Abstract
Purpose of Review
The last decade has seen significant improvements in the management and understanding of the pathogenesis of CNS germ cell tumors (GCTs) by studies on genomic and epigenomic analyses, and published results of clinical trials. This review highlights the new findings to stay up-to-date on the knowledge and better inform the future directions.
Recent Findings
CNS GCTs are characterized by either MAPK or PI3K pathway mutations. Germinoma has a striking global hypo-methylation, analogous to its hypothesized cell-of-origin; primordial germ cell. Micro RNA cluster mir-371–373 and mir-302/367 are characteristic of GCTs, which have potential for liquid biopsy. Clinical trials have revealed whole-ventricular irradiation for germinoma and local radiotherapy for localized non-germinomatous GCTs seem to be sufficient for tumor control.
Summary
Advancements in basic, translational, and clinical studies are improving our understanding of this rare disease. Further studies are needed, especially in the field of radiomics, liquid biopsy, genomic structural variants, and treatment stratification, to better structure the future management scheme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central nervous system (CNS) germ cell tumor (GCT) is a rare neoplasm which mainly affects pediatric, adolescent and young adult population, with a peak age of teenagers [1••, 2]. There is a male predominance, but the gender distribution is dependent on the site of the tumor. The most common location is the pineal gland, followed by neurohypophysis and ventricular system, and GCTs at the basal ganglia, thalamus, cerebral and cerebellar hemispheres, brainstem, and spinal cord are rare. While there is an even distribution between genders for GCTs located in the neurohypophysis [3], there is a strikingly higher incidence in male patients for pineal GCTs [4]. There is also a difference in incidence based on geographic distribution; GCTs are more common in East Asia, compared with North America and Europe [5, 6]. The site of tumor location is also different geographically: basal ganglia GCTs are more common in East Asia and bifocal (neurohypophysis and pineal gland) tumors are more common in Europe and North America [7•].
GCTs are sensitive to chemotherapy and radiotherapy. Long-term survival can be achieved in germinoma, with 10-year overall survival (OS) of ≥ 90% [1••, 8,9,10]. Prognosis for non-germinomatous GCTs (NGGCTs) is also improving, with recent data showing 5-year OS of around 70% per clinical trials by Clinical Oncology Group (COG) and International Society of Paediatric Oncology (SIOP) [11, 12••]. However, long-term survivors can suffer from late sequelae due to chemotherapy and radiotherapy, including secondary neoplasms such as glioma and meningioma, and cavernous malformation, vascular damage, endocrinological insufficiency, and intellectual decline [10, 13•, 14]. These long-term side effects of current standard of care treatment regimens prompt the need for new treatment algorithms that would reduce treatment-related side effects including a smaller radiation field and dose, targeted therapy, and stratified treatment intensity.
Mainly due to its rareness, basic research into the pathogenesis of GCTs and clinical trials are relatively uncommon compared with other more common pediatric CNS tumors such as medulloblastoma or ependymoma. However, in the past 10 years, we have seen progress in understanding the disease mechanism through genomic and epigenomic profiles, diagnostic methods, imaging studies, and clinical trials across the globe. Furthermore, five international GCT symposia have been held to discuss the best clinical management, and consensus was achieved in many aspects of clinical practice [15]. Although we have not yet developed molecularly targeted therapy, recent basic and clinical findings are promising. This paper illustrates and summarizes the up-to-date knowledge for both basic and clinical findings focusing on articles published within the last 5 years, and presents potential future methods to better manage this rare disease.
Molecular Investigations Into Pathogenesis
Mutational Analysis
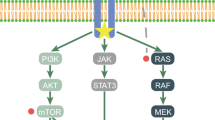
A study evaluating the mutational profile of 124 CNS GCTs has revealed that MAPK pathway (KIT/RAS signaling pathway) alterations are common, including KRAS and NRAS in 48% of samples, and PI3K pathway alterations including MTOR in 13% of samples were the dominant features in tumorigenesis [16]. Among all the mutations, KIT and RAS mutations, which were mutually exclusive, were the most frequent, accounting for approximately 60% in germinoma and 8.6% in NGGCTs. Irrespective of KIT mutational status, KIT expression was immunohistochemically positive in all germinoma [17].
Another interesting finding was that the JMJD1c germline variant was found to be 4.8 times higher in the Japanese patient cohort compared with other cohorts of different ethnicity. JMJD1c codes a histone demethylation enzyme, which is involved in the maintenance of primordial germ cells (PGCs) in mice. This was suspected to be one of the factors responsible for the difference in the incidence between ethnicities [18]. Inhibitors for MTOR and KIT are potential molecularly targeted drugs, the former of which was partially verified in vitro [19] and both of which are already in clinical use for other types of tumors. Other tyrosine-kinase inhibitors such as MEK or ERK inhibitors are also potentially effective [18]. So far, however, no biomarker-based clinical trial has been conducted using targeted drugs for CNS GCTs.
