Abstract
Purpose of Review
The purpose of this review is to examine the proposed role of immune modulation in the development and progression of diabetic kidney disease (DKD).
Recent Findings
Diabetic kidney disease has not historically been considered an immune-mediated disease; however, increasing evidence is emerging in support of an immune role in its pathophysiology. Both systemic and local renal inflammation have been associated with DKD. Infiltration of immune cells, predominantly macrophages, into the kidney has been reported in a number of both experimental and clinical studies. In addition, increased levels of circulating pro-inflammatory cytokines have been linked to disease progression. Consequently, a variety of therapeutic strategies involving modulation of the immune response are currently being investigated in diabetic kidney disease.
Summary
Although no current therapies for DKD are directly based on immune modulation many of the therapies in clinical use have anti-inflammatory effects along with their primary actions. Macrophages emerge as the most likely beneficial immune cell target and compounds which reduce macrophage infiltration to the kidney have shown potential in both animal models and clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
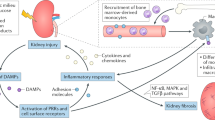
Diabetic kidney disease (DKD) is currently the most prevalent chronic kidney disease and the leading cause of end-stage renal disease (ESRD) in adults [1]. DKD has not historically been considered an immune-mediated disease as metabolic and haemodynamic factors have been thought to be the main causes of injury. However, recent studies support a role for the immune system in both the development and progression of this disease. Emerging data indicate that DKD is associated with both systemic and local renal inflammation. Elevated levels of inflammatory cytokines including tumour necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, interleukin (IL)-1 and IL-6 have been found in the peripheral blood of patients with DKD in comparison to healthy control individuals [2]. For a number of these cytokines, expression was found to increase with disease progression. In addition, analysis of renal biopsies has confirmed the presence of inflammatory cells in both glomeruli and the interstitium at all stages of DKD [3, 4••].
In this review, we discuss the role of the immune system in both the development and progression of DKD. We examine the role of leukocyte recruitment to the kidney and also the effects of pro-inflammatory cytokines and chemokines. Throughout, we highlight implications for new therapeutic strategies and discuss those which are currently under consideration particularly those which have proceeded to clinical trials.
Infiltrating Immune Cells
Inflammation in the kidney involves complex interactions between resident kidney cells and infiltrating leukocytes. There is growing evidence that immune cells accumulate in the kidney even in the early stages of DKD. While there is substantial evidence for the infiltration of macrophages, the potential role of other immune cells including neutrophils, mast cells and lymphocytes is less clear. Below we review the potential role of each immune cell type in DKD progression:
Macrophages
Macrophages are the most numerous infiltrating leukocytes found in renal biopsies from patients with DKD and in experimental animal models, with both glomerular and interstitial accumulation reported [5]. Macrophage accumulation in the kidneys of diabetic patients predicts declining renal function [3]. A number of features of the diabetic kidney may enhance the recruitment of macrophages. These include increased expression of ICAM-1 and MCP-1 by renal tubular cells responding to high circulating levels of glucose and advanced glycation end products (AGEs) [6, 7]. Once recruited to the diabetic kidney, these macrophages respond to local high levels of glucose, AGEs and oxidised low-density lipoprotein (Ox-LDL) to execute inflammatory cytokine secretion [8, 9]. Other mechanisms by which macrophages have been proposed to progress DKD include stimulation of the production of reactive oxygen species (ROS) and proteases. This will lead to tissue damage and ultimately to renal fibrosis [10].
