thrombus in the inferior vena cava. J Urol. 2002;168:1374–7. https://doi.org/10.1097/01.ju.0000023441.00587.02.
Blute ML, Boorjian SA, Leibovich BC, Lohse CM, Frank I, Karnes RJ. Results of inferior vena caval interruption by greenfield filter, ligation or resection during radical nephrectomy and tumor thrombectomy. J Urol. 2007;178:440–4. https://doi.org/10.1016/j.juro.2007.03.121.
Lawindy SM, Kurian T, Kim T, Mangar D, Armstrong PA, Alsina AE, et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int. 2012;110:926–39. https://doi.org/10.1111/j.1464-410X.2012.11174.x.
Trombetta C, Liguori G, Bucci S, Benvenuto S, Garaffa G, Belgrano E. Evaluation of tumor thrombi in the inferior vena cava with intraoperative ultrasound. World J Urol. 2007;25:381–4. https://doi.org/10.1007/s00345-007-0191-6.
• Li Q, Wang Z, Ma X, Tang J, Luo Y. Diagnostic accuracy of contrast-enhanced ultrasound for detecting bland thrombus from inferior vena cava tumor thrombus in patients with renal cell carcinoma. Int Braz J Urol. 2020;46:92–100. https://doi.org/10.1590/S1677-5538.IBJU.2019.0304. Thirty patients included in this retrospective study. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of CEUS in detecting bland thrombus from IVC tumor thrombus were 87.5%, 100%, 96.7%, 100%, and 95.6%, respectively.
Nazim SM, Ather MH, Hafeez K, Salam B. Accuracy of multidetector CT scans in staging of renal carcinoma. Int J Surg. 2011;9:86–90. https://doi.org/10.1016/j.ijsu.2010.07.304.
Renard AS, Nedelcu C, Paisant A, Saulnier P, le Bigot J, Azzouzi AR, et al. Is multidetector CT-scan able to detect T3a renal tumor before surgery? Scand J Urol. 2019;53:350–5. https://doi.org/10.1080/21681805.2019.1675756.
Woodruff DY, Van Veldhuizen P, Muehlebach G, Johnson P, Williamson T, Holzbeierlein JM. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol Oncol. 2013;31:517–21. https://doi.org/10.1016/j.urolonc.2011.03.006.
Psutka SP, Leibovich BC. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol. 2015;7:216–29. https://doi.org/10.1177/1756287215576443.
Lee AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27:4895–1. https://doi.org/10.1200/JCO.2009.22.3958.
Dimitropoulos K, Omar MI, Chalkias A, Arnaoutoglou E, Douketis J, Gravas S. Perioperative antithrombotic (antiplatelet and anticoagulant) therapy in urological practice: a critical assessment and summary of the clinical practice guidelines. World J Urol. 2020. https://doi.org/10.1007/s00345-020-03078-2.
Gunn AJ, Patel AR, Rais-Bahrami S. Role of angio-embolization for renal cell carcinoma. Curr Urol Rep. 2018;19:76. https://doi.org/10.1007/s11934-018-0827-7.
Zielinski H, Szmigielski S, Petrovich Z. Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000;23:6–12. https://doi.org/10.1097/00000421-200002000-00002.
Subramanian VS, Stephenson AJ, Goldfarb DA, Fergany AF, Novick AC, Krishnamurthi V. Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74:154–9. https://doi.org/10.1016/j.urology.2008.12.084.
Chan AA, Abel EJ, Carrasco A, Zainfeld DE, Ifokwe JI, Vaporciyan AA, et al. Impact of preoperative renal artery embolization on surgical outcomes and overall survival in patients with renal cell carcinoma and inferior vena cava thrombus. J Urol. 2011;185:e707–8. https://doi.org/10.1016/j.juro.2011.02.2092.
Agochukwu N, Shuch B. Clinical management of renal cell carcinoma with venous tumor thrombus. World J Urol. 2014;32:581–9. https://doi.org/10.1007/s00345-014-1276-7.
Sun Y, de Castro Abreu AL, Gill IS. Robotic inferior vena cava thrombus surgery: novel strategies. Curr Opin Urol. 2014;24:140–7. https://doi.org/10.1097/MOU.0000000000000033.
González J, Andrés G, Martínez-Salamanca JI, Ciancio G. Improving surgical outcomes in renal cell carcinoma involving the inferior vena cava. Expert Rev Anticancer Ther. 2013;13:1373–87. https://doi.org/10.1586/14737140.2013.858603.