Copy Number Analysis
GCTs are characterized by highly enriched copy number abnormalities and imbalanced chromosomes. Array comparative genome hybridization (aCGH), SNP array, and fluorescent in situ hybridization (FISH) revealed frequent gains in 1q, 12p, 21q, X and loss in 13q. Chromosome 12p contains a representative gene, KRAS, which often has mutation in CNS GCTs. So far, however, no specific gene has been linked with the pathogenesis, in line with corresponding chromosomal aberrations [17]. Congenitally acquired diseases associated with CNS GCTs include Klinefelter syndrome (47, XXY) and Down syndrome (21 trisomy) [20, 21]. Higher incidence of CNS GCTs in these syndromes suggests the presence of potential causative genes in chromosomes X and 21, but this has not been established.
Methylation Analysis
According to the methylation profiling of 61 CNS GCTs analyzed by Illumina 450 K array, germinoma was found to be highly characterized by whole genome demethylation, which shows a striking contrast to any other tumors in the body. This demethylation status is analogous to that of the hypothesized cell-of-origin, PGCs [22]. Hypomethylation in germinoma was also evidenced by negative immunohistochemistry with 5-methylcytocine at tumor cell nuclei [23].
Other CNS GCTs showed similar methylation patterns to somatic tissues, and depended on the level of differentiation of the tissue. Another interesting finding was that microdissection of each histological subtype in mixed GCTs revealed that mutational status was shared throughout the tumor, contrary to the different methylation status between different histological components. This suggests that cell-of-origin firstly acquires mutation, followed by the change in methylation, resulting in different phenotypes [22].
Expression and Transcriptome Analysis
Immunohistochemical analyses demonstrated that genes related to pluripotency including KIT, Oct3/4, NANOG, and TFAP2c and genes related to germ cells including MAGEA4, NY-ESO-1, and TSPY were highly expressed in GCTs, suggesting that GCTs represent both primitive and gonad-destined cells [24]. Microarray gene expression analysis corroborated the above findings, demonstrating that pluripotency-related genes including KLF4 and immune-related genes were highly expressed in germinoma. NGGCTs were characterized by the genes related to Wnt/beta-catenin pathway, invasion, and epithelial-mesenchymal transition [25].
GWAS
Genome-wide association study (GWAS), which is investigating the statistical association between single-nucleotide polymorphism (SNP) and disease, is now being conducted for CNS GCTs in Japan, the results of which are pending. For testicular GCTs, there have been several reports on identifying representative SNPs in association with disease by GWAS, and the genes were found to be related to germ cell development, differentiation, telomere function microtubule assembly, and DNA repair [26]. Furthermore, there have been reports about familial testicular GCTs, with the risk elevated to 8–10 times between brothers and 4–6 times between father and son [27]. The findings of SNPs in CNS GCTs are awaited.
New Diagnostic Methods
Tumor Marker
Standard tumor markers examined in daily clinical practice include human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) in the blood serum and CSF. These can be used as diagnostic methods to differentiate germinoma and NGGCTs, the latter of which usually have elevated levels, especially HCG in choriocarcinomas and AFP in yolk sac tumors and occasionally teratomas. HCG-producing germinoma including germinoma with syncytiotrophoblastic giant cell (STGC) is usually treated as pure germinoma as all germinomas secrete HCG to varying extent [28], but there is a report about worse prognosis with germinoma with elevated HCG [29]. Tumor markers were also found to be useful in differentiating tumor progression and “growing teratoma syndrome” during chemo- and radiotherapy, as the latter is defined as normalization of tumor markers despite tumor growth [30]. Additionally, monitoring of tumor markers is essential in detecting a relapse in follow-ups [31••, 32]. Another tumor marker, placental alkaline phosphatase (PLAP), was recently investigated, which in combination with other standard tumor markers can differentiate germinomas, choriocarcinomas, and other NGGCTs, and can also be useful in immunohistochemistry in the diagnosis of germinoma [33].
Micro RNA
MicroRNAs from miR-371a-373p and miR-302/367 clusters have been detected in the serum and CSF of patients with CNS malignant NGGCTs. These biomarkers were found to have higher sensitivity and specificity compared with standard tumor markers in cases of testicular counterparts [34]. MicroRNA detection has potential as diagnostic measures and gauging treatment response and relapse [35•]. Another study found 27 differentially expressed microRNAs between germinoma and NGGCTs, some of which were related to epigenetic status and platinum-agent resistance [36]. Establishment of standardized measurement methodology and clinical feasibility of microRNA assessment are warranted.
White Blood Cell Differential in CSF
A recent study revealed that the white blood cell fraction in CSF was closely related to the histopathological entity. Germinoma was associated with high percentage of lymphocytes and NGGCT with monocytes. Also, the multiplicity of tumors in CNS was related to a high percentage of lymphocytes [13•]. This finding is seemingly reflecting the well-known pathological feature of germinoma harboring coexistent lymphocytes in its tissue, which is called a “two-cell pattern” [37]. Furthermore, transcriptomic analysis on NGGCTs in preliminary studies found that they have abundant macrophage in their tissue. These features reflect another perspective of the pathogenesis of GCTs from the angle of the microenvironment, and can aid in the diagnosis of histopathological categorization, and potentially can be lead to consideration of treatments such as immunotherapy. These findings warrant external validation before their practical use.