Depletion of macrophages using diphtheria toxin (DT) in CD11b–DT receptor (CD11b-DTR) transgenic mice confirmed the direct role of macrophages in the progression of DKD. In this study, CD11b-DTR mice were treated with DT in order to deplete macrophages after induction of diabetes using streptozotocin (STZ). This macrophage depletion significantly reduced albuminuria, kidney macrophage recruitment and glomerular histological changes [11]. Mice lacking chemokine-CC motif ligand 2 (Ccl2) demonstrated significantly reduced capacity to recruit and activate kidney macrophages and were substantially protected from the development of DKD [7, 12]. Similarly, chemokine-CC motif receptor (CCR)2 antagonists have been shown to prevent glomerulosclerosis and renal failure in the db/db mouse model of type 2 diabetes and also in diabetic human transgenic CCR2 knockin mice [13,14,15]. These findings have led to phase 2 clinical trials of such compounds in type 2 diabetes patients with DKD. In addition, co-treatment with one such compound, CCX140-B, provided additional protection against the development of albuminuria and declining renal function compared with current standard of care therapy alone [16•]. Phase 3 clinical trials are now planned for the CCR2 antagonist CCX140-B.
Macrophages are classified based on their inflammatory phenotype into M1 (classically activated macrophages, secreting pro-inflammatory cytokines) and M2 (alternatively activated macrophages, capable of modulating immune responses through anti-inflammatory or pro-resolution mechanisms) [17, 18] (Fig. 1). Macrophages located at the site of diabetic kidney injury are predominantly M1 [7, 11, 19]. The relevance of M1 macrophages to the development of DKD has been shown using mice with macrophages deficient in cyclooxygenase-2 (Cox-2). Macrophages in these mice demonstrate increased M1 polarisation which is associated with increased renal injury [20]. Conversely, adoptive transfer of pro-resolution, M2 macrophages to STZ-induced type 1 diabetic mice led to decreased macrophage infiltration to the kidney along with decreased renal damage including tubular atrophy, glomerular hypertrophy and interstitial expansion [21]. Pentraxin-3 has also been shown recently to attenuate renal damage in DKD by promoting M2 macrophage differentiation [22••]. These data suggest that therapeutic strategies which reduce the M1 phenotype and promote the M2 phenotype in kidney macrophages could have significant potential in the treatment and management of DKD.
M1 versus M2 macrophages. Local cytokine production can polarise macrophages to an M1 or M2 phenotype. M1 macrophages are largely pro-inflammatory and secrete ROS and nitric oxide (NO) along with pro-inflammatory cytokines including IL-12, TNF-1α, IL-1β and MCP-1. M1 macrophages have been shown to contribute to the renal damage seen in DKD [20]. Conversely, M2 macrophages are anti-inflammatory and promote resolution of inflammation and healing. They secrete regulatory cytokines including IL-10 and TGF-β. Promotion of M2 macrophage polarisation has been shown to attenuate renal damage in DKD [21, 22••]. IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; NO, nitric oxide; ROS, reactive oxygen species; TNF, tumour necrosis factor; TGF, transforming growth factor
One potential class of compounds which have been shown to promote pro-resolution bioactions are Lipoxins (LXs). One member of the Lipoxin family, LXA4 has been shown to act on macrophages by blocking ICAM-1 expression along with the production of inflammatory cytokines including IL-6 and TNF-α [23,24,25]. LXA4 has also been shown to reduce fibrotic and inflammatory responses in the unilateral ureteric obstruction (UUO) model of renal fibrosis [26].
T Cells
While there is little evidence that T cells play a direct role in the development of DKD, activated T cells may enhance progression of the disease. Modest increases in both CD4+ and CD8+ T cells have been reported in the interstitium of patients with type 2 diabetes, with numbers of these cells correlating with proteinuria [27]. Similarly, the number of T cells infiltrating the kidneys of DKD animals have been shown to correlate with the degree of albuminuria [27,28,29]. Studies on RAG1−/− mice, which lack both mature T and B cells, demonstrated that while these mice were not protected from STZ-induced renal fibrosis or histological injury, preservation of podocytes and a reduction in albuminuria was seen [28]. Another study using the STZ mouse model demonstrated that treatment with CTLA4-Ig (abatacept) effectively reduced the accumulation of activated CD4+ T cells in the kidney. However, no differences were found in albumin excretion rate (AER) or albumin/creatinine ratio, or mesangial expansion suggesting that renal T cell inflammation is not a driving factor in the pathology of DKD, at least in this model [30].
