Abstract
Human chorionic gonadotropin (hCG) production has been utilized as a diagnostic marker for germinoma with syncytiotrophoblastic giant cells (STGC) and choriocarcinoma. Elevated hCG in germinoma is considered to predict less favorable prognosis, and an intensive treatment strategy may accordingly be applied. However, there is some evidence that any germinoma may produce hCG to varying extent. We investigated mRNA expression of the hCG β subunit (hCGβ) using real time quantitative polymerase chain reaction in 94 germ cell tumors (GCTs). Most (93.3 %) GCTs showed higher expression levels compared with that of normal brain tissue (1.09 × 100–1.40 × 105 fold). The expression was the highest in GCTs which harbor choriocarcinoma or STGC components. The expression level of hCGβ in germinoma was highly variable (1.09 × 100–5.88 × 104 fold) in linear but not bimodal distribution. hCG concentrations in serum and CSF correlated with gene expression, especially when GCTs with single histological component were analyzed separately. The expression was not significantly associated with recurrence in pure germinoma. These results suggest that the serum/CSF hCG levels may need to be interpreted with caution as most GCTs appear to have the capacity of producing hCG irrespective of their histology. The clinical significance of ubiquitous hCG expression in GCTs needs further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial germ cell tumors (GCTs) develop mostly in the pediatric and young adult population with a strong male preponderance [1]. The current World Health Organization (WHO) classification recognizes five main histological subtypes of intracranial GCTs; germinoma, teratoma, choriocarcinoma, yolk sac tumor, and embryonal carcinoma [2]. The tumors often appear as a mixture of any combination of these components. Germinoma, which is the most common subtype, usually means pure germinoma but it also includes germinoma with syncytiotrophoblastic giant cells (STGC) as its subtype.
Human chorionic gonadotropin (hCG) is one of the most commonly tested tumor markers in the clinical settings along with alpha-fetoprotein (AFP). Elevation of the hCG level in serum and/or cerebrospinal fluid (CSF), or positive immunohistochemistry of the tumor tissue has been used to make a diagnosis of choriocarcinoma or germinoma with STGC. Some consider that most GCTs can be diagnosed solely based on laboratory tests of those tumor markers together with the clinical presentation and imaging examinations without histopathological analyses [3].
However, it has been known that a subgroup of germinoma without STGC also secrete hCG, and is therefore called hCG-producing germinoma. The prognostic significance of hCG in germinoma is controversial; some argue that the elevation of hCG has a negative impact on the outcome in germinoma [4, 5], while others reported the opposite [6, 7]. While patients with pure germinoma are categorized into the good prognosis group of the therapeutic classification proposed by a Japanese group [8, 9], those with hCG-producing germinoma are classified into the intermediate prognosis group and accordingly receive more intense chemo-radiotherapy than pure germinoma. In European clinical trials, they are treated as a poor prognosis tumor together with non-germinomatous GCTs (NGGCTs) if the concentration of hCGβ in the serum or CSF exceeds 50 IU/l (https://www.skion.nl/workspace/uploads/2_siop_cns_gct_ii_final_version_2_15062011_unterschrift_hoppenheit.pdf).
However, an ultrasensitive enzyme immunoassay (EIA) method to measure the hCG titer in CSF developed by Katakami et al. revealed that almost all germinoma produced hCGβ [10, 11]. These data suggest that not only pure germinoma but also at least some NGGCTs in addition to choriocarcinomas may potentially produce hCG, which could be undetected by conventional laboratory tests.
In order to validate the significance of hCG as a tumor marker for GCTs and to find the possible association with prognosis, we investigated hCG production in intracranial GCT cells by quantitatively evaluating hCGβ mRNA expression using real time reverse polymerase chain reaction (PCR) in 94 GCTs. We here show that hCGβ mRNA is expressed virtually in all intracranial GCTs of any histological subtypes.
Materials and methods
Tumor materials
A total of 94 primary intracranial GCTs from 94 patients were included in this study. The samples were collected from the neurosurgical departments of 10 hospitals that participate in the Intracranial Germ Cell Tumor Genome Analysis Consortium of Japan (the iGCT Consortium). A non-neoplastic adult brain tissue sample (Clontech Laboratories, Mountain View, CA) and two adult testis samples (Cell Applications, San Diego, CA and Clontech Laboratories) were included as controls for the experiment. The investigation was approved by the ethical committee of the National Cancer Center, Tokyo, Japan and the respective local institutional review boards.
Among the 94 cases, 84 were primary and 10 recurrent lesions. Eighty-two patients were male and 12 female. The age at the diagnosis ranged from 2 months to 45 years (median 15 years, mean 17 years). Pure germinoma are typically infiltrated by T lymphocytes coexisting in the tumor, generating the characteristic “two-cell pattern” observed by histopathology [12]. Since the co-existing non-neoplastic cells in the tumor tissue may compromise the results of the expression analyses, only samples that were histologically shown to contain at least 10 % of tumor cells were included in the current study.
Clinical information including the serum and/or CSF concentration of total hCG and hCGβ, therapeutic records (operation, radiation, and chemotherapy) and events (recurrence and death) in the follow-up periods were available in all cases (Supplementary Table). Serum and CSF measurements of total hCG and hCGβ were performed by various methods including chemiluminescence immunoassay (CLIA), enzyme immunoassay (EIA), fluorescence EIA (FEIA), immunoradiometric assay (IRMA) and radioimmunoassay (RIA), depending on the institutions. CSF samples were obtained either before or during operation by ventricular or lumbar drainage. This information is presented in the supplementary table.
Tumor histology
The histopathological diagnosis was reviewed and re-classified according to the WHO classification of tumors of the central nervous system by a single expert neuropathologist (YN) for all 94 tumors who was blind to the hCG expression results [2]. As a result, the 94 tumor samples consisted of the following histological diagnoses; 48 pure germinoma, 12 mature teratoma, 7 immature teratoma, 19 mixed germinoma, 1 mixed GCT without a germinoma component, 3 yolk sac tumors, 1 choriocarcinoma, 1 embryonal carcinoma, and 2 unspecified high grade GCTs.
RNA extraction and cDNA synthesis
Total RNA was extracted from the frozen samples using a miRNeasy Mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer’s recommendations. First-strand cDNA was synthesized from 500 ng total RNA with Superscript III (Invitrogen Life Technologies, Carlsbad, CA).
Real-time PCR
The mRNA expression levels of hCGβ were determined in each sample by real-time quantitative PCR (qPCR) using LightCycler 480 SYBR Green I Master (Roche Diagnostics, Indianapolis, IN) and the SYBR Green I (483-533 nm) detection format on a CFX96 (Bio-Rad Laboratories, Inc. Hercules, CA, USA) according to the manufacturer’s recommendations. The following primer pair was designed to amplify the specific sequence to the coding regions of the hCGβ gene shared by CGB3, 5, 7 and 8 but not CGB1 or 2: forward primer 5′- TAGCACTCGACGACTGAGTCTC -3′ (P0285) and reverse primer 5′- GACAACGACGACGACTCGTA -3′ (P0286). The following thermal cycling conditions were employed; 5 min at 95 °C; 45 cycles of 10 s at 95 °C, 5 s at 58 °C, 15 s at 72 °C and 5 s at 82 °C. A standard curve was generated using a serial dilution of the subcloned PCR products of the target and the reference sequence as described [13]. Expression was measured relative to human adult brain RNA. The expression of hCGβ was compared with the expression of H6PD, which was used as the reference gene [14]. The primer pair 5′- GATCCTGCCTTTCCGAGAC -3′ (P0114) and 5′- GACCTCCGTCAGATGGTTC -3′ (P0115) was used.
Immunohistochemistry
Immunohistochemistry was performed for 40 cases in which formalin-fixed paraffin-embedded samples were available. Immunostaining with a primary antibody against hCG (1:500, Dako, Glostrup, Denmark) was carried out using an auto-immunostainer (Ventana Benchmark XT, Roche Tissue Diagnostics, Tokyo, Japan). Percentage and intensity of immunostaining was quantified under a light microscopy by a single neuropathologist (YN) who was blind to the mRNA expression data using “Allred score” which is commonly used for assessing the estrogen and progesterone receptor expression in breast cancer in clinic [15]. Briefly, the proportion of the positive staining tumor cells was scored as 0 = none; 1 ≤ 1/100; 2 = 1/100 to < 1/10; 3 = 1/10 to < 1/3; 4 = 1/3-2/3; 5 ≥ 2/3. The intensity was scored as 0 = none, 1 = weak; 2 = intermediate; 3 = strong. The proportion and intensity scores were added to calculate a total score that would range between 0-8, which is defined as “Allred score” [16].