Clinical Trials
There are three major organizations where clinical trials targeting germinoma and NGGCTs in a large scale have been conducted: COG in North America, SIOP in Europe, and CNS Germ Cell Tumor Study Group in Japan. Clinical trials in these three independent groups have historically shaped current management schemes. While there have been differences in the classification and treatment regimens of these tumors, the medications used and the radiation coverage and dose are mostly congruent.
COG
In ACNS0122 clinical trial targeting NGGCTs, alternating the chemotherapy agents carboplatin and etoposide with ifosfamide and etoposide, followed by craniospinal irradiation (CSI) 36 Gy and local radiotherapy (LRT) 18 Gy, were employed. The study achieved 5-year event-free survival and overall survival (OS) of 84 ± 4% and 93 ± 3%, respectively [38]. The next clinical trial was ACNS1123, and the result of the study for localized NGGCT (stratum1) has already been published. The chemotherapy regimen was about the same with an increase in the dose of etoposide, but the radiation field was limited to whole ventricular irradiation (WVI) of 30.6 Gy instead of CSI and LRT of 23.4 Gy. Three-year progression-free survival (PFS) and OS were reported to be 88 ± 4% and 92 ± 3%, respectively. This is comparable to the results of trial ACNS0122, from which WVI was found to be sufficient for a selected cohort of localized NGGCTs. In ACNS1123, patients with germinoma in stratum 2 were treated with WVI 18 Gy and LRT 12 Gy if tumor showed CR to carboplatin and etoposide, and WVI 24 Gy and LRT 12 Gy if the tumor showed non-CR. Though data from stratum 2 has not officially been published yet, 3-year PFS was reported to be 94 ± 3% [39].
SIOP
In CNS GCT-96 clinical trial, patients with germinoma were treated either with CSI 24 Gy and LRT 16 Gy or LRT 40 Gy and carboplatin and etoposide alternating with ifosfamide and etoposide (“CarboPEI”) for non-disseminated cases. Five-year event-free survival was 97 ± 2% and 88 ± 4%, respectively. This study revealed that most of the recurrences in the LRT group were outside of the radiation field, giving us evidence that LRT was insufficient in treating germinoma [40]. The recently closed CNS GCTII trial employed WVI 24 Gy for cases with CR to “CarboPEI” and combination WVI 24 Gy with LRT 16 Gy for non-CR cases. The results are yet to be published. For NGGCTs, cisplatin, etoposide, and ifosfamide (“PEI”) and LRT 54 Gy for localized NGGCTs and PEI chemotherapy, CSI 30 Gy and LRT 24 Gy for disseminated cases were employed. Five-year PFS and OS were 72 ± 4% and 82 ± 4% for localized cases and 68 ± 9% and 75 ± 8% for disseminated cases, respectively. They also showed that AFP > 1000 ng/ml at presentation and residual disease at the end of treatment were unfavorable prognostic factors in NGGCTs [11]. The following CNS GCTII clinical trial uses upfront high-dose chemotherapy for these high-risk cases.
Japan
In the multicenter prospective study (1995–2003) funded by the former Ministry of Health and Welfare Cancer Research Grant, germinoma was treated with extended LRT 24 Gy (equal to WVI except for not covering the lower half of the fourth ventricle) and carboplatin and etoposide (“CARE”), resulting in 10-year OS of 98% [41]. The current clinical trial (jRCTs031180223) which has already completed enrollment is using WVI 23.4 Gy and CARE regimen. Japanese clinical trials are unique in the classification of NGGCTs into further two groups: an intermediate and a poor prognosis group. The former is defined by patients with mixed GCTs with a dominant germinoma component or immature teratoma, and the latter includes patients with dominant malignant components (yolk sac tumor, embryonal carcinoma, and choriocarcinoma) or excessively-high tumor markers (HCG > 2000 IU/l or AFP > 2000 ng/ml). A multicenter prospective study conducted from 1995–2003 treated an intermediate prognosis group with extended LRT 30 Gy and LRT 20 Gy with CARE regimen, and the poor prognosis group was treated with CSI 30 Gy and LRT 30 Gy with ifosfamide, cisplatin, and etoposide (“ICE”) regimen. Patients in this study had a 5-year PFS and OS of 85 and 95%, respectively, for the intermediate prognosis group, and 59% for both PFS and OS in the poor prognosis category. In an ongoing clinical trial, patients in the intermediate prognosis group are treated with WVI 23.4 Gy and 27 Gy with CARE regimen, and the poor prognosis group CSI 30.6 Gy and LRT 30.6 Gy with ICE. The final results of this study is expected soon.
