Abstract
Purpose of Review
Osteoarthritis (OA) is the most common form of joint disease globally and is associated with significant morbidity and disability. Increasing evidence points to an important inflammatory component in the development and progression of OA. The precise pathways involved in OA inflammatory processes remain to be clarified. Basic calcium phosphate (BCP) and calcium pyrophosphate dihydrate (CPP) crystals can induce inflammation and arthritis and recent studies point to a potential pathogenic role in OA. In the light of this evidence, we explore the relationship and potential mechanistic pathways linking calcium-containing crystals and OA.
Recent Findings
CPP crystals induce inflammation through the NLRP3 inflammasome while BCP crystals mediate both NLRP3 dependent and independent effects. BCP crystals have been demonstrated to induce key mitogenic and inflammatory pathways and contribute to cartilage degradation.
Summary
Calcium-containing crystals induce key inflammatory pathways and may represent an attractive novel target in OA, a condition devoid of effective treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common form of arthritis worldwide [1]. The increase in prevalence with ageing and with obesity means that it is an even greater problem in the developed world [2]. Anticipated demographic changes suggest that we will experience an exponential increase in the current OA epidemic over the coming years [3]. OA is associated with both significant morbidity and disability [4]. Previous concepts of OA as a purely degenerative process of “wear-and-tear” are outdated and incorrect. Evidence from laboratory, imaging, and synovial biopsy studies demonstrate the importance of inflammatory processes in the OA joint [5,6,7,8]. Basic calcium phosphate (BCP) and calcium pyrophosphate dihydrate (CPP) crystals are commonly found in the synovial fluid and tissue of joints affected by OA [9]. BCP crystals are the cause of the extremely destructive Milwaukee shoulder syndrome, while CPP crystals are the causative agent in acute and chronic CPP arthritis [10, 11]. The potential importance of these two types of calcium crystals in the pathogenesis of OA remain to be fully elucidated. In this article, we present a conceptual framework for the role of BCP and CPP in OA, as well as discussing key relevant research which is progressing our understanding in this area.
Osteoarthritis
Worldwide, OA is the most common form of joint pathology [1]. The majority of individuals over the age of 55 have radiographic evidence of OA; 67% of women and 55% of men have radiographic evidence of hand OA alone [12]. The prevalence of symptomatic OA is considerably less than that of the radiographic changes. One fifth of those with radiographic hand OA have symptoms [12, 13]. Globally, symptomatic radiographic OA at the knee affects 3.8% and at the hip 0.85% of the world’s population [1]. In addition, OA at other sites contributes significantly to other common health conditions such as low back pain, which is the leading global cause of years lived with disability [14]. When symptomatic, OA presents with symptoms of joint pain and stiffness. This can be sufficiently severe to lead to significant debility and difficulties with living independently [15]. Ultimately, symptoms of OA can be sufficiently severe to necessitate joint replacement surgery with all the attendant risk and costs entailed in major surgery [16].
The traditional view of OA as joint degeneration as an inevitable sequela of ageing is inaccurate. While joint biomechanics play a role, increasing evidence points to important roles for other aetiological factors such as genetics, and particularly joint inflammation [5,6,7,8, 17]. Serum C-reactive protein (CRP) is associated with both the development and progression of OA [7, 18]. How much of this effect is explained by the correlation between CRP and obesity remains uncertain [18, 19]. Other pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumour necrosis factor alpha (TNF-α) have also been associated with the rate of OA progression [20]. The presence of inflammation in OA joints has been demonstrated by a number of different imaging modalities including ultrasound and magnetic resonance imaging (MRI) [21, 22]. MRI detected synovitis is a predictor of OA progression [21]. Synovitis is also detectable on arthroscopic biopsies from OA joints, and histological synovitis is a predictor of OA progression [5]. While there is increasing evidence that low-grade inflammation is important in OA pathogenesis, the pathways responsible for this inflammatory process are less clear. Calcium crystals, both BCP and CPP, are one proposed link between inflammation and OA.