Statistical analysis
All statistical analyses were performed using a JMP version 10 software (SAS Institute, Cary, NC). The data were analyzed using Wilcoxon’s test for mRNA expression evaluated by binary logarithm and Pearson’s Chi square test for comparing two subsets. The association between mRNA expression and immunohistochemistry score was analyzed using an analysis of variance. A p-value below 0.05 was considered statistically significant.
Results
hCGβ expression across histological subtypes
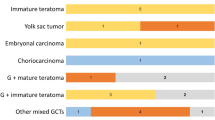
hCGβ was expressed at variable levels across all the histological subtypes in all tumors but four, which include one pure germinoma, two mature teratoma and one germinoma mixed with immature teratoma and STGC. Most cases (84/90 cases, 93.3 %) showed higher levels of hCGβ expression than normal brain tissue (relative ratio to the brain tissue: 1.09 × 100 – 1.40 × 105). Many of them also showed higher expression than normal adult testis tissue (77/90 cases, 85.6 %). Figure 1 shows the expression levels in each histological subtype. Among them, a single case of choriocarcinoma showed by far the highest level of expression, followed by mixed germinoma which have components of immature teratoma, STGC and choriocarcinoma. Pure germinoma showed significantly higher expression compared with mature teratoma, immature teratoma or yolk sac tumors (p = 0.01, 0.02 and 0.02, respectively, Wilcoxon’s test).
The expression levels of hCGβ according to all histological subtypes are shown. The highest expression was scored by a choriocarcinoma (GCT66, Supplementary Table), followed by germinoma with mixed components. The hCGβ expression in mixed germinoma exhibited a variable and bimodal pattern; those harboring STGC or a choriocarcinoma component (Pink circles) tended to demonstrate higher expression than those without. The expression levels of pure germinoma were significantly higher than that of mature and immature teratoma and yolk sac tumors (p = 0.01, 0.02, 0.02, respectively, Wilcoxon’s test). (*p = 0.01, **p = 0.02)
hCGβ expression in germinoma
Among the 49 germinoma included in the study, there were two germinoma with STGC. Their hCGβ expression levels were among the highest along with another case of pure germinoma (Figs. 1 and 2). The expression levels in other pure germinoma displayed a wide range of variation; the relative ratio to the brain ranged from 1.09 × 100 to 5.88 × 104. The distribution of the expression levels was linear but not bimodal (Fig. 2).
The expression of hCGβ in germinoma (pure germinoma and germinoma with syncytiotrophoblastic giant cells (STGC)) and two normal adult testis samples are presented in an ascending order. All tumors showed higher expression compared with normal brain tissue (>0), and most also showed higher expression than normal testis samples (48/50 cases, 96 %). Among them, two germinoma with STGC scored the first and third highest expression levels. The expression levels showed a linear but not a bimodal distribution
Correlation between the expression and the concentration of hCG in serum and CSF
The hCG data was available for serum in 55 cases and for CSF in 25, in which the concentration of total hCG was measurable in 30 and 19 cases, respectively. We investigated the correlation between hCGβ expression and the concentrations in serum and CSF in these cases. Tumoral hCGβ mRNA expression showed a trend of positive correlation to the total hCG concentration in both serum and CSF. The R square values of the correlation between the logarithm of expression and serum/CSF concentration were 0.34 and 0.11 in linear correlation, respectively (Fig. 3).