Other New Findings
Bifocal tumors are regarded as highly likely germinoma and often treated as such as long as tumor markers are negative and the imaging features are compatible, although there is a certain degree of disagreement among clinicians to this approach [15]. A recent study on bifocal tumors among patients with diabetes insipidus and negative tumor markers revealed 3.4% (3 out of 89 cases) were histopathologically NGGCTs [42•]. Another study found 3 NGGCT cases out of 14 patients with bifocal tumors, which showed normal or only mildly-elevated tumor markers [43]. Other tumors can also present in the bifocal location; for instance, two cases of bifocal PNET were also reported [44]. These studies raise concern about treating bifocal tumors as germinoma without histopathological confirmation, and biopsy should be given consideration for confirmation.
There is also controversy over treating patients with germinoma with CSI when the cytology of CSF is positive, as whether positive cytology reflects spinal dissemination has not been verified. A recent study of 66 patients with germinoma showed that a spinal lesion was present almost equally in cases with and without positive cytology results. They further demonstrated that the prognosis was the same when treated with or without CSI among the patients with positive cytology results. CSI might be unnecessary for patients with positive cytology results without evidence of spinal lesion on imaging [45].
A recent study on relapsed GCTs reported dismal prognosis despite treatment. One well-known phenomenon is that GCTs can relapse as either the same histology, or transform to NGGCTs. In one small study, patients with relapsed GCTs were treated with salvage standard-dose chemotherapy and irradiation or high-dose chemotherapy and autologous stem cell rescue with or without re-irradiation. Patients who had an original diagnosis of germinoma, no matter the histology on relapse, faired much better than those with an original diagnosis of NGGCT, who had a 5-year OS of 9%. No valid treatment option is available, whether chemotherapy or radiotherapy [46]. Urgent need is recognized for a treatment strategy beyond high-dose chemotherapy.
Potential Future Clinical Trials
Further efforts should be made to reduce the treatment burden for the young patients. One way of treatment reduction is to identify a subgroup with a certain molecular or histopathological feature that can be safely treated with reduced treatment. Recent studies have shown that low tumor cell content percentage in the pathological specimen can be a favorable prognostic factor in germinoma. The study demonstrated that tumor cell content varied significantly in the range of < 5 to 90%, with the median of 50%. Those harboring tumor cell content < 50% showed longer PFS than those who had higher percentages [47••]. Though limited by small cohort size, no patients experienced disease recurrence who had < 50% tumor cell content after treatment with platinum-based chemotherapy and LRT [48]. Another study has shown that chromosomal 12p gain was prevalent in NGGCTs especially with a malignant component, and was a poor prognostic factor among NGGCTs (in submission). These histopathological and molecular features can be leveraged in identifying cases for which treatment intensity can be modified, although these findings need validation before clinically employed.
Recent studies have revealed that 74–90% of germinoma cells show positivity for PD-L1 [47••, 49]. The microenvironment of germinoma is rich in T-cells in some cases. Although the mutation burden does not seem to be high in general [19], these findings suggest a possible therapeutic role for immune checkpoint inhibitors [50].
Unresolved Issues
One of the unestablished matters surrounding GCTs is the most appropriate cutoff levels for tumor markers to distinguish germinoma and NGGCTs. NGGCTs are often diagnosed based on the elevated tumor markers without histopathological confirmation: βhCG > 100 IU/ml or AFP > 10 ng/ml in COG ACNS1123 and βhCG > 50 IU/ or AFP > 25 ng/ml in SIOP CNS GCTII. Omitting confirmation of diagnosis with a histopathological specimen can potentially lead to over-treatment, such as in patients with HCG-producing germinomas and marker-positive teratomas. Furthermore, different cutoffs in tumor markers make it difficult to compare the treatment outcomes between different clinical trials. In contrast, diagnosis on surgical specimens obtained by biopsy has an inherent limitation, as it only represents part of the entire tumor and can lead to misdiagnosis in cases of mixed tumors. Verifications of the appropriate cutoff levels to minimize the possibility of under-or over-treatment are thus necessary.
Another important issue to be resolved is the development of treatment for relapsed GCTs, especially relapsed NGGCTs, in the framework of clinical trials. As described previously, the current treatment regimen involving high-dose chemotherapy with autologous stem cell implantation and re-irradiation has shown only a limited effect. Completely new modalities or drugs might be necessary, including the introduction of immunotherapy such as immune-checkpoint inhibitors. The promising news is that a research group at Baylor College established the first patient-derived xenograft model of germinoma. This will surely help in testing new drugs in the preclinical setting [51].