Ayyathurai R, Garcia-Roig M, Gorin MA, González J, Manoharan M, Kava BR, et al. Bland thrombus association with tumour thrombus in renal cell carcinoma: analysis of surgical significance and role of inferior vena caval interruption. BJU Int. 2012;110:E449–5. https://doi.org/10.1111/j.1464-410X.2012.11128.x.
Ciancio G, Vaidya A, Soloway M. Early ligation of the renal artery using the posterior approach: a basic surgical concept reinforced during resection of large hypervascular renal cell carcinoma with or without inferior vena cava thrombus. BJU Int. 2003;92:488–9. https://doi.org/10.1046/j.1464-410x.2003.04372.x.
Desai MM, Gill IS, Ramani AP, Matin SF, Kaouk JH, Campero JM. Laparoscopic radical nephrectomy for cancer with level I renal vein involvement. J Urol. 2003;169:487–91. https://doi.org/10.1097/01.ju.0000041955.93458.f5.
Varkarakis IM, Bhayani SB, Allaf ME, Inagaki T, Gonzalgo ML, Jarrett TW. Laparoscopic-assisted nephrectomy with inferior vena cava tumor thrombectomy: preliminary results. Urology. 2004;64:925–9. https://doi.org/10.1016/j.urology.2004.05.044.
Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol. 2011;59:652–6. https://doi.org/10.1016/j.eururo.2010.08.038.
Wang B, Li H, Ma X, Zhang X, Gu L, Li X, et al. Robot-assisted laparoscopic inferior vena cava thrombectomy: different sides require different techniques. Eur Urol. 2016;69:1112–9. https://doi.org/10.1016/j.eururo.2015.12.001.
• Loh-Doyle J, Bhanvadia S, Patil MB, Djaladat H, Daneshmand S. Vena cavoscopy in the assessment of intraluminal vena caval tumor involvement. Urology. 2018;113:105–9. https://doi.org/10.1016/j.urology.2017.11.020. Among 36 patients who were included in this study, 8 patients (22%) were found to have residual tumor thrombus during cavoscopy. Five of these patients had residual mass and caval invasion that ultimately resulted in cavectomy.
Duty B, Daneshmand S. Venous resection in urological surgery. J Urol. 2008;180:2338–42. https://doi.org/10.1016/j.juro.2008.08.028.
•• Chopra S, Simone G, Metcalfe C, et al. Robot-assisted level II-III inferior vena cava tumor thrombectomy: step-by-step technique and 1-year outcomes. Eur Urol. 2017;72:267–74. https://doi.org/10.1016/j.eururo.2016.08.066. Detailed surgical techniques and outcomes of 25 patients who underwent level II-III IVC thrombectomy were reported in this study. Median operative time, EBL, and LOS were 4.5 h, 240 ml, and 4 days, respectively. Complication rate was 17% and all patients were alive at a 16-month median follow-up.
•• Gu L, Ma X, Gao Y, et al. Robotic versus open level I-II inferior vena cava thrombectomy: a matched group comparative analysis. J Urol. 2017;198:1241–6. https://doi.org/10.1016/j.juro.2017.06.094. In this study, the authors reported 31 and 37 robotic and open IVC thrombectomies, respectively. Robotic cohort had significantly lower median operative time, EBL, blood transfusion rate, and LOS. The postoperative complication rate was lower in the robotic group, though the oncologic outcomes were similar between the two groups.
Pouliot F, Shuch B, Larochelle JC, Pantuck A, Belldegrun AS. Contemporary management of renal tumors with venous tumor thrombus. J Urol. 2010;184:833–5. https://doi.org/10.1016/j.juro.2010.04.071.
Ciancio G, Gonzalez J, Shirodkar SP, Angulo JC, Soloway MS. Liver transplantation techniques for the surgical management of renal cell carcinoma with tumor thrombus in the inferior vena cava: step-by-step description. Eur Urol. 2011;59:401–6. https://doi.org/10.1016/j.eururo.2010.07.028.
Boorjian SA, Sengupta S, Blute ML. Renal cell carcinoma: vena caval involvement. BJU Int. 2007;99:1239–44. https://doi.org/10.1111/j.1464-410X.2007.06826.x.
Kwon TW, Kim H, Moon KM, Cho YP, Song C, Kim CS, et al. Surgical treatment of inferior vena cava tumor thrombus in patients with renal cell carcinoma. J Korean Med Sci. 2010;25:104–9. https://doi.org/10.3346/jkms.2010.25.1.104.
van Riel WG, van Golen RF, Reiniers MJ, Heger M, van Gulik TM. How much ischemia can the liver tolerate during resection? Hepatobiliary Surg Nutr. 2016;5:58–71. https://doi.org/10.3978/j.issn.2304-3881.2015.07.05.