Basic Calcium Phosphate Crystals
BCP is an umbrella term for a heterogeneous group of crystalline non-acidic calcium phosphates [23]. BCP is composed predominantly of hydroxyapatite with lesser quantities of other substances including octacalcium phosphate, tricalcium phosphate, and magnesium whitlockite [24]. BCP crystals are individually small, 20–100 nm in size, and therefore not detectable by conventional or polarised light microscopy which has a limit of resolution of approximately 1 μm [25]. BCP crystals have a tendency to clump when present in large volumes, and these aggregates may be sufficiently large to be visualised [25]. Notwithstanding this, both their small size and the inherent difficulties with visualisation mean that BCP crystals are frequently unidentified even when present in synovial fluid. An overview of methods utilised for the detection of BCP crystals, and their advantages and limitations is shown in Table 1. At present, due to their individual disadvantages and limited availability, none of these methods can be used in routine clinical practice, and there remains a need for a simple, reliable, and inexpensive method for BCP crystal identification (Table 1).
Intra-articular BCP crystals were first identified in cases of OA with inflammatory features and subsequently in the rapidly destructive form of OA known as the Milwaukee shoulder syndrome [10, 26]. Originally described as a dramatic destructive shoulder arthropathy, this condition may also affect other large joints [27]. Large quantities of BCP crystals are identifiable in joint aspirates from the affected joint [10]. A presumptive role for BCP in the pathogenesis of the Milwaukee shoulder syndrome is suggested by this abundance of crystals and by commonalities in clinical presentation with other forms of crystal arthropathy. BCP crystals are also frequently identified in joints without any overt inflammatory disease; detailed examination has revealed their presence in 58% of OA synovial fluid samples and 100% of cartilage samples at the time of joint replacement for OA [28, 29]. Studies such as these demonstrating the ubiquity of BCP crystals have led to considerable debate as to whether their presence represents a pathogenic mechanism or merely a secondary consequence of joint damage.
Calcium Pyrophosphate Dihydrate Crystals
The identification of CPP crystals, while still requiring significant expertise, is considerably less complicated than BCP crystals. Expert and diligent use of polarised light microscopy will correctly identify CPP crystals in the vast majority of affected cases [30]. The nature of the pathogenic role of CPP crystals has therefore been somewhat easier to define. CPP is the most common cause of chondrocalcinosis and CPP crystals were first identified in 1962 in knee synovial fluid from patients with chondrocalcinosis and acute arthritis [31]. CPP arthritis takes on a variety of clinical presentations from an acute monoarthritis (pseudogout) to an inflammatory polyarthritis (pseudo-rheumatoid) and a more chronic degenerative arthropathy (pseudo-osteoarthritis) [11]. CPP crystals are found considerably less frequently than BCP crystals in OA but are identifiable in 20% of menisci at the time of joint replacement [28]. Both types of calcium crystals are also frequently found together in OA joints [28]. The intra-articular injection of CPP crystals in animal models of OA accelerates disease progression [32].
Mechanisms Linking Calcium Crystals and OA
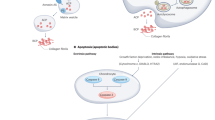
Both BCP and CPP crystals are commonly detected in OA joints and cartilage, are associated with the severity of OA, and may become easier to detect as OA progresses [28, 29]. As a general rule, CPP crystals appear to be more overtly inflammatory than BCP crystals, for example in acute CPP arthritis [11]. However, BCP has the capacity to induce significant inflammatory reactions such as those seen in the Milwaukee shoulder syndrome and in calcific periarthritis [33]. At the same time, both types of calcium crystals can be apparently inert bystanders in and around the joint [34]. The reasons behind this dichotomy remain to be fully clarified but may involve other proteins and cytokines synergistically interacting with the crystals. The pathways currently hypothesised to link calcium crystals and osteoarthritis are summarised in Fig. 1.