Future Directions and Perspectives
Although the pathogenesis of CNS GCTs has been partially disclosed with the discovery of characteristic mutational profiles, highly-sensitive miRNAs, global demethylation status of germinomas, prognostic significance of tumor cell content in H-E specimen and 12p gain, to date, none of these has led to the development of new treatment strategies. One of the issues is that a genomic variant has not been unraveled yet. The next step would be to analyze the whole genome sequence to probe non-coding regions or structural variants that cannot be clarified by RNA sequence. As pediatric tumors are often associated with single genomic structural abnormality, this might be true for CNS GCTs as well. Molecularly targeted therapy is strongly needed, especially for approximately 10% of germinoma that are resistant to conventional chemotherapy and radiotherapy, NGGCTs that need intensified chemotherapy and high-dose radiotherapy and relapsed GCTs.
Radiomics analysis is being developed and studied in the diagnostic imaging of brain tumors, and will likely to have a bigger role in the daily clinical practice [52]. Potential ways of its usage in the field of CNS GCTs include differentiation of GCTs from other entities, germinoma from NGGCT, or specifying the histopathological components in the mixed GCTs on imaging. These technologies would be helpful when combined with tumor markers, to minimize the possibility of under- or over-treatment caused by sampling errors in biopsy cases, or diagnostic errors when skipping histopathological confirmation.
Conclusions
The last decade has seen a significant improvement in understanding the pathogenesis of GCTs with the development of genomic and epigenomic technologies, including mutational, epigenomic, transcriptomic, and miRNA profiles. Furthermore, clinical trial results have been published worldwide, leading to the reduction of the treatment burden on the generally young patient population. Still, there remains a gap in the knowledge, and though potential molecular targets have been identified, none has been studied in clinical trials to date. Hopefully, this decade will see further unraveling of the origins of these tumors, improved imaging and body fluid diagnoses supported by developing technologies, and new modalities and drugs to more accurately target the key elements in the pathogenesis with the aim of curing disease without risk of long-term sequela related to treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Takami H, Fukuoka K, Fukushima S, Nakamura T, Mukasa A, Saito N, Yanagisawa T, Nakamura H, Sugiyama K, Kanamori M. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21:1565–77. The study included 190 CNS GCTs, and revealed germinoma prognosis depended on tumor location, marker-positive germinoma had worse prognosis, female GCTs had fewer MAPK pathway mutation, and basal ganglia GCTs had a higher percentage of PI3K pathway mutations.
Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–67. https://doi.org/10.3171/jns.1985.63.2.0155.
Takami H, Graffeo CS, Perry A, Giannini C, Daniels DJ. Epidemiology, natural history, and optimal management of neurohypophyseal germ cell tumors. J Neurosurg. 2020;1:1–9.
Takami H, Graffeo CS, Perry A, Giannini C, Daniels DJ. The third eye sees double: cohort study of clinical presentation, histology, surgical approaches, and ophthalmic outcomes in pineal region germ cell tumors. World Neurosurg. 2021;150:e482–90.
Gittleman H, Cioffi G, Vecchione-Koval T, Ostrom QT, Kruchko C, Osorio DS, Finlay JL, Barnholtz-Sloan JS. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J Neurooncol. 2019;143:251–60.
McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 2012;14:1194–200.
• Takami H, Perry A, Graffeo CS, Giannini C, Narita Y, Nakazato Y, Saito N, Nishikawa R, Matsutani M, Ichimura K. Comparison on epidemiology, tumor location, histology, and prognosis of intracranial germ cell tumors between Mayo Clinic and Japanese consortium cohorts. J Neurosurg. 2020;1:1–11. The study demonstrated that basal ganglia GCTs were more frequent in East Asia, while bifocal tumors were more frequent in Western countries, suggesting the difference in tumor location in addition to its general frequency.
Lo AC, Hodgson D, Dang J, Tyldesley S, Bouffet E, Bartels U, Cheng S, Hukin J, Bedard PL, Goddard K. Intracranial germ cell tumors in adolescents and young adults: a 40-year multi-institutional review of outcomes. Int J Radiat Oncol Biol Phys. 2020;106:269–78.
Hong KT, Lee DH, Kim BK, An HY, Choi JY, Phi JH, Cheon J-E, Kang HJ, Kim S-K, Kim J-Y. Treatment outcome and long-term follow-up of central nervous system germ cell tumor using upfront chemotherapy with subsequent photon or proton radiation therapy: a single tertiary center experience of 127 patients. BMC Cancer. 2020;20:1–10.
Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015;17:741–6. https://doi.org/10.1093/neuonc/nou311.
Calaminus G, Frappaz D, Kortmann RD, Krefeld B, Saran F, Pietsch T, Vasiljevic A, Garre ML, Ricardi U, Mann JR. Outcome of patients with intracranial non-germinomatous germ cell tumors—lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 2017;19:1661–72.
•• Fangusaro J, Wu S, MacDonald S, Murphy E, Shaw D, Bartels U, Khatua S, Souweidane M, Lu H-M, Morris D. Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: a Children’s Oncology Group Study. J Clin Oncol. 2019;37:3283–90. This paper reported the outcome of stratum 1 of ACNS1123 by COG, targeting 107 NGGCT cases. The study showed favorable 3y-PFS and OS of 84 and 93%, respectively, with whole-ventricular and local radiotherapy and alternating chemotherapy.
• Takami H, Perry A, Graffeo CS, Giannini C, Daniels DJ. Novel diagnostic methods and posttreatment clinical phenotypes among intracranial germ cell tumors. Neurosurgery. 2020;87:563–72. The study showed that the cell fraction in cerebrospinal fluid predicted the histopathological diagnosis; lymphocyte-rich fluid was a sign of multiple lesions and germinoma, and monocyte-rich fluid reflects non-germinoma.
Cheng S, Kilday J-P, Laperriere N, Janzen L, Drake J, Bouffet E, Bartels U. Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J Neurooncol. 2016;127:173–80.
Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16:e470–7. https://doi.org/10.1016/S1470-2045(15)00244-2.
Ichimura K, Fukushima S, Totoki Y, Matsushita Y, Otsuka A, Tomiyama A, Niwa T, Takami H, Nakamura T, Suzuki T, Fukuoka K, Yanagisawa T, Mishima K, Nakazato Y, Hosoda F, Narita Y, Shibui S, Yoshida A, Mukasa A, Saito N, Kumabe T, Kanamori M, Tominaga T, Kobayashi K, Shimizu S, Nagane M, Iuchi T, Mizoguchi M, Yoshimoto K, Tamura K, Maehara T, Sugiyama K, Nakada M, Sakai K, Kanemura Y, Nonaka M, Asai A, Yokogami K, Takeshima H, Kawahara N, Takayama T, Yao M, Kato M, Nakamura H, Hama N, Sakai R, Ushijima T, Matsutani M, Shibata T, Nishikawa R. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016. https://doi.org/10.1007/s00401-016-1557-x.
Fukushima S, Otsuka A, Suzuki T, Yanagisawa T, Mishima K, Mukasa A, Saito N, Kumabe T, Kanamori M, Tominaga T, Narita Y, Shibui S, Kato M, Shibata T, Matsutani M, Nishikawa R, Ichimura K. Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127:911–25. https://doi.org/10.1007/s00401-014-1247-5.
Wang L, Yamaguchi S, Burstein MD, Terashima K, Chang K, Ng HK, Nakamura H, He Z, Doddapaneni H, Lewis L, Wang M, Suzuki T, Nishikawa R, Natsume A, Terasaka S, Dauser R, Whitehead W, Adekunle A, Sun J, Qiao Y, Marth G, Muzny DM, Gibbs RA, Leal SM, Wheeler DA, Lau CC. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014;511:241–5. https://doi.org/10.1038/nature13296.
Ichimura K, Fukushima S, Totoki Y, Matsushita Y, Otsuka A, Tomiyama A, Niwa T, Takami H, Nakamura T, Suzuki T. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016;131:889–901.
Miyake Y, Adachi JI, Suzuki T, Mishima K, Sasaki A, Nishikawa R. Craniospinal Germinomas in Patient with Down Syndrome Successfully Treated with Standard-Dose Chemotherapy and Craniospinal Irradiation: Case Report and Literature Review. World Neurosurg. 2017. https://doi.org/10.1016/j.wneu.2017.09.024.
Queipo G, Aguirre D, Nieto K, Pena YR, Palma I, Olvera J, Chavez L, Najera N, Kofman-Alfaro S. Intracranial germ cell tumors: association with Klinefelter syndrome and sex chromosome aneuploidies. Cytogenet Genome Res. 2008;121:211–4. https://doi.org/10.1159/000138887.
Fukushima S, Yamashita S, Kobayashi H, Takami H, Fukuoka K, Nakamura T, Yamasaki K, Matsushita Y, Nakamura H, Totoki Y, Kato M, Suzuki T, Mishima K, Yanagisawa T, Mukasa A, Saito N, Kanamori M, Kumabe T, Tominaga T, Nagane M, Iuchi T, Yoshimoto K, Mizoguchi M, Tamura K, Sakai K, Sugiyama K, Nakada M, Yokogami K, Takeshima H, Kanemura Y, Matsuda M, Matsumura A, Kurozumi K, Ueki K, Nonaka M, Asai A, Kawahara N, Hirose Y, Takayama T, Nakazato Y, Narita Y, Shibata T, Matsutani M, Ushijima T, Nishikawa R, Ichimura K. Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. 2017;133:445–62. https://doi.org/10.1007/s00401-017-1673-2.
Schulte SL, Waha A, Steiger B, Denkhaus D, Dörner E, Calaminus G, Leuschner I, Pietsch T. CNS germinomas are characterized by global demethylation, chromosomal instability and mutational activation of the Kit-, Ras/Raf/Erk-and Akt-pathways. Oncotarget. 2016;7:55026.
Hoei-Hansen CE, Carlsen E, Jorgensen N, Leffers H, Skakkebaek NE, Rajpert-De Meyts E. Towards a non-invasive method for early detection of testicular neoplasia in semen samples by identification of fetal germ cell-specific markers. Hum Reprod. 2007;22:167–73. https://doi.org/10.1093/humrep/del320.
Wang HW, Wu YH, Hsieh JY, Liang ML, Chao ME, Liu DJ, Hsu MT, Wong TT. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genomics. 2010;11:132. https://doi.org/10.1186/1471-2164-11-132.
Litchfield K, Levy M, Orlando G, Loveday C, Law PJ, Migliorini G, Holroyd A, Broderick P, Karlsson R, Haugen TB, Kristiansen W, Nsengimana J, Fenwick K, Assiotis I, Kote-Jarai Z, Dunning AM, Muir K, Peto J, Eeles R, Easton DF, Dudakia D, Orr N, Pashayan N, Bishop DT, Reid A, Huddart RA, Shipley J, Grotmol T, Wiklund F, Houlston RS, Turnbull C. Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat Genet. 2017;49:1133–40. https://doi.org/10.1038/ng.3896.
Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br J Cancer. 2004;90:1765–70.
Takami H, Fukushima S, Fukuoka K, Suzuki T, Yanagisawa T, Matsushita Y, Nakamura T, Arita H, Mukasa A, Saito N, Kanamori M, Kumabe T, Tominaga T, Kobayashi K, Nagane M, Iuchi T, Tamura K, Maehara T, Sugiyama K, Nakada M, Kanemura Y, Nonaka M, Yokogami K, Takeshima H, Narita Y, Shibui S, Nakazato Y, Nishikawa R, Ichimura K, Matsutani M. Human chorionic gonadotropin is expressed virtually in all intracranial germ cell tumors. J Neurooncol. 2015;124:23–32. https://doi.org/10.1007/s11060-015-1809-y.
Fukuoka K, Yanagisawa T, Suzuki T, Shirahata M, Adachi JI, Mishima K, Fujimaki T, Katakami H, Matsutani M, Nishikawa R. Human chorionic gonadotropin detection in cerebrospinal fluid of patients with a germinoma and its prognostic significance: assessment by using a highly sensitive enzyme immunoassay. J Neurosurg Pediatr. 2016;18:573–7. https://doi.org/10.3171/2016.4.PEDS1658.
Michaiel G, Strother D, Gottardo N, Bartels U, Coltin H, Hukin J, Wilson B, Zelcer S, Hansford JR, Hassall T. Intracranial growing teratoma syndrome (iGTS): an international case series and review of the literature. J Neurooncol. 2020;147:721–30.
•• Fonseca A, Xia C, Lorenzo AJ, Krailo M, Olson TA, Pashankar F, Malogolowkin MH, Amatruda JF, Billmire DF, Rodriguez-Galindo C. Detection of relapse by tumor markers versus imaging in children and adolescents with nongerminomatous malignant germ cell tumors: a report from the Children’s Oncology Group. J Clin Oncol. 2019;37:396. The study revealed tumor markers were a sensitive method in detecting a relapse in the surveillance after standard treatment for CNS GCTs.
Cheung V, Segal D, Gardner SL, Zagzag D, Wisoff JH, Allen JC, Karajannis MA. Utility of MRI versus tumor markers for post-treatment surveillance of marker-positive CNS germ cell tumors. J Neurooncol. 2016;129:541–4.
Aihara Y, Watanabe S, Amano K, Komatsu K, Chiba K, Imanaka K, Hori T, Ohba T, Dairoku H, Okada Y. Placental alkaline phosphatase levels in cerebrospinal fluid can have a decisive role in the differential diagnosis of intracranial germ cell tumors. J Neurosurg. 2018;131:687–94.
Dieckmann K-P, Radtke A, Spiekermann M, Balks T, Matthies C, Becker P, Ruf C, Oing C, Oechsle K, Bokemeyer C. Serum levels of microRNA miR-371a-3p: a sensitive and specific new biomarker for germ cell tumours. Eur Urol. 2017;71:213–20.
• Murray MJ, Ajithkumar T, Harris F, Williams RM, Jalloh I, Cross J, Ronghe M, Ward D, Scarpini CG, Nicholson JC. Clinical utility of circulating miR-371a-3p for the management of patients with intracranial malignant germ cell tumors. Neuro-Oncol Adv. 2020;2:vdaa048. The study revealed that miR-371a-3p can be a clinically useful and sensitive marker in diagnosing non-germinoma as detected in blood serum and cerebrospinal fluid.
Hsieh T-H, Liu Y-R, Chang T-Y, Liang M-L, Chen H-H, Wang H-W, Yen Y, Wong T-T. Global DNA methylation analysis reveals miR-214-3p contributes to cisplatin resistance in pediatric intracranial nongerminomatous malignant germ cell tumors. Neuro Oncol. 2018;20:519–30.
Zapka P, Dorner E, Dreschmann V, Sakamato N, Kristiansen G, Calaminus G, Vokuhl C, Leuschner I, Pietsch T. Type, frequency, and spatial distribution of immune cell infiltrates in CNS germinomas: evidence for inflammatory and immunosuppressive mechanisms. J Neuropathol Exp Neurol. 2017. https://doi.org/10.1093/jnen/nlx106.
Goldman S, Bouffet E, Fisher PG, Allen JC, Robertson PL, Chuba PJ, Donahue B, Kretschmar CS, Zhou T, Buxton AB. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: A Children’s Oncology Group Study. J Clin Oncol. 2015;33:2464.
Bartels U, Fangusaro J, Shaw D, Bhatia A, Omar-Thomas A, Wu S, MacDonald S, Murphy E, Souweidane M, Fouladi M. GCT-41. Response-based radiation therapy in patients with newly diagnosed central nervous system localized germinoma: a children’s oncology group (cog) prospective phase 2 clinical trial. Neuro-Oncology. 2020;22:iii336.
Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garre ML, Patte C, Ricardi U, Saran F, Frappaz D. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15:788–96. https://doi.org/10.1093/neuonc/not019.
Matsutani M, Group JPBTS. Combined chemotherapy and radiation therapy for CNS germ cell tumors–the Japanese experience. J Neurooncol. 2001;54:311–6.
• Kanamori M, Takami H, Yamaguchi S, Sasayama T, Yoshimoto K, Tominaga T, Inoue A, Ikeda N, Kambe A, Kumabe T. So-called bifocal tumors with diabetes insipidus and negative tumor markers: are they all germinoma? Neuro Oncol. 2021;23:295–303. The study showed that 3.4% of bifocal tumors with diabetes insipidus and negative tumor marker were histopathologically non-germinoma, suggesting the necessity of surgical biopsy.
Aizer AA, Sethi RV, Hedley-Whyte ET, Ebb D, Tarbell NJ, Yock TI, Macdonald SM. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro Oncol. 2013;15:955–60. https://doi.org/10.1093/neuonc/not050.
Phuakpet K, Larouche V, Hawkins C, Huang A, Tabori U, Bartels UK, Bouffet E. Rare Presentation of Supratentorial Primitive Neuroectodermal Tumors Mimicking Bifocal Germ Cell Tumors: 2 Case Reports. J Pediatr Hematol Oncol. 2016;38:e67-70. https://doi.org/10.1097/MPH.0000000000000402.
Kanamori M, Takami H, Suzuki T, Tominaga T, Kurihara J, Tanaka S, Hatazaki S, Nagane M, Matsuda M, Yoshino A. Necessity for craniospinal irradiation of germinoma with positive cytology without spinal lesion on MR imaging—a controversy. Neuro-Oncol Adv. 2021;3:vdab086.
Murray MJ, Bailey S, Heinemann K, Mann J, Göbel UK, Saran F, Hale JP, Calaminus G, Nicholson JC. Treatment and outcomes of UK and German patients with relapsed intracranial germ cell tumors following uniform first-line therapy. Int J Cancer. 2017;141:621–35.
•• Takami H, Fukushima S, Aoki K, Satomi K, Narumi K, Hama N, Matsushita Y, Fukuoka K, Yamasaki K, Nakamura T. Intratumoural immune cell landscape in germinoma reveals multipotent lineages and exhibits prognostic significance. Neuropathol Appl Neurobiol. 2020;46:111–24. The study showed that germinoma with low tumor cell content showed favorable prognosis than those with high tumor cell content, and PD-1 and PD-L1 were highly expressed in lymphocytes and germinoma cells, respectively.
Takami H, Satomi K, Fukuoka K, Fukushima S, Matsushita Y, Yamasaki K, Nakamura T, Tanaka S, Mukasa A, Saito N. Low tumor cell content predicts favorable prognosis in germinoma patients. Neuro-Oncol Adv. 2021;3(1).
Wildeman ME, Shepard MJ, Oldfield EH, Lopes MBS. Central nervous system germinomas express programmed death ligand 1. J Neuropathol Exp Neurol. 2018;77:312–6.
Zschäbitz S, Lasitschka F, Jäger D, Grüllich C. Activity of immune checkpoint inhibition in platinum refractory germ-cell tumors. Ann Oncol. 2016;27:1356–60.
Lindsay H, Huang Y, Du Y, Braun FK, Teo WY, Kogiso M, Qi L, Zhang H, Zhao S, Mao H. Preservation of KIT genotype in a novel pair of patient-derived orthotopic xenograft mouse models of metastatic pediatric CNS germinoma. J Neurooncol. 2016;128:47–56.
Parekh V, Jacobs MA. Radiomics: a new application from established techniques. Expert Rev Precis Med Drug Dev. 2016;1:207–26.
Funding
This study was supported by Grant-in-Aid for Young Scientists, KAKENHI No. 20K17918 from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Takami, H. Advances in Molecular Profiling and Developing Clinical Trials of CNS Germ Cell Tumors: Present and Future Directions. Curr Oncol Rep 24, 105–112 (2022). https://doi.org/10.1007/s11912-022-01195-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01195-2