Gill IS, Metcalfe C, Abreu A, Duddalwar V, Chopra S, Cunningham M, et al. Robotic level III inferior vena cava tumor thrombectomy: initial series. J Urol. 2015;194:929–38. https://doi.org/10.1016/j.juro.2015.03.119.
de Castro Abreu AL, Chopra S, Azhar RA, Berger AK, Metcalfe C, Minetti M, et al. Robotic transabdominal control of the suprahepatic, infradiaphragmatic vena cava to enable level 3 caval tumor thrombectomy: pilot study in a perfused-cadaver model. J Endourol. 2015;29:1177–81. https://doi.org/10.1089/end.2015.0081.
Abaza R, Shabsigh A, Castle E, Allaf M, Hu JC, Rogers C, et al. Multi-institutional experience with robotic nephrectomy with inferior vena cava tumor thrombectomy. J Urol. 2016;195:865–71. https://doi.org/10.1016/j.juro.2015.09.094.
•• Wang B, Huang Q, Liu K, et al. Robot-assisted level III-IV inferior vena cava thrombectomy: initial series with step-by-step procedures and 1-yr outcomes. Eur Urol. 2020;78:77–86. https://doi.org/10.1016/j.eururo.2019.04.019. In this study, surgical techniques and outcomes of 13 patients who underwent level III–IV robotic IVC thrombectomy have been reported. The authors reported robotic surgery as a feasible approach for well-selected patients, despite high complication (23% grade IV) and 7.7% perioperative mortality rates.
• Shen D, Du S, Huang Q, et al. A modified sequential vascular control strategy in robot-assisted level III-IV inferior vena cava thrombectomy: initial series mimicking the open ‘milking’ technique principle. BJU Int. 2020. https://doi.org/10.1111/bju.15094. Twelve patients underwent a modified technique of robotic level III–IV IVC thrombectomy with early release of porta hepatis and stopping CPB. This modified technique resulted in shorter pringle and CPB times as well as a lower rate of grade II–IV perioperative complications and better postoperative hepatorenal and coagulation function, compared to the conventional technique.
Wang B, Li H, Huang Q, Liu K, Fan Y, Peng C, et al. Robot-assisted retrohepatic inferior vena cava thrombectomy: first or second porta hepatis as an important boundary landmark. Eur Urol. 2018;74:512–20. https://doi.org/10.1016/j.eururo.2017.11.017.
Kundavaram C, Abreu AL, Chopra S, et al. Advances in robotic vena cava tumor thrombectomy: intracaval balloon occlusion, patch grafting, and vena cavoscopy. Eur Urol. 2016;70:884–90. https://doi.org/10.1016/j.eururo.2016.06.024.
Olumi AF, Blute ML. Open surgery of the kidney. In: Wein AJ, Kavoussi LR, Partin AW, et al., editors. Campbell-Walsh urology. 11th ed. Philadelphia: Elsevier; 2020. p. 2248–78.
Patil MB, Montez J, Loh-Doyle J, Cai J, Skinner EC, Schuckman A, et al. Level III-IV inferior vena caval thrombectomy without cardiopulmonary bypass: long-term experience with intrapericardial control. J Urol. 2014;192:682–8. https://doi.org/10.1016/j.juro.2014.03.112.
Ciancio G, Shirodkar SP, Soloway MS, Livingstone AS, Barron M, Salerno TA. Renal carcinoma with supradiaphragmatic tumor thrombus: avoiding sternotomy and cardiopulmonary bypass. Ann Thorac Surg. 2010;89:505–10. https://doi.org/10.1016/j.athoracsur.2009.11.025.
Ahmadi N, Cunningham M, Duddalwar V, et al. Robotic assisted level IV inferior vena cava tumor thrombectomy. J Urol. 2018;199:e744. https://doi.org/10.1016/j.juro.2018.02.1769.
González J, Gorin MA, Garcia-Roig M, Ciancio G. Inferior vena cava resection and reconstruction: technical considerations in the surgical management of renal cell carcinoma with tumor thrombus. Urol Oncol. 2014;32:34.e19–34.e3.4E26. https://doi.org/10.1016/j.urolonc.2013.01.004.
Adams LC, Ralla B, Bender YY, et al. Renal cell carcinoma with venous extension: prediction of inferior vena cava wall invasion by MRI. Cancer Imaging. 2018;18:17. https://doi.org/10.1186/s40644-018-0150-z.
Psutka SP, Boorjian SA, Thompson RH, Schmit GD, Schmitz JJ, Bower TC, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int. 2015;116:388–96. https://doi.org/10.1111/bju.13005.