Proposed pathways linking calcium crystals and osteoarthritis. Basic calcium phosphate (BCP), calcium pyrophosphate dihydrate (CPP), interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-α), matrix metalloproteinase (MMP), prostaglandin E (PGE), nucleotide-binding oligomerisation domain-like receptor protein 3 (NLRP3), interleukin-1 beta (IL-1β)
Translational research has demonstrated the ability of both BCP and CPP crystals to induce inflammatory pathways in resident articular cells. CPP crystals constitute a damage-associated molecular pattern (DAMP), and are capable of activating the NLRP3 inflammasome through Toll-like receptors (TLRs), the same mechanistic pathway as occurs in gout [35, 36]. In contrast, BCP crystals have the ability to operate through both NLRP3 dependent and independent pathways [37, 38]. Pazar et al. reported the key role of the NLRP3 inflammasome in BCP-induced IL-1β secretion from monocytes and macrophages in cell line and ex vivo models [37].
Intra-articular injection of BCP or CPP crystals has been shown to result in synovial inflammation with infiltration of macrophages and the formation of multinucleated giant cells [27, 32, 38]. BCP crystals increase mitogenesis and the secretion of the matrix metalloproteinases (MMP), MMP-1, and MMP-13 from human fibroblasts [39, 40]. Cunningham et al. showed in a murine model that the exposure of macrophages to BCP crystals leads to increased release of pro-inflammatory cytokines and the damage-associated molecule, S100A8, through activation of Syk and PI3 kinase [41]. These results were subsequently confirmed in human macrophages and dendritic cells by Corr et al. [42•]. BCP particles have also been shown to induce TNF-α release through TLR-4 mediated mechanisms in the context of hydroxyapatite coated implants [43]. BCP crystals increase PGE2 production through upregulation of COX-1 and COX-2 in OA synovial fibroblasts [44, 45]. The co-incubation of cultured cells with BCP and known pro-inflammatory cytokines such as TNF-α and IL-1α has a synergistic effect on mitogenic and inflammatory pathways [39].
Sun et al. demonstrated that meniscal cells from OA patients calcify more readily than normal meniscal cells, and have a corresponding upregulation of many genes involved in biomineralisation including ENPP1 and ANKH [46]. Chondrocytes were long considered an inactive bystander in the pathogenesis of inflammatory joint disease; however, recent experimental work suggests that they are an active participant in inflammatory pathways within the joint. McCarthy et al. showed that BCP crystals increase MMP-13 at both gene and protein level in chondrocytes [39]. BCP crystals increase nitric oxide production, expression of inducible nitric oxide synthase mRNA, and IL-1β mRNA expression [47]. BCP crystals stimulate IL-6 secretion from chondrocytes and result in proteoglycan loss in human cartilage explants [48••]. Interestingly, IL-6 has also been shown to stimulate BCP crystal formation and chondrocyte mineralisation [48••]. The end-stage of OA is characterised by loss of articular cartilage; the mechanisms behind which are unclear. BCP crystals have been shown to increase chondrocyte apoptosis and cartilage degradation [38, 49]. The exact pathways involved remain to be fully elucidated but BCP-induced fluctuations in intracellular ionised calcium concentrations in chondrocytes have been associated with cartilage matrix degradation [50].
A key feature of many crystal arthropathies including gout and the Milwaukee shoulder syndrome is bone erosion and destruction. Bone erosion is also a prominent feature of some forms of OA, particularly erosive hand OA. In this context, BCP and CPP crystals have both recently been shown to have stimulatory effects on osteoclasts. In an in vitro murine cell line model, Chang et al. demonstrated that calcium-containing crystals enhance RANKL/M-CSF mediated osteoclastogenesis and bone resorption through extracellular-signal-regulated kinase and p38 pathways [51•]. BCP crystals have also been demonstrated to inhibit anti-osteoclastogenic cytokine signalling [52•].
Targeting Calcium Crystals in OA
The identification of the potential importance of calcium-containing crystals in osteoarthritis progression has opened up the consideration of new therapeutic avenues in a disease which has suffered from a dearth of treatment options. Therapeutic strategies targeting various inflammatory pathways have been trialled in OA with limited success. A 12-month study of intra-articular administration of an autologous interleukin-1 receptor antagonist suggested modest potential benefits in 167 patients with symptomatic knee OA in a double-blind randomised controlled trial (RCT) [53]. In a subsequent study intra-articular IL-1 blockade with anakinra was well tolerated but showed no significant improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score in a 12-week double-blind RCT of 170 patients with symptomatic knee OA [54]. This suggests that targeting the ability of BCP crystals to induce inflammatory pathways through non-NLRP3 dependent pathways may be of greater importance in addressing the inflammatory component of OA [38••]. Phosphocitrate (PC) is a compound which has attracted attention due to its potential beneficial effects on pathologic calcification. PC is a naturally occurring small molecule which inhibits the formation of calcium-containing crystals without influencing basal calcium levels [55]. In animal models of atherosclerosis PC effectively inhibited the formation of calcium-containing crystalline structures without any evident toxic effects [56]. In addition to its direct effects on calcification, PC has also been shown to inhibit calcium crystal-induced mitogenesis, cell death, and the expression of MMPs and other genes important in OA pathogenesis [57,58,59,60,61]. In a guinea pig model, PC was shown to inhibit meniscal calcification and OA progression in a crystal-induced OA model but not in a control traumatic OA model [62]. The majority of animal studies to date have focused on the intraperitoneal administration of PC as a means of systemically delivering the drug [57, 62]. Intra-articular administration of PC should be feasible in humans and would have the added theoretical benefits of increasing the PC concentration at the target site while minimising adverse systemic events.
Conclusion
In conclusion, the precise nature of the contribution of calcium-containing crystals to the pathogenesis of OA remains to be fully defined. Recent developments in clinical and translational research evidence provide support for a role, particularly for BCP crystals. The relative importance of crystals in overall OA initiation and propagation requires further investigation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602..
Conway R, McCarthy GM. Obesity and osteoarthritis: more than just mechanics. EMJ Rheumatol. 2015;2(1):75–83.
Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69.
Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–58..
Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil. 2005;13(5):361–7. https://doi.org/10.1016/j.joca.2005.01.005.
Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema M, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69(10):1779–83. https://doi.org/10.1136/ard.2009.121426.
Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40(4):723–7. https://doi.org/10.1002/art.1780400419.
Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24(2):365–71.
Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29(3):570–4.
McCarty DJ, Halverson PB, Carrera GF, Brewer BJ, Kozin F. “Milwaukee shoulder”--association of microspheroids containing hydroxyapatite crystals, active collagenase, and neutral protease with rotator cuff defects. I. Clinical aspects. Arthritis Rheum. 1981;24(3):464–73. https://doi.org/10.1002/art.1780240303.
Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis f. 2011;70(4):563–70. https://doi.org/10.1136/ard.2010.139105.
Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682–7. https://doi.org/10.1136/ard.2004.023564.
Dahaghin S, Bierma-Zeinstra SM, Reijman M, Pols HA, Hazes JM, Koes BW. Prevalence and determinants of one month hand pain and hand related disability in the elderly (Rotterdam study). Ann Rheum Dis. 2005;64(1):99–104. https://doi.org/10.1136/ard.2003.017087.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. https://doi.org/10.1016/S0140-6736(12)61729-2.
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. https://doi.org/10.1136/annrheumdis-2013-204763.
Piscitelli P, Iolascon G, Di Tanna G, Bizzi E, Chitano G, Argentiero A, et al. Socioeconomic burden of total joint arthroplasty for symptomatic hip and knee osteoarthritis in the Italian population: a 5-year analysis based on hospitalization records. Arthritis Care Res (Hoboken). 2012;64(9):1320–7. https://doi.org/10.1002/acr.21706.
Rodriguez-Fontenla C, Calaza M, Evangelou E, Valdes AM, Arden N, Blanco FJ, et al. Assessment of osteoarthritis candidate genes in a meta-analysis of nine genome-wide association studies. Arthritis Rheumatol (Hoboken, NJ). 2014;66(4):940–9.
Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthr Cartil. 2002;10(8):595–601. https://doi.org/10.1053/joca.2002.0800.
Kerkhof HJ, Bierma-Zeinstra SM, Castano-Betancourt MC, de Maat MP, Hofman A, Pols HA, et al. Serum C reactive protein levels and genetic variation in the CRP gene are not associated with the prevalence, incidence or progression of osteoarthritis independent of body mass index. Ann Rheum Dis. 2010;69(11):1976–82. https://doi.org/10.1136/ard.2009.125260.
Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil. 2010;18(11):1441–7. https://doi.org/10.1016/j.joca.2010.08.016.
Damman W, Liu R, Bloem JL, Rosendaal FR, Reijnierse M, Kloppenburg M. Bone marrow lesions and synovitis on MRI associate with radiographic progression after 2 years in hand osteoarthritis. Ann Rheum Dis. 2017;76(1):214–7. https://doi.org/10.1136/annrheumdis-2015-209036.
Sarmanova A, Hall M, Moses J, Doherty M, Zhang W. Synovial changes detected by ultrasound in people with knee osteoarthritis - a meta-analysis of observational studies. Osteoarthr Cartil. 2016;24(8):1376–83.
Yavorskyy A, Hernandez-Santana A, McCarthy G, McMahon G. Detection of calcium phosphate crystals in the joint fluid of patients with osteoarthritis - analytical approaches and challenges. Analyst. 2008;133(3):302–18. https://doi.org/10.1039/b716791a.
McCarty DJ, Lehr JR, Halverson PB. Crystal populations in human synovial fluid. Identification of apatite, octacalcium phosphate, and tricalcium phosphate. Arthritis Rheum. 1983;26(10):1220–4. https://doi.org/10.1002/art.1780261008.
MacMullan P, McMahon G, McCarthy G. Detection of basic calcium phosphate crystals in osteoarthritis. Joint Bone Spine. 2011;78(4):358–63. https://doi.org/10.1016/j.jbspin.2010.10.008.
Dieppe PA, Crocker P, Huskisson EC, Willoughby DA. Apatite deposition disease. A New Arthropathy. Lancet. 1976;1(7954):266–9.
Halverson PB, McCarty DJ, Cheung HS, Ryan LM. Milwaukee shoulder syndrome: eleven additional cases with involvement of the knee in seven (basic calcium phosphate crystal deposition disease). Semin Arthritis Rheum. 1984;14(1):36–44. https://doi.org/10.1016/0049-0172(84)90007-6.
Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60(9):2694–703. https://doi.org/10.1002/art.24774.
Nalbant S, Martinez JA, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR Jr. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthr Cartil. 2003;11(1):50–4. https://doi.org/10.1053/joca.2002.0861.
Lumbreras B, Pascual E, Frasquet J, Gonzalez-Salinas J, Rodriguez E, Hernandez-Aguado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64(4):612–5. https://doi.org/10.1136/ard.2004.027268.
Kohn NN, Hughes RE, Mc CD Jr, Faires JS. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: the “pseudogout syndrome”. II. Identification of crystals. Ann Intern Med. 1962;56(5_Part_1):738–45. https://doi.org/10.7326/0003-4819-56-5-738.
Fam AG, Morava-Protzner I, Purcell C, Young BD, Bunting PS, Lewis AJ. Acceleration of experimental lapine osteoarthritis by calcium pyrophosphate microcrystalline synovitis. Arthritis Rheum. 1995;38(2):201–10. https://doi.org/10.1002/art.1780380208.
Rosenthal AK. Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol. 2011;23(2):170–3.
Low C, Conway R, O'Shea FD. Pseudo pseudogout. Ir Med J. 2014;107(10):333–4.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. https://doi.org/10.1038/nature04516.
Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science (New York, NY). 2010;327(5963):296–300.
Pazar B, Ea HK, Narayan S, Kolly L, Bagnoud N, Chobaz V, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186(4):2495–502. https://doi.org/10.4049/jimmunol.1001284.
•• Ea HK, Chobaz V, Nguyen C, Nasi S, van Lent P, Daudon M, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PloS One. 2013;8(2):e57352. This paper demonstrates that BCP crystal effects may be mediated by pathways independent of the NLRP3 inflammasome.
McCarthy GM, Westfall PR, Masuda I, Christopherson PA, Cheung HS, Mitchell PG. Basic calcium phosphate crystals activate human osteoarthritic synovial fibroblasts and induce matrix metalloproteinase-13 (collagenase-3) in adult porcine articular chondrocytes. Ann Rheum Dis. 2001;60(4):399–406. https://doi.org/10.1136/ard.60.4.399.
Molloy ES, Morgan MP, Doherty GA, McDonnell B, O'Byrne J, Fitzgerald DJ, et al. Mechanism of basic calcium phosphate crystal-stimulated matrix metalloproteinase-13 expression by osteoarthritic synovial fibroblasts: inhibition by prostaglandin E2. Ann Rheum Dis. 2008;67(12):1773–9. https://doi.org/10.1136/ard.2007.079582.
Cunningham CC, Mills E, Mielke LA, O'Farrell LK, Lavelle E, Mori A, et al. Osteoarthritis-associated basic calcium phosphate crystals induce pro-inflammatory cytokines and damage-associated molecules via activation of Syk and PI3 kinase. Clin Immunol (Orlando, Fla). 2012;144(3):228–36.
• Corr EM, Cunningham CC, Helbert L, McCarthy GM, Dunne A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res Ther. 2017;19(1):23. This paper demonstrates that the effects of BCP crystals are mediated by the membrane-proximal tyrosine kinases Syk and PI3K.
Grandjean-Laquerriere A, Tabary O, Jacquot J, Richard D, Frayssinet P, Guenounou M, et al. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials. 2007;28(3):400–4. https://doi.org/10.1016/j.biomaterials.2006.09.015.
Molloy ES, Morgan MP, Doherty GA, McDonnell B, O'Byrne J, Fitzgerald DJ, et al. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: role of prostaglandin E2 and the EP4 receptor. Osteoarthr Cartil. 2009;17(5):686–92. https://doi.org/10.1016/j.joca.2008.09.014.
Molloy ES, Morgan MP, Doherty GA, McDonnell B, Hilliard M, O'Byrne J, et al. Mechanism of basic calcium phosphate crystal-stimulated cyclo-oxygenase-1 up-regulation in osteoarthritic synovial fibroblasts. Rheumatology (Oxford). 2008;47(7):965–71. https://doi.org/10.1093/rheumatology/ken144.
Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton HJ, Zinchenko N, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12(2):R56. https://doi.org/10.1186/ar2968.
Ea HK, Uzan B, Rey C, Liote F. Octacalcium phosphate crystals directly stimulate expression of inducible nitric oxide synthase through p38 and JNK mitogen-activated protein kinases in articular chondrocytes. Arthritis Res Ther. 2005;7(5):R915–26. https://doi.org/10.1186/ar1763.
•• Nasi S, So A, Combes C, Daudon M, Busso N. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2016;75(7):1372–9. Key paper showing the feedback loop between IL-6 and BCP.
Ea HK, Monceau V, Camors E, Cohen-Solal M, Charlemagne D, Liote F. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann Rheum Dis. 2008;67(11):1617–25. https://doi.org/10.1136/ard.2008.087718.
Nguyen C, Lieberherr M, Bordat C, Velard F, Come D, Liote F, et al. Intracellular calcium oscillations in articular chondrocytes induced by basic calcium phosphate crystals lead to cartilage degradation. Osteoarthr Cartil. 2012;20(11):1399–408. https://doi.org/10.1016/j.joca.2012.07.017.
• Chang CC, Tsai YH, Liu Y, Lin SY, Liang YC. Calcium-containing crystals enhance receptor activator of nuclear factor kappaB ligand/macrophage colony-stimulating factor-mediated osteoclastogenesis via extracellular-signal-regulated kinase and p38 pathways. Rheumatology (Oxford). 2015;54(10):1913–22. This paper shows that calcium-containing crystals enhance RANKL/M-CSF-induced osteoclastogenesis through extracellular-signal-regulated kinase and p38 pathways.
• Cunningham CC, Corr EM, McCarthy GM, Dunne A. Intra-articular basic calcium phosphate and monosodium urate crystals inhibit anti-osteoclastogenic cytokine signalling. Osteoarthritis Cartilage. 2016;24(12):2141–52. This important paper demonstrates the effects of BCP in inhibiting anti-osteoclastogenic signalling through both IL-6 and IFN-γ pathways through activation of p38 and ERK MAPK as well as JNK.
Auw Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartil. 2008;16(4):498–505. https://doi.org/10.1016/j.joca.2007.07.008.
Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–52. https://doi.org/10.1002/art.24096.
Tew WP, Malis CD, Howard JE, Lehninger AL. Phosphocitrate inhibits mitochondrial and cytosolic accumulation of calcium in kidney cells in vivo. Proc Natl Acad Sci U S A. 1981;78(9):5528–32. https://doi.org/10.1073/pnas.78.9.5528.
Shankar R, Crowden S, Sallis JD. Phosphocitrate and its analogue N-sulpho-2-amino tricarballylate inhibit aortic calcification. Atherosclerosis. 1984;52(2):191–8. https://doi.org/10.1016/0021-9150(84)90117-5.
Sun Y, Haines N, Roberts A, Ruffolo M, Mauerhan DR, Mihalko KL, et al. Disease-modifying effects of phosphocitrate and phosphocitrate-β-ethyl ester on partial meniscectomy-induced osteoarthritis. BMC Musculoskelet Disord. 2015;16(1):270. https://doi.org/10.1186/s12891-015-0724-x.
Cheung HS, Sallis JD, Struve JA. Specific inhibition of basic calcium phosphate and calcium pyrophosphate crystal-induction of metalloproteinase synthesis by phosphocitrate. Biochim Biophys Acta. 1996;1315(2):105–11. https://doi.org/10.1016/0925-4439(95)00106-9.
Nair D, Misra RP, Sallis JD, Cheung HS. Phosphocitrate inhibits a basic calcium phosphate and calcium pyrophosphate dihydrate crystal-induced mitogen-activated protein kinase cascade signal transduction pathway. J Biol Chem. 1997;272(30):18920–5. https://doi.org/10.1074/jbc.272.30.18920.
Sun Y, Reuben P, Wenger L, Sallis JD, Demadis KD, Cheung HS. Inhibition of calcium phosphate-DNA coprecipitates induced cell death by phosphocitrates. Front Biosci. 2005;10(1-3):803–8. https://doi.org/10.2741/1574.
Sun Y, Franklin AM, Mauerhan DR, Hanley EN. Biological effects of phosphocitrate on osteoarthritic articular chondrocytes. Open Rheumatol J. 2017;11(1):62–74. https://doi.org/10.2174/1874312901711010062.
Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54(8):2452–61. https://doi.org/10.1002/art.22017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Financial Disclosures
No funding was received for this manuscript. The authors have no conflicts of interest in relation to the current work.
Additional information
This article is part of the Topical Collection on Crystal Arthritis
Rights and permissions
About this article
Cite this article
Conway, R., McCarthy, G.M. Calcium-Containing Crystals and Osteoarthritis: an Unhealthy Alliance. Curr Rheumatol Rep 20, 13 (2018). https://doi.org/10.1007/s11926-018-0721-9
Published:
DOI: https://doi.org/10.1007/s11926-018-0721-9