The correlation between the expression level of hCGβ and the concentration of total hCG in serum (a, c) and cerebrospinal fluid (CSF) (b, d) in all GCTs (a, b) and tumors of a single histological component (i.e. pure germinoma, yolk sac tumors, choriocarcinoma, and embryonal carcinoma) (c, d). Although an association between expression and the concentration in serum was observed (a, R2 = 0.34), that of CSF was obscure (b, R2 = 0.11). The association was stronger when the tumors of a single histological component were analyzed separately (c: blood serum, R2 = 0.62, d: CSF, R2 = 0.40)
Since many of the GCTs are heterogeneous and of mixed histological subtypes (mixed germinoma or mixed NGGCT) or teratoma which are composed of the three germ cell layers (endoderm, mesoderm, and ectoderm), the measured mRNA expression levels only reflect the small piece of the tumor tissue used for analysis but may not necessarily reflect all of the histological components of the entire tumor. We therefore performed a subset analysis on pure germinoma and NGGCTs with a single histological component, for which hCG concentrations in serum (21 cases, Fig. 3c) and CSF (11 cases, Fig. 3d) were available. The correlation between hCGβ mRNA expression and serum/CSF hCG concentration was stronger in this subset than the above analysis in which all histological subtypes were included, the R square values being 0.62 for serum and 0.40 for CSF in linear correlation. The separate analyses of the correlation between hCGβ mRNA expression and serum/CSF hCG concentration were performed, yielding a similar result to the above comparison (Supplementary Fig. 1).
Correlation between the expression and immunohistochemistry
The Allred score (the sum of the proportion and intensity scores) ranged from 0 to 8 in the 40 cases of various histologies of GCTs analyzed for immunohistochemistry. The highest score was recorded in 3 cases, which were 2 germinoma mixed with STGC and a germinoma with a choriocarcinoma component. As with the mRNA expression result, pure germinoma cases also showed various scores ranging from 0 to 5. A positive correlation, though only marginally significant, was found between the expression and immunohistochemistry score by linear regression (p = 0.0502, analysis of variance). The scores are shown in the supplementary table and the correlation figure displayed in the supplementary Fig. 2.
hCGβ expression according to the tumor location
The hCGβ expression levels were then compared according to the site of the tumors in 41 primary pure germinoma cases. Since GCTs tend to occur in the midline structures including neurohypophyseal and pineal regions, the expression was compared between tumors in the midline structures and those in other areas including thalamus, basal ganglia, cerebral hemisphere, and posterior fossa. Tumors located at ventricles concomitant with midline regions were categorized as midline tumors. 32 cases were categorized in the midline tumor group and 9 cases in the non-midline tumor group. The tumors which developed in the midline structures showed lower expression than tumors in other locations (6.0 vs. 8.4 in log2 scale, p = 0.007, Wilcoxon’s test) (Fig. 4a).
a The comparison of hCGβ expression levels between two groups of primary pure germinoma based on location. Tumors located outside the midline structures showed higher expression than those of the midline structures (neurohypophyseal and pineal regions) (p = 0.007). b The hCGβ expression levels of the pure germinoma cases which recurred (n = 4) or did not recur (n = 25) were compared. The recurrent cases showed a tendency for higher hCG expression than non-recurrent cases, although the difference was not significant (p = 0.12, Wilcoxon’s test)
hCGβ expression and prognosis
We then investigated the impact of hCGβ expression on the prognosis of pure germinoma patients who were followed-up for more than 3 years after initiation of the treatment. Among the total of 32 cases, 6 cases recurred, in which one died. The clinical characteristics of the two groups with or without recurrence are shown in Table 1. Age, sex, tumor locations, operations, and chemotherapy did not differ significantly between the two groups. However, local irradiation, compared with extended local irradiation encompassing the tumor site and the third and fourth ventricles, was more frequently performed in recurrence cases (2/6) than no recurrence cases (1/26) (p = 0.03, Chi square test).
In order to exclude the effect of radiation field difference in analyzing the association of hCGβ expression and recurrence, we focused on 29 cases (including 4 that recurred) who received radiotherapy covering more than a local field. A significant difference in hCGβ expression was not observed between cases with or without recurrence (7.7 vs. 6.0 in log2 scale, p = 0.16, Wilcoxon’s test) (Fig. 4b).
Discussion
Elevated serum and/or CSF levels of hCG have been used as a tumor marker for choriocarcinoma and germinoma with STGC amongst GCTs. The SIOP CNS GCT II clinical trials recommend that increased levels of hCG and/or AFP in serum and/or CSF are sufficient to make a diagnosis of NGGCT without histopathological confirmation by biopsy (https://www.skion.nl/workspace/uploads/2_siop_cns_gct_ii_final_version_2_15062011_unterschrift_hoppenheit.pdf). The rationale behind this is the assumption that pure germinoma do not produce hCG. However, there is some evidence of hCG production in germinoma [10, 11, 17–19]. To address this controversy, we determined the levels of hCGβ mRNA in intracranial GCT tissues of various histological subtypes. GCTs have been hypothesized to originate from totipotent primordial germ cells (PGCs), which normally develop into gonadal organs [20]. Intracranial GCTs are considered to have originated from mis-migrated PGCs during development [3, 21]. As human PGCs are not available, we used normal adult human brain and testis samples as surrogate reference for expression analyses. The current study clearly showed that the great majority of GCTs expressed higher levels of hCGβ than normal brain tissue (93.3 %) and normal adult testis tissue (85.6 %). Thus, hCGβ expression is not limited to choriocarcinoma and germinoma with STGC but observed across all histological subtypes.
In this study, hCGβ mRNA expression was detected in almost all pure germinoma at a higher level than normal adult brain and testis (Fig. 2). This indicates that germinoma cells have at least the potential of producing the hCG protein. Moreover, the hCGβ expression levels across all germinoma did not show a bimodal pattern, a finding that does not support the idea of subdividing germinoma into two groups according to hCG-producing capacity. This fact corroborates many previous studies reporting the existence of pure germinoma which secrete hCG in the serum and CSF. Katakami et al. developed an ultra-sensitive assay to detect hCG in serum or CSF at picogram level and found hCG elevation in the CSF in every case of pure germinoma [10, 11]. Ikura et al. investigated 6 autopsy cases of iGCT and reported that all four cases with germinoma but without choriocarcinoma components stained positive for hCG by IHC [18]. hCG production by pure germinoma was also suggested by a case presented by Tamaki et al., which showed an extreme elevation of hCGβ in the cyst fluid of a pure germinoma [19].
hCGβ expression was also detected in NGGCTs such as teratoma, yolk sac tumors and embryonal carcinoma, which are histopathologically devoid of these hCG-producing cells. Teratoma contain components originating from each of the three germ layers [20] and accordingly it is understandable that teratoma may contain hCG-producing cells within their entire tumor contents. With regard to embryonal carcinoma, although several reports have demonstrated hCG secretion [8] or hCG immunostaining [8, 22] in these tumors, whether hCG producing cells are present in embryonal carcinoma is currently unknown.
The ubiquitous expression of hCG in all types of GCTs may be explained partly by the tumor heterogeneity. In our series, 22 cases (22.9 %) were of mixed histologies, among which 9 cases were composed of 3 or more histological subtypes. This finding reflects the histological diversity of GCTs.
GCTs are also known to be chronologically heterogeneous. GCTs sometimes recur as a tumor of different histological subtype from the original, and this phenomenon suggests that the tumors may undergo a dynamic change in histology over time irrespective of the influence of radiation and chemotherapy [23, 24]. Seminoma, the gonadal counterpart of intracranial germinoma, is also known to change its morphology once it metastasizes [25]. In the light of such dynamism affecting the tumor identity, it comes as no surprise that every GCT would have a component of hCG–producing cells within its content. In seminoma, serum hCG level may reflect the STGC contents in the tumor [26]. Thus, the varying degrees of hCGβ expression might indicate the presence of those hCG–producing cells in iGCT as well. The hCGβ expression across NGGCT irrespective of histology might reflect the core nature of GCTs, although this hypothesis remains to be proven.
Pure germinoma consists of undifferentiated cells that resemble primordial germ cells, which are regarded as the cell of origin. NGGCT comprises tumors with embryonic lineage (embryonal carcinoma and teratoma) and extra-embryonic lineage (choriocarcinoma and yolk sac tumor) [21]. Whereas it is understandable that hCG expression is exceptionally high in choriocarcinoma considering that hCG is mostly produced at cytotrophoblast and syncytiotrophoblast at the embryonic stage, it is intriguing that some pure germinoma, which are the most undifferentiated subtype of GCTs, also express hCGβ as described above. Surani et al. recently investigated human PGC-like cell induced from embryonal stem cell by RNA sequencing [27]. According to their data available from a public database, the expression of hCG was almost none in those induced PGC-like cells. Considering the generally believed hypothesis that PGCs are the cell of origin of germinomas, their data suggest that a germinoma cell itself may not produce hCG but some germinoma tissues may contain histopathologically unidentified hCG-producing non-germinomatous cells. However, there still remains a possibility that germinoma cell has a potential to differentiate into the syncytiotrophoblastic lineage, producing hCG.
hCGβ expression has been considered as a marker for the presence of pluripotent stem/germ cells. However, hCGβ expression has been observed in many non-germ cell tumors in recent studies including colorectal and ovarian cancers, in which a strong association between hCGβ expression and poor prognosis has been reported [28, 29], suggesting that hCGβ may play an active role in these cancers.
It has been known that hCG comprises 4 isoforms, all of which have different biological functions; the regular and hyperglycosylated hCG (hCG-H) are respectively produced by villous syncytiotrophoblast cells and cytotrophoblast cells in placenta, free β subunit by various non-trophoblastic tumors, and pituitary hCG by pituitary glands [30]. The regular hCG has hormonal functions including promotion of progesterone production by corpus luteum, angiogenesis, suppression of immune reactions, growth of fetal organs, etc. hCG-H has recently been shown to have autocrine but not hormonal property and promotes placental implantation in pregnancy, as well as growth and invasion of choriocarcinoma and other germ cell tumors. hCGβ also functions as an autocrine fashion, promoting growth, invasion, and metastases of cancer cells. The mechanism of the production of these separate isoforms is based on post-translational modifications such as glycosylation [31].
Although it thus seems probable that choriocarcinoma produces the regular hCG while STGC within germinoma produces hCG-H, an immunohistochemistry study on testicular GCTs denoted that hCGβ was positive in small number of seminomas and all types (hCG, hCG-H, hCGβ) were positive in non-seminomatous GCTs [32]. Although the mRNA expression analysis in our study cannot distinguish the production of each subtype, our investigations into the subtypes of hCG produced by pure germinoma and other germ cell tumors may provide a clue to understand their significance in tumor biology. An independent study on hCG isoforms on blood or CSF specimens would be of interest.
While the expression of hCGβ was only loosely associated with the level of total hCG in serum and CSF when all GCTs were included, the correlation became stronger when the tumors with a single histology including pure germinoma, yolk sac tumors, and embryonal carcinoma were separately analyzed. This suggests that hCGβ expression at the RNA level may reflect the amount of protein secretion.
The great majority of GCTs develop in the midline of the body, e.g., mediastinum, testis and ovary [33], as well as intracranial GCTs [34]. Pure germinoma which occur outside the midline structures such as in the neurohypophyseal and pineal regions show higher expression of hCGβ compared with those arising in those structures. Our results somewhat corroborate the report by Ogino et al. who studied 103 pure germinoma cases and showed that tumors with high serum hCG concentrations were more frequently found in basal ganglia compared with tumors with normal concentrations, although the tumor size was not considered as a confounding factor [6]. The elevation in mRNA expression in pure germinoma outside the typical sites may also suggest a different pathogenesis depending on the tumor location.
The prognostic significance of hCG production by germinoma has been debated in the literature. In the current study, comparison of hCGβ expression between the cases with or without recurrence, after excluding the 3 cases who received local radiotherapy, showed higher levels in cases with recurrence, although not significant (p = 0.16).
The impact of the level of mRNA expression on the tendency for recurrence remains equivocal in the present study and consequently we cannot draw a firm conclusion as to whether it is appropriate to divide germinoma into good and intermediate prognosis groups based on hCG production capability [8]. Germinoma patients generally have a favorable prognosis when given appropriate radiotherapy with or without chemotherapy. Recurrence is relatively uncommon, hence the limited number of cases available for comparison studies. In order to evaluate the significance of hCG expression on the tumor prognosis, a larger number of cases followed up for longer periods would be required.
Our investigation has a number of limitations inherent to all studies on intracranial GCTs. GCTs can be histologically highly heterogeneous and GCT cells are intermingled with many other non-GCT components including lymphocytes, granulomatous tissue, etc. Such factors may interfere with the true expression pattern of the tumor cells in the analysis. Although a micro-dissection from a FFPE section could potentially improve the sampling accuracy, such procedure might not be suitable for mRNA expression analysis. Tumor specimen is often gained by means of biopsy and a small biopsy specimen may not always represent all of the components of the tissue. The source of the CSF samples was either ventricular or lumbar drainage depending on each case, which may act as a confounding factor in assessing the relationship with the mRNA expression. These restrictions limit the power of our study. Nevertheless, the current analysis employed 94 frozen samples covering all histological subtypes of these rare tumors, which makes it the largest as well as the most thorough among other similar studies.
In conclusion, we have demonstrated that hCGβ mRNA is expressed across all histological subtypes of GCTs, suggesting that GCTs are capable of producing hCG regardless of the histology. How the elevated mRNA expression in tumor tissue translates into the detection of serum/CSF hCG is currently unknown. The impact of hCG expression on the prognosis of germinoma needs further investigation in a larger cohort. The diagnostic value of the high levels of serum/CSF hCG in diagnosing choriocarcinomas remains the same. Nonetheless, our findings prompt a cautious interpretation of hCG data for preoperative diagnosis of intracranial GCTs. A comprehensive investigation combining all genetic/epigenetic/serological/histopathological data will hopefully lead to a better understanding of this enigmatic class of tumors.
References
Shibui S (2014) Report of the brain tumor registry of Japan, 2001–2004
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Göbel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D (2000) Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 11:263–271
Inamura T, Nishio S, Ikezaki K, Fukui M (1999) Human chorionic gonadotrophin in CSF, not serum, predicts outcome in germinoma. J Neurol Neurosurg Psychiat 66:654–657
Utsuki S, Kawano N, Oka H, Tanaka T, Suwa T, Fujii K (1999) Cerebral germinoma with syncytiotrophoblastic giant cells: feasibility of predicting prognosis using the serum hCG level. Acta Neurochir 141:975 discussion 977-978
Ogino H, Shibamoto Y, Takanaka T, Suzuki K, Ishihara S, Yamada T, Sugie C, Nomoto Y, Mimura M (2005) CNS germinoma with elevated serum human chorionic gonadotropin level: clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys 62:803–808. doi:10.1016/j.ijrobp.2004.10.026
Shibamoto Y, Takahashi M, Sasai K (1997) Prognosis of intracranial germinoma with syncytiotrophoblastic giant cells treated by radiation therapy. Int J Radiat Oncol Biol Phys 37:505–510
Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T (1997) Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86:446–455. doi:10.3171/jns.1997.86.3.0446
Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H (1998) Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer 34:104–110
Fujimaki T, Matsutani M, Nishikawa R, Mishima K, Suzuki T, Katakami H (2011) All germinomas are capable of producing hCG-beta and might be treated in the same protocol. Neuro-oncology 13:I13
Katakami H, Hashida S, Yamaguchi H, Yazawa S, Nakano S, Wakisaka S, Matsutani M (2003) Diagnosis and follow-up of CNS germ cell tumors using an ultrasensitive assay of HCG-beta. Horm Clin 51:196–206
Utsuki S, Oka H, Tanizaki Y, Kondo K, Kawano N, Fujii K (2005) Pathological features of intracranial germinomas with reference to fibrous tissue and granulomatous change. Brain Tumor Pathol 22:9–13. doi:10.1007/s10014-004-0171-0
Zeng N, Liu L, McCabe MG, Jones DT, Ichimura K, Collins VP (2009) Real-time quantitative polymerase chain reaction (qPCR) analysis with fluorescence resonance energy transfer (FRET) probes reveals differential expression of the four ERBB4 juxtamembrane region variants between medulloblastoma and pilocytic astrocytoma. Neuropathol Appl Neurobiol 35:353–366. doi:10.1111/j.1365-2990.2008.01001.x
Fukushima S, Otsuka A, Suzuki T, Yanagisawa T, Mishima K, Mukasa A, Saito N, Kumabe T, Kanamori M, Tominaga T, Narita Y, Shibui S, Kato M, Shibata T, Matsutani M, Nishikawa R, Ichimura K (2014) Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. doi:10.1007/s00401-014-1247-5
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Modern Pathol: An Off J United States and Can Acad of Pathol Inc 11:155–168
Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, le Roanh D, To TV, Qian Z, Love RR, Allred DC (2004) Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Modern Pathol: An Off J United States and Can Acad Pathol Inc 17:1545–1554. doi:10.1038/modpathol.3800229
Bjornsson J, Scheithauer BW, Okazaki H, Leech RW (1985) Intracranial germ cell tumors: pathobiological and immunohistochemical aspects of 70 cases. J Neuropathol Exp Neurol 44:32–46
Ikura Y, Sasaki M, Ohgami M, Ikebe T, Gotoh K, Bettoh H, Sakurai M (1996) Mixed germ-cell tumor of the brain. Pathologic study of six autopsy cases. Pathol Res Ractice 192:595–603. doi:10.1016/S0344-0338(96)80111-7
Tamaki N, Lin T, Shirataki K, Hosoda K, Kurata H, Matsumoto S, Ito H (1990) Germ cell tumors of the thalamus and the basal ganglia. Child’s Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 6:3–7
Teilum G (1965) Classification of endodermal sinus tumour (mesoblatoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathologica et Microbiologica Scandinavica 64:407–429
Sano K (1999) Pathogenesis of intracranial germ cell tumors reconsidered. J Neurosurg 90:258–264. doi:10.3171/jns.1999.90.2.0258
Yamagami T, Handa H, Yamashita J, Okumura T, Paine J, Haebara H, Furukawa F (1987) An immunohistochemical study of intracranial germ cell tumours. Acta Neurochir 86:33–41
Tan C, Scotting PJ (2013) Stem cell research points the way to the cell of origin for intracranial germ cell tumours. J Pathol 229:4–11. doi:10.1002/path.4098
Wong JM, Chi SN, Marcus KJ, Levine BS, Ullrich NJ, MacDonald S, Lechpammer M, Goumnerova LC (2010) Germinoma with malignant transformation to nongerminomatous germ cell tumor. J Neurosurg Pediatr 6:295–298. doi:10.3171/2010.6.PEDS09541
Mostofi FK (1973) Tumors of the male genital system. Armed Forces Institute of Pathology, Washington, D.C.
Madersbacher S, Kratzik C, Gerth R, Dirnhofer S, Berger P (1994) Human chorionic gonadotropin (hCG) and its free subunits in hydrocele fluids and neoplastic tissue of testicular cancer patients: insights into the in vivo hCG-secretion pattern. Cancer Res 54:5096–5100
Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA (2015) SOX17 is a critical specifier of human primordial germ cell fate. Cell 160:253–268. doi:10.1016/j.cell.2014.12.013
Louhimo J, Carpelan-Holmstrom M, Alfthan H, Stenman UH, Jarvinen HJ, Haglund C (2002) Serum HCG beta, CA 72-4 and CEA are independent prognostic factors in colorectal cancer. Int J Cancer 101:545–548. doi:10.1002/ijc.90009
Vartiainen J, Lassus H, Lehtovirta P, Finne P, Alfthan H, Butzow R, Stenman UH (2008) Combination of serum hCG beta and p53 tissue expression defines distinct subgroups of serous ovarian carcinoma. Int J Cancer 122:2125–2129. doi:10.1002/ijc.23322
Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol: RB&E 8:102. doi:10.1186/1477-7827-8-102
Cole LA, Butler S (2012) Hyperglycosylated hCG, hCGbeta and Hyperglycosylated hCGbeta: interchangeable cancer promoters. Mol Cell Endocrinol 349:232–238. doi:10.1016/j.mce.2011.10.029
Lempiainen A, Sankila A, Hotakainen K, Haglund C, Blomqvist C, Stenman UH (2014) Expression of human chorionic gonadotropin in testicular germ cell tumors. Urol Oncol 32:727–734. doi:10.1016/j.urolonc.2013.11.007
Oosterhuis JW, Stoop H, Honecker F, Looijenga LH (2007) Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl 30:256. discussion 263-254 doi:10.1111/j.1365-2605.2007.00793.x
Jennings MT, Gelman R, Hochberg F (1985) Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63:155–167. doi:10.3171/jns.1985.63.2.0155
Acknowledgments
This study was supported by a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. We thank Dr Hideki Katakami for sharing valuable data and inspiring the project, and Dr Sylvia Kocialkowski for critical reading of the manuscript.
Conflict of interest
The authors declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Intracranial Germ Cell Tumor Genome Analysis Consortium (the iGCT Consortium).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takami, H., Fukushima, S., Fukuoka, K. et al. Human chorionic gonadotropin is expressed virtually in all intracranial germ cell tumors. J Neurooncol 124, 23–32 (2015). https://doi.org/10.1007/s11060-015-1809-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1809-y