Duty B, Daneshmand S. Resection of the inferior vena cava without reconstruction for urologic malignancies. Urology. 2009;74:1257–62. https://doi.org/10.1016/j.urology.2009.06.092.
Caso J, Seigne J, Back M, Spiess PE, Pow-Sang J, Sexton WJ. Circumferential resection of the inferior vena cava for primary and recurrent malignant tumors. J Urol. 2009;182:887–93. https://doi.org/10.1016/j.juro.2009.05.015.
•• Djaladat H, Ghoreifi A, Basin MF, et al. Perioperative outcome of suprarenal resection of vena cava without reconstruction in urologic malignancies: a case series and review of the literature. Urology. 2020;S0090–4295(20):30445–3. https://doi.org/10.1016/j.urology.2020.02.042. Twenty-eight patients included in this study, including 22 level 3, 3 level 2, and 3 level 4 IVC thrombus. Ninety-day complication and mortality rates were 35% and 7%, respectively.
• Medina LG, Ghoreifi A, Thaker H, Duddalwar V, Djaladat H. Robotic suprarenal cavectomy in a patient with kideny tumor and level III tumor thrombosis. J Urol. 2020;203(Supplement 4):e93–4. https://doi.org/10.1097/JU0000000000000826.012. This is the first robotic level III IVC thrombectomy and cavectomy without reconstruction reported in the literature. The authors presented a video demonstrating the technique of the aforementioned surgery. The surgical margins were negative, and no perioperative complication was reported.
Abel EJ, Thompson RH, Margulis V, Heckman JE, Merril MM, Darwish OM, et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: a contemporary multicenter experience. Eur Urol. 2014;66:584–92. https://doi.org/10.1016/j.eururo.2013.10.029.
Shirodkar SP, Soloway MS, Ciancio G. Budd-Chiari syndrome in urology: impact on nephrectomy for advanced renal cell carcinoma. Indian J Urol. 2011;27:351–6. https://doi.org/10.4103/0970-1591.85439.
Shuch B, Larochelle JC, Onyia T, Vallera C, Margulis D, Pantuck AJ, et al. Intraoperative thrombus embolization during nephrectomy and tumor thrombectomy: critical analysis of the University of California-Los Angeles experience. J Urol. 2009;181:492–9. https://doi.org/10.1016/j.juro.2008.10.036.
Toren P, Abouassaly R, Timilshina N, Kulkarni G, Alibhai S, Finelli A. Results of a national population-based study of outcomes of surgery for renal tumors associated with inferior vena cava thrombus. Urology. 2013;82:572–7. https://doi.org/10.1016/j.urology.2013.04.054.
• Freifeld Y, Woldu SL, Singla N, et al. Impact of hospital case volume on outcomes following radical nephrectomy and inferior vena cava thrombectomy. Eur Urol Oncol. 2019;2:691–8. https://doi.org/10.1016/j.euo.2018.10.005. Among 2664 patients with pT3b-c RCC from National Cancer Database who underwent RN and IVC thrombectomy, treatment at high-volume centers (performing > 3 procedures per year) was associated with better overall survival and 24% relative risk reduction for all-cause mortality.
Lardas M, Stewart F, Scrimgeour D, Hofmann F, Marconi L, Dabestani S, et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus. Eur Urol. 2016;70:265–80. https://doi.org/10.1016/j.eururo.2015.11.034.
• Beksac AT, Shah QN, Paulucci DJ, et al. Trends and outcomes in contemporary management renal cell carcinoma and vena cava thrombus. Urol Oncol. 2019;37:576.e17–23. https://doi.org/10.1016/j.urolonc.2019.05.010. In this study, 872 patients from National Cancer Database who underwent open or robotic RN and level IIIb thrombectomy were included. Robotic approach was associated with 26% reduction in LOS but no difference in readmissions or 30-day mortality rate.
•• Rose KM, Navaratnam AK, Faraj KS, et al. Comparison of open and robot assisted radical nephrectomy with level I and II inferior vena cava tumor thrombus: the Mayo Clinic experience. Urology. 2020;136:152–7. https://doi.org/10.1016/j.urology.2019.11.002. Patients in the robotic group (n = 27), compared to the open group (n = 24), demonstrated decreased LOS, EBL, and transfusion rate. The robotic group had 26% fever complications compared to the open, though the overall and recurrence-free survivals were comparable between the two groups.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alireza Ghoreifi and Hooman Djaladat each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
About this article
Cite this article
Ghoreifi, A., Djaladat, H. Surgical Tips for Inferior Vena Cava Thrombectomy. Curr Urol Rep 21, 51 (2020). https://doi.org/10.1007/s11934-020-01007-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11934-020-01007-9