Forkhead box P3 (FoxP3)+ T-regulatory cells (Tregs) have also been reported in kidneys of diabetic mice. One study in db/db mice has shown that depletion of Tregs using an anti-CD25 monoclonal antibody resulted in accelerated progression of renal injury including increased glomerular hyperfiltration and albuminuria. Conversely, adoptive transfer of immunosuppressive Tregs resulted in decreased albuminuria and glomerular diameter [31]. Another study in patients with type 2 diabetes has demonstrated an inverse correlation between the number of Tregs in the kidney and urinary AER [32]. Zhang et al. examined the relative proportions of Th1/Th2/Th17/Treg cells in serum from patients with various degrees of DKD and controls. They found a skew towards Th1 and Th17 cells in DKD and that the urine albumin/creatinine ratio (ACR) was positively correlated with proportions of Th1 and Th17 cells and also the ratio of Th17:Treg cells [33•].
Renal IL-17-producing T helper cells (Th17 cells) have also been identified in the STZ mouse model [34]. One study has suggested that Th17 cells may protect diabetic kidneys by reducing and modifying the inflammatory response. Type 1 diabetic mice genetically deficient in IL-17A developed more severe nephropathy than controls, while administration of IL-17A to wild-type mice effectively prevented the development of nephropathy and reversed established disease [35]. However, Kim et al. have conversely demonstrated a significant increase in the number of IL-17A+ CD4+ T cells in the kidneys of STZ-treated mice and reported that this increase is associated with progression of DKD [34]. They have also shown that modulating Th17 cells using mycophenolate mofetil (MMF) was associated with a decrease in albuminuria. Similarly, Kuo et al. found increased numbers of CD4+ IL-17+ T cells in kidney biopsies from patients with type 2 diabetes. The number of these cells was positively correlated with deterioration in estimated glomerular filtration rate (eGFR) [36]. As such, the role of T cells in DKD remains controversial.
Other Immune Cells
There is little evidence that B cells play a direct role in the pathogenesis of DKD. One study in non-obese diabetic (NOD) mice reported a modest increase in the numbers of B cells in the glomeruli [37]. More recently Moon et al. reported an increase in B cells in the kidneys of patients with type 2 diabetes. Converse to the mouse model, this increase was seen in the interstitium. The authors also found that the numbers of these cells correlated with levels of proteinuria [27]. However, given a lack of any further evidence, it is most likely that the role of B cells in DKD is in the production of immunoglobulins directed against antigens produced in diabetes, e.g., oxLDL and AGEs, leading to increased levels of immune complexes [38, 39].
Mast cells have also been shown to be increased in the interstitium of DKD patients, with the numbers of mast cells correlating with serum creatinine [40]. Mast cell degranulation has also been reported in renal biopsies of patients with type 2 diabetes at varying stages of nephropathy [41]. Mast cell stabilising agents have been shown to significantly improve kidney function in terms of renal collagen content, urinary protein and creatinine clearance in STZ rats [42]. These data suggest that mast cells may contribute to the development, or more likely to the progression of DKD.
TNF-α
Increased serum levels of TNF-α have been reported in patients with type 2 diabetes where increases have been shown to correlate with the degree of albuminuria [45, 46•]. Urinary TNF-α has also been shown to be increased in diabetic patients and correlates with clinical markers of DKD and disease progression [47]. Increased levels of TNF-α in the kidney lead to increased ROS production, increased endothelial cell permeability, cytotoxicity and recruitment of macrophages, further perpetuating inflammation [48, 49]. From a therapeutic perspective, TNF-α inhibitors have been shown to decrease albumin excretion in diabetic rats [50]. A similar study using Ins2(Akita) mice, demonstrated that blockade of TNF-α with an anti-TNF-α antibody led to kidney protection as measured by reductions in albuminuria, plasma creatinine, histopathologic changes, kidney macrophage recruitment and plasma inflammatory cytokine levels. Using macrophage-specific TNF-α-deficient mice, this study further demonstrated that macrophages are the primary source of renal TNF-α in the STZ model of diabetes. Loss of macrophