Abstract
Purpose of Review
Although intra-articular (IA) mineralization resulting from calcium crystal deposition is common in osteoarthritis (OA), its clinical significance remains unclear, including whether it is a cause or consequence of OA. This article reviews the current understanding of IA mineralization in OA including epidemiology, pathogenesis, and associations with clinical and structural OA outcomes.
Recent Findings
Many gaps in knowledge remain regarding the role in OA of calcium crystal deposition resulting in IA mineralization. However, recent studies have begun to further elucidate this role, including through use of advanced imaging modalities that can differentiate calcium crystal types, as well as basic science studies investigating whether calcium pyrophosphate (CPP) and basic calcium phosphate (BCP) depositions represent different phenotypes and endotypes in OA.
Summary
An improved understanding of the role of IA mineralization in OA may lead to development of targeted therapies with potential clinical and structural benefits for knee OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra-articular (IA) mineralization resulting from calcium crystal deposition is common in osteoarthritis (OA), with articular cartilage calcification universally present in advanced or end-stage OA [1,2,3]. However, the clinical significance of this IA mineralization remains unclear. Crystals that can deposit intra-articularly include calcium pyrophosphate (CPP) and basic calcium phosphate (BCP). The term chondrocalcinosis refers to articular cartilage calcification that is identified on imaging or histological examination, but is not synonymous with the deposition of CPP crystals [4].
Clinical presentations of CPP are broadly defined by the umbrella term calcium pyrophosphate deposition disease (CPPD) [4], and includes asymptomatic CPPD, OA with CPPD, acute CPP crystal arthritis, and chronic CPP crystal arthritis. BCP clinical manifestations include calcific tendinitis (also called periarthritis), most commonly affecting the shoulder, and a destructive, erosive monoarthritis termed Milwaukee shoulder [5].
The common pathophysiology underlying both CPP and BCP crystal arthritis is crystal-induced low-grade inflammation. Whether this crystal-induced inflammation also contributes to the pathogenesis of OA remains to be fully elucidated. Chondrocalcinosis, as well as the presence of BCP and CPP crystals, have been associated with OA, particularly with more advanced stages of radiographic disease [6,7,8,9,10,11]. However, gaps in the understanding of IA mineralization with OA remain, such as whether it is a cause or consequence of OA. It remains unclear whether this calcium deposition increases the risk of cartilage damage or other structural abnormalities. Additionally, whether targeting the presence of IA mineralization as an OA phenotype may be of benefit for clinical and structural outcomes requires further study.
Epidemiology
Prevalence
The prevalence of chondrocalcinosis visualized on radiographs depends on the anatomic site studied. In the community-based Framingham study, the prevalence of knee chondrocalcinosis was 8.1% among a cohort with age > 60 years [6]. In a UK, community-based study (mean age 63.7 years), the prevalence was similar (7.0%) [7]. In autopsy studies, the prevalence of chondrocalcinosis on gross inspection and histopathologic examination was higher, with a prevalence of 14–20% in the knee [12, 13].
Risk factors
Radiographic chondrocalcinosis is more prevalent with increasing age, from 25% in those aged 55–59 to 60% in those aged 80–84[7]. Among adults 18 years and older, an Italian study estimated a prevalence of 0.42% [14], while an Italian study of those 65 and older found a prevalence of 10.4% [8]. A Spanish primary care-based study of those 60 and older found a similar prevalence of chondrocalcinosis of either the knee or wrist [15].
Female sex has been associated with higher frequency chondrocalcinosis in some studies [6, 15] though not others [7]. A 2011 meta-analysis did not support a sex predisposition [4]. The prevalence of knee chondrocalcinosis in a Beijing cohort was much lower compared to the US-based Framingham cohort (1.8% vs 6.2% in men; 2.7% vs 7.7% in women) [16], suggesting differences by race.
Other risk factors for CPPD include metabolic conditions such as hyperparathyroidism, hypomagnesemia, hemochromatosis, and hypophosphatasia [17,18,19,20]. Hypomagnesemia from gastrointestinal and renal losses are associated with CPPD, such as with rare conditions like Bartter’s and Gitleman’s syndromes [20]. Some studies have suggested associations between CPPD and medications that induce hypomagnesemia (diuretics, proton pump inhibitors) [21,22,23].
There are rare, familial forms of CPPD, from which some of the pathobiology has been elucidated. These include mutations in osteoprotegerin, the decoy receptor for RANK ligand, and ANKH, a transmembrane protein that increases the transport of inorganic pyrophosphate to the extracellular space [24].
OA and IA mineralization
The presence of radiographic chondrocalcinosis is associated with radiographic OA [6,7,8, 15]. In synovial fluid studies of knee OA, the prevalence of BCP or hydroxyapatite crystals ranges from 26–57% of knees in older studies [9,10,11, 25], while the prevalence of CPP crystals ranges from 12 to 52% of knees [9,10,11, 25, 26].
Articular cartilage calcification has been associated with worsening OA severity [3, 9,10,11]. Studies have suggested that articular calcification is associated with increasing age as well as OA severity [9,10,11, 27]. However, in a study of 106 knees from 56 donors at the time of autopsy (mean age, 52 years), older age, but not OA severity, was correlated with the presence of calcification [28]. No age correlation was found in other studies [3, 29]. A potential explanation, discussed later in this review, may be that mineralization from CPP crystals is associated with older age (cellular senescence), and mineralization from BCP crystals with OA severity [9, 11].
Studies from Fuerst and colleagues, using multiple methods of visualization including electron microscopy and digital contact radiography, have also demonstrated that articular cartilage calcification is universally present in end-stage OA joints at the time of joint replacement [3, 29]. Further, older autopsy studies showed that articular cartilage calcification is also present in normal joints [30]. These findings suggest that IA mineralization may not simply be a byproduct of severe OA, or advanced age, but that there may be a pathogenic role for calcium crystal deposition in the joint.
Identification and imaging
The identification and diagnosis of IA mineralization using clinically available modalities remains challenging. While CPP crystals are more easily identified in aspirated synovial fluid using polarized light microscopy, BCP crystals require Alizarin red staining and large aggregates to be seen on light microscopy [31]. Conventional radiograph only has a sensitivity of approximately 40% for the detection of chondrocalcinosis [32,33,34].
In a 2016 meta-analysis of 13 studies using ultrasound to detect CPPD, the sensitivity ranged from 34 to 77% and the specificity from 92 to 100% [35]. An OMERACT working group developed definitions for the detection of knee and wrist CPPD in the hyaline cartilage and fibrocartilage [36, 37]. These were validated [38] and studied in another independent cohort [32]. There are no consensus criteria for the detection of BCP on ultrasound, although ultrasound has been used for diagnosis and guided therapies.
Conventional CT may also be used to detect IA mineralization, although dual energy CT (DECT) has become increasingly studied [39]. Using DECT, the sensitivity and specificity for detecting CPPD were 77.8% and 93.8%, respectively, in one study using polarized light microscopy visualization of synovial fluid aspirate as a reference standard [34]. A novel CT scoring system, the Boston University Calcium Knee Score (BUCKS) was developed in the Multicenter Osteoarthritis Study (MOST) cohort for the semi-quantitative scoring of IA mineralization in the knee, with good inter- and intra-reader reliability [40]. DECT has also been studied for its ability to discriminate CPP from BCP crystals in both ex vivo and in vivo samples [41,42,43]. However, limitations remain. DECT is unable to detect early CPPD, when crystal deposits are too small [44]. In a phantom study, while DECT was able to discriminate other crystal compositions from monosodium urate, it failed to discriminate CPP from BCP with small crystal diameters [45].
Improved methods for the detection of IA mineralization, as well as the differentiation of CPP from BCP deposits, are essential for research efforts to understand the role of calcium crystal deposition in OA pathogenesis and progression, and whether its presence may serve as a suitable imaging biomarker for use in clinical trials.
Chondrocalcinosis and osteoarthritis
OA structural outcomes
The presence of chondrocalcinosis has been associated with OA severity, but longitudinal studies have been conflicting as to whether there is increased risk of OA structural progression. Neogi et al. studied two OA cohorts, the Boston OA Knee Study (n = 265 knees) and the Health ABC cohort (n = 373 knees), in which chondrocalcinosis prevalence in the knee was 9%, and 18.5%, respectively [46]. Knee MRIs were obtained at baseline and follow-up, but no association of baseline chondrocalcinosis was seen with the risk of cartilage loss on MRI. Similar findings were seen in the Knee and Hip OsteoArthritis Long-term Assessment (KHOALA) cohort of 636 participants with symptomatic knee OA with or without hip OA [47•]. At 5-year follow-up, baseline radiographic chondrocalcinosis was not associated with radiographic progression or risk of total joint replacement. Conversely, in a small sample of 70 participants from the Osteoarthritis Initiative (OAI), the presence of chondrocalcinosis was associated with worsening of the MRI score at 4-year follow-up [48•]. Although data from observational studies have been mixed, they suggest that IA mineralization, when visualized as radiographic chondrocalcinosis, may place knees at higher risk for cartilage loss.
OA clinical outcomes
The presence of chondrocalcinosis has been associated with OA clinical outcomes. In the KHOALA cohort, Latourte and colleagues demonstrated that baseline chondrocalcinosis was associated with worse pain and physical function scores measured on the Western Ontario and McMaster Universities Arthritis Index (WOMAC), although there was no association with OA structural changes [47•]. Similarly, Han and colleagues found associations in OAI with pain on the WOMAC and Intermittent and Constant Osteoarthritis Pain (ICOAP) questionnaire, though not with synovitis on MRI [49]. These studies suggest that IA mineralization may contribute to the development of symptoms and functional limitations in people with OA. However, as with studies of OA structural outcomes, current published evidence has been limited to use of radiographic chondrocalcinosis as a proxy for the presence of IA mineralization.
Pathophysiology
Although observational studies in cohorts of adults with or at risk for OA have not provided conclusive evidence that radiographic chondrocalcinosis (as a surrogate for IA mineralization) is associated with higher risk of OA incidence or progression, animal models have demonstrated this link with structural progression. In a New Zealand white rabbit model of OA, the repeated injection of CPP crystals into the knee resulted in severe OA [50]. In a Hartley guinea pig model of OA, BCP calcification of the meniscus resulted in cartilage degeneration [51].
The pathophysiology underlying calcium crystal deposition effects on OA structural and clinical outcomes may involve inflammation and the biomechanical effects of crystals [52]. Formation of crystals depends upon several factors. Briefly, inorganic pyrophosphate blocks the formation of BCP crystals while high levels of inorganic pyrophosphate lead to CPP crystal formation [53]. In a study of human ankles ranging from normal to radiographic OA, Muehleman and colleagues found that crystal deposition caused biomechanical damage to the cartilage, leading to OA structural progression [27]. From their animal study, Cheung, et al. also concluded that meniscal calcification may result in altered joint biomechanics, contributing to structural damage in the joint [51].
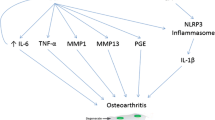
CPP crystals lead to inflammation through activation of the NLRP3 inflammasome, leading to release of the pro-inflammatory cytokine IL-1β, similar to what is seen in gout via monosodium urate crystals [54]. It is reasonable to hypothesize that calcium crystal induced inflammation may contribute to pain development in OA, including to pain fluctuations.
The understanding of how BCP crystals contribute to inflammation and possibly to structural damage is more complex. Hydroxyapatite crystals have been shown to induce IL-1β and IL-18 release in vitro [55], but this may be via alternative pathways independent of NLRP3 inflammasome activation [56, 57]. The membrane proximal kinases Syk and PI3 have been implicated in pro-inflammatory cytokine release by BCP crystals [58]. BCP crystals also promote TNF release from macrophages [59] and IL-6 from chondrocytes [60]. The up-regulation of select matrix metalloproteinases from OA synovial fibroblasts and the increase of prostaglandin 2 production via COX-1 and -2 [61, 62] may also contribute to structural damage of the joint through degradation of extracellular matrix components in the cartilage.
Animal models and other basic science studies support the pathogenic role of calcium crystals in OA, particularly through the elaboration of pro-inflammatory cytokines. Further work is needed to determine whether targeting cytokine-specific pathways may be a viable treatment strategy for the subset of individuals with knee OA who also have IA mineralization on imaging.
Treatment of osteoarthritis with IA mineralization
There are no specific guidelines for the management of OA with chondrocalcinosis [63]. The management is largely derived from the treatment of CPPD [64].
For acute CPP arthritis, recommendations include steroids (intra-articular preferred over systemic dosing), colchicine, and nonsteroidal anti-inflammatory drugs, although the evidence is limited to older, small studies. The available evidence, and therefore therapies, for chronic or recurrent CPP arthritis are also limited. A small (n = 36) double-blind, randomized study of hydroxychloroquine demonstrated better response in reduction of tender and swollen joints in chronic CPP arthritis [65]. A double-blind, randomized crossover study of methotrexate (15 mg weekly for 3 months) in 26 participants with chronic CPP arthritis did not demonstrate benefit in disease activity 44 (DAS44) improvement [66].
The treatment of BCP depends on the clinical presentation. Calcific tendinitis may be treated with NSAID or corticosteroid injection. If refractory, barbotage and extracorporeal shockwave therapy may be considered [5]. For Milwaukee shoulder, surgery may be considered if refractory to more conservative measures.
Given the role of inflammation in CPP arthritis, biologic therapies have been studied for its management. A systematic review performed in 2020 found only 11 studies (all case series or case reports) totaling 74 patients with data reported following the receipt of the IL-1 receptor antagonist anakinra for either acute or chronic CPP arthritis [67]. Of those with acute arthritis episodes, 81% had a response (variably defined) and of those with chronic arthritis, 43% had a response, with adverse events reported in 4% overall. IL-6 inhibition with tocilizumab has also been studied in a small pilot study of 11 participants with recurrent acute and chronic CPP arthritis [68].
Although prior studies of IL-1β inhibition for knee OA were negative [69,70,71], there has been renewed interest in targeting inflammatory pathways for OA. An exploratory analysis of a large trial of canakinumab, an IL-1β inhibitor, in individuals with cardiovascular disease and elevated high sensitivity C-reactive protein levels found significant reductions in total joint replacements [72•]. This finding supports the hypothesis that there is a subset of individuals with knee OA, perhaps those with IA mineralization demonstrated on imaging, in whom directly targeting inflammation might be helpful. Further work needs to be done in defining this phenotype.
Gaps in knowledge and future work
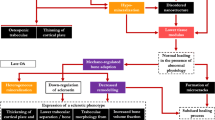
Many gaps in the understanding of IA mineralization and its role in OA remain (Fig. 1). A major question to disentangle is whether IA mineralization is a cause or consequence of OA. With advanced imaging modalities, studies can be better designed to address whether there are location- and/or tissue-specific effects of calcium crystal deposition, and whether CPP and BCP represent different endotypes/phenotypes. All of this work would lead to more precise OA phenotyping and targeted treatment.
Novel advanced imaging
Advancement of research that can determine whether location- and tissue-specific effects exist for OA structural and clinical outcomes depend in part on usage of improved imaging. If crystal deposits are sufficiently large within knees, then the differentiation of CPP from BCP may be possible using DECT [44]. However, limitations remain, especially with smaller deposits. Photon-counting detector CT is a novel advanced imaging technique which has been capability to discriminate CPP from BCP compared with DECT [73,74,75]. It is non-invasive and is being studied for clinical use, and has a benefit over DECT of finer spatial resolution [76].
Location- and tissue-specific effects
Earlier work characterizing calcium crystal deposition was unable to properly study the distribution of CPP vs BCP [7]. Nguyen et al. looked at 20 knees with primary OA at the time of total knee replacement [77]. Consistent with the work of Fuerst and colleagues demonstrating that articular cartilage calcification is widespread within the OA knee [3], all knees had calcium containing crystals and all had BCP crystals. Crystals were present in all compartments. It has not been clearly shown whether there are location-specific effects of IA mineralization within the knee, that is, whether compartments or subregions of the joint with a larger burden of mineralization is at higher risk of cartilage damage.
Studies have not yet provided clear insights into whether IA mineralization in different anatomic tissues within the joint may have differential effects on cartilage damage. Calcification of the meniscus in knee OA was investigated by Sun et al. in an in vitro study using cultured meniscal cells from individuals with knee OA [78]. They demonstrated that these cells produced more calcium deposition than did non-OA controls. With CT-detected IA mineralization in MOST, Misra, et al. found that the most common site was the meniscus [79]. Whether there is a difference in the effect of IA mineralization in the meniscus versus the hyaline articular cartilage on cartilage damage or worsening remains to be seen.
CPP VS BCP: different phenotypes and endotypes?
IA mineralization resulting from CPP vs BCP deposition may represent different phenotypes (disease subtypes defined by observable characteristics) and endotypes (disease subtypes defined by pathobiological mechanisms). The association of BCP crystals and chondrocyte hypertrophy via the Wnt signalling pathway has long been known. Meyer et al. studied CPP crystals in individuals with chondrocalcinosis compared with BCP crystals in individuals with OA, and defined two different endotypes: one of chondrocyte hypertrophy in BCP (universally present in OA articular cartilage) and another of cellular senescence in CPP [80•]. This is consistent with older work that links chondrocalcinosis or CPP with increasing age and BCP with increasing OA severity [3, 9,10,11].
Few studies have evaluated the differences in inflammation via cytokine production by crystal type. Scanu, et al. studied MSU, CPP, and BCP crystals and their effect of pro-inflammatory cytokine production from polymorphonuclear cells, monocytes, and lymphocytes [81]. They found that BCP crystals, compared to the other crystal types, required higher concentrations to cause the same effects. In a small study of 110 individuals with knee OA, the presence of BCP crystals detected by synovial fluid and scanning electron microscopy was associated with higher WOMAC scores but the presence of CPP with higher Kellgren-Lawrence grading [82].
Potential differences in structural outcomes by crystal type remains to be elucidated. While CPP has been understood to be more inflammatory and thus linked to more structural severity, BCP can also be destructive as evidenced by Milwaukee shoulder. A recent DECT study demonstrated BCP-based calcifications with intraosseous migration, supporting the erosive nature of BCP crystals [43].
Conclusions
In conclusion, the renewed interest in elucidating the role of IA mineralization in OA may lead to the development of novel targeted treatment strategies benefiting both structural and symptoms. This is much needed in this condition that affects 528 million adults worldwide and which, despite being a leading cause of pain and disability, has limited effective treatment options.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Schumacher HR. Crystals, inflammation, and osteoarthritis. Am J Med. 1987;83:11–6.
Ea HK, Nguyen C, Bazin D, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–8.
Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703.
Zhang W, Doherty M, Bardin T, et al. European league against rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70:563–70.
Rosenthal AK. Basic calcium phosphate crystal-associated musculoskeletal syndromes: an update. Vol. 30, Current Opinion in Rheumatology. Lippincott Williams and Wilkins. 2018;168–72.
Felson D, Anderson J, Naimark A, et al. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–5.
Neame RL, Carr AJ, Muir K, et al. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003;62:513–8.
Ramonda R, Musacchio E, Perissinotto E, et al. Prevalence of chondrocalcinosis in Italian subjects from northeastern Italy. The Pro.V.A. (PROgetto Veneto Anziani) study. Clin Exp Rheumatol 27:981–4.
Halverson PB, McCarty DJ. Patterns of radiographic abnormalities associated with basic calcium phosphate and calcium pyrophosphate dihydrate crystal deposition in the knee. Ann Rheum Dis. 1986;45:603–5.
Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4.
Nalbant S, Martinez JAM, Kitumnuaypong T, et al. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthr Cartil. 2003;11:50–4.
Mitrovic D, Stankovic A, Iriarte-Borda O, et al. The prevalence of chondrocalcinosis in the human knee joint: an autopsy survey. J Rheumatol. 1988;15:633–41.
Ryu K, Iriuchishima T, Oshida M, et al. The prevalence of and factors related to calcium pyrophosphate dihydrate crystal deposition in the knee joint. Osteoarthr Cartil. 2014;22:975–9.
Salaffi F, de Angelis R, Grassi W. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. Clin Exp Rheumatol. 2005;23:819–28.
Sanmarti R, Panella D, Brancos MA, et al. Prevalence of articular chondrocalcinosis in elderly subjects in a rural area of Catalonia. Ann Rheum Dis. 1993;52:418–22.
Zhang Y, Terkeltaub R, Nevitt M, et al. Lower prevalence of chondrocalcinosis in Chinese subjects in Beijing than in white subjects in the United States: the Beijing osteoarthritis study. Arthritis Rheum. 2006;54:3508–12.
Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology. 2009;48:711–5.
Richette P, Ayoub G, Lahalle S, et al. Hypomagnesemia associated with chondrocalcinosis: a cross-sectional study. Arthritis Care Res. 2007;57:1496–501.
Zeng C, Wei J, Terkeltaub R, et al. Dose-response relationship between lower serum magnesium level and higher prevalence of knee chondrocalcinosis. Arthritis Res Ther. 2017;19:236.
Jones AC, Chuck AJ, Arie EA, et al. Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 1992;22:188–202.
Felson DT, Rabasa G, Chen X, et al. The association of diuretics and proton pump inhibitors with chondrocalcinosis. ACR Open Rheumatol. 2021;0:1–5.
KleiberBalderrama C, Rosenthal AK, Lans D, et al. Calcium pyrophosphate deposition disease and associated medical comorbidities: a national cross-sectional study of US veterans. Arthritis Care Res. 2017;69:1400–6.
Liew JW, Peloquin C, Tedeschi SK, et al. Proton-pump inhibitors and risk of calcium pyrophosphate deposition in a population-based study. Arthritis Care Res. 2022.
Williams CJ, Rosenthal AK. Pathogenesis of calcium pyrophosphate deposition disease. Vol. 35, Best Practice and Research: Clinical Rheumatology. Bailliere Tindall Ltd; 2021.
Pattrick M, Hamilton E, Wilson R, et al. Association of radiographic changes of osteoarthritis, symptoms, and synovial fluid particles in 300 knees. Ann Rheum Dis. 1993;52:97–103.
Ledingham J, Regan M, Jones A, et al. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52:520–6.
Muehleman C, Li J, Aigner T, et al. Association between crystals and cartilage degeneration in the ankle. J Rheumatol. 2008;35:1108–17.
Mitsuyama H, Healey RM, Terkeltaub RA, et al. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthr Cartil. 2007;15:559–65.
Fuerst M, Niggemeyer O, Lammers L, et al. Articular cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet Disord. 2009;10.
Scotchford CA, Greenwald S, Ali SY. Calcium phosphate crystal distribution in the superficial zone of human femoral head articular cartilage. J Anat. 1992;181:293–300.
MacMullan PA, McCarthy GM. The meniscus, calcification and osteoarthritis: a pathologic team. Arthritis Res Ther. 2010;12:12–3.
Lee KA, Lee SH, Kim HR. Diagnostic value of ultrasound in calcium pyrophosphate deposition disease of the knee joint. Osteoarthr Cartil. 2019;27:781–7. https://doi.org/10.1016/j.joca.2018.11.013.
Sirotti S, Becce F, Sconfienza LM, et al. Reliability and diagnostic accuracy of radiography for the diagnosis of calcium pyrophosphate deposition: performance of the novel definitions developed by an international multidisciplinary working group. Arthritis Rheumatol. 2022.
Tanikawa H, Ogawa R, Okuma K, et al. Detection of calcium pyrophosphate dihydrate crystals in knee meniscus by dual-energy computed tomography. J Orthop Surg Res. 2018;13.
Filippou G, Adinolfi A, Cimmino MA, et al. Diagnostic accuracy of ultrasound, conventional radiography and synovial fluid analysis in the diagnosis of calcium pyrophosphate dihydrate crystal deposition disease. Clin Exp Rheumatol. 2016;34:254–60.
Filippou G, Scirè CA, Damjanov N, et al. Definition and reliability assessment of elementary ultrasonographic findings in calcium pyrophosphate deposition disease: a study by the OMERACT calcium pyrophosphate deposition disease ultrasound subtask force. J Rheumatol. 2017;44:1744–9.
Filippou G, Scirè CA, Adinolfi A, et al. Identification of calcium pyrophosphate deposition disease (CPPD) by ultrasound: reliability of the OMERACT definitions in an extended set of joints - an international multiobserver study by the OMERACT calcium pyrophosphate deposition disease ultrasound subtask force. Ann Rheum Dis. 2018;77:1195–200.
Filippou G, Scanu A, Adinolfi A, et al. Criterion validity of ultrasound in the identification of calcium pyrophosphate crystal deposits at the knee: an OMERACT ultrasound study. Ann Rheum Dis. 2021;80:261–7.
Filippou G, Pascart T, Iagnocco A. Utility of ultrasound and dual energy CT in crystal disease diagnosis and management. Curr Rheumatol Rep. 2020;22:1–8.
Guermazi A, Jarraya M, Lynch JA, et al. Reliability of a new scoring system for intraarticular mineralization of the knee: Boston University Calcium Knee Score (BUCKS). Osteoarthr Cartil. 2020;28:802–10.
Pascart T, Norberciak L, Legrand J, et al. Dual-energy computed tomography in calcium pyrophosphate deposition: initial clinical experience. Osteoarthr Cartil. 2019;27:1309–14.
Pascart T, Falgayrac G, Norberciak L, et al. Dual-energy computed-tomography-based discrimination between basic calcium phosphate and calcium pyrophosphate crystal deposition in vivo. Ther Adv Musculoskelet Dis. 2020;12:1–9.
Collinot JA, Pascart T, Budzik JF, et al. Non-invasive characterization of intra-articular mineralization using dual-energy computed tomography. Rheumatology. 2020;59:3997–8.
Budzik J, Marzin C, Legrand J, et al. Can dual-energy computed tomography be used to identify early calcium crystal deposition in the knees of patients with calcium pyrophosphate deposition? Arthritis Rheumatol. 2021;73:687–92.
Døssing A, Müller FC, Becce F, et al. Dual-energy computed tomography for detection and characterization of monosodium urate, calcium pyrophosphate, and hydroxyapatite. Invest Radiol. 2021;1–8.
Neogi T, Nevitt M, Niu J, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum. 2006;54:1822–8.
Latourte A, Rat AC, Ngueyon Sime W, et al. Chondrocalcinosis of the knee and the risk of osteoarthritis progression: data from the knee and hip osteoarthritis long-term assessment cohort. Arthritis and Rheumatology 2020;72:726–32. A longitudinal study of radiographic chondrocalcinosis with risk of structural and clinical OA outcomes.
Foreman SC, Gersing AS, von Schacky CE, et al. Chondrocalcinosis is associated with increased knee joint degeneration over 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2020;28:201–7. A longitudinal study of radiographic chondrocalcinosis with risk of structural OA outcomes.
Han BK, Kim W, Niu J, et al. Chondrocalcinosis in knee joints is associated with pain but not with synovitis: data from the osteoarthritis initiative. Arthritis Care Res. 2017;69:1651–8.
Fam AG, Morava-Protzner I, Purcell C, et al. Acceleration of experimental lapine osteoarthritis by calcium pyrophosphate microcrystalline synovitis. Arthritis Rheum. 1995;38:201–10.
Cheung HS, Sallis JD, Demadis KD, et al. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54:2452–61.
Ea HK, Nguyen C, Bazin D, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–8.
Thouverey C, Bechkoff G, Pikula S, et al. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr Cartil. 2009;17:64–72.
Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41.
Jin C, Frayssinet P, Pelker R, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108:14867–72.
Pazár B, Ea H-K, Narayan S, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186:2495–502.
Ea HK, Chobaz V, Nguyen C, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8:1–8.
Corr EM, Cunningham CC, Helbert L, et al. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res Ther. 2017;19:1–13. https://doi.org/10.1186/s13075-017-1225-0.
Grandjean-Laquerriere A, Tabary O, Jacquot J, et al. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials. 2007;28:400–4.
Nasi S, So A, Combes C, et al. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2016;75:1372–9.
Molloy ES, Morgan MP, Doherty GA, et al. Mechanism of basic calcium phosphate crystal-stimulated matrix metalloproteinase-13 expression by osteoarthritic synovial fibroblasts: Inhibition by prostaglandin E2. Ann Rheum Dis. 2008;67:1773–9.
Molloy ES, Morgan MP, Doherty GA, et al. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: role of prostaglandin E2 and the EP4 receptor. Osteoarthr Cartil. 2009;17:686–92.
Abhishek A, Neogi T, Choi H, et al. Review: unmet needs and the path forward in joint disease associated with calcium pyrophosphate crystal deposition. Arthritis Rheumatol. 2018;70:1182–91.
Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011;70:571–5.
Rothschild B, Yakubov L. Prospective 6-month, double-blind trial of hydroxychloroquine treatment of CPDD. Compr Ther. 1997;23:327–31.
Finckh A, McCarthy G, Madigan A, et al. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther. 2014;16:1–8.
Cipolletta E, di Matteo A, Scanu A, et al. Biologics in the treatment of calcium pyrophosphate deposition disease: systematic literature review. Clin Exp Rheumatol. 2020;38:1001–7.
Latourte A, Ea HK, Frazier A, et al. Tocilizumab in symptomatic calcium pyrophosphate deposition disease: a pilot study. Vol. 79, Annals of the Rheumatic Diseases. BMJ Publishing Group. 2020;1126–8.
Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. 2009;61:344–52.
Cohen SB, Proudman S, Kivitz AJ, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. 2011;13.
Fleischmann RM, Bliddal H, Blanco FJ, et al. A phase II trial of Lutikizumab, an anti–interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. 2019;71:1056–69.
Schieker M, Conaghan PG, Mindeholm L, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2020;173:509–15. The post-hoc analysis of the CANTOS trial identifying a possible role for IL-1β inhibition in reducing the risk of progression to end-stage knee OA
Huber FA, Becce F, Gkoumas S, et al. Differentiation of crystals associated with arthropathies by spectral photon-counting radiography. Invest Radiol 2020;Publish Ah:1–6.
Stamp LK, Anderson NG, Becce F, et al. Clinical utility of multi-energy spectral photon-counting computed tomography in crystal arthritis. Arthritis Rheumatol. 2019;71:1158–62.
Bernabei I, Sayous Y, Raja A, et al. Multi-energy photon-county CT versus other clinical imaging techniques for the identification of articular calcium crystal deposition. Rheumatology. 2021.
Baffour FI, Glazebrook KN, Ferrero A, et al. Photon-counting detector CT for musculoskeletal imaging: a clinical perspective. Am J Roentgenol. 2022.
Nguyen C, Bazin D, Daudon M, et al. Revisiting spatial distribution and biochemical composition of calcium-containing crystals in human osteoarthritic articular cartilage. Arthritis Res Ther. 2013;15.
Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12.
Misra D, Guermazi A, Sieren JP, et al. CT imaging for evaluation of calcium crystal deposition in the knee: initial experience from the Multicenter Osteoarthritis (MOST) study. Osteoarthr Cartil. 2015;23:244–8.
Meyer F, Dittmann A, Kornak U, et al. Chondrocytes from osteoarthritic and chondrocalcinosis cartilage represent different phenotypes. Front Cell Dev Biol 2021;9. Study defining two different phenotypes for chondrocalcinosis reflecting calcium pyrophosphate deposition and OA with basic calcium phosphate depiosition.
Scanu A, Oliviero F, Luisetto R, et al. Effect of pathogenic crystals on the production of pro- and anti-inflammatory cytokines by different leukocyte populations. Immunobiology. 2021;226.
Frallonardo P, Ramonda R, Peruzzo L, et al. Basic calcium phosphate and pyrophosphate crystals in early and late osteoarthritis: relationship with clinical indices and inflammation. Clin Rheumatol. 2018;37:2847–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jean W. Liew declares that he has no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liew, J.W. Intra-articular Mineralization and Association with Osteoarthritis Development and Outcomes. Curr Treat Options in Rheum 9, 70–81 (2023). https://doi.org/10.1007/s40674-023-00203-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-023-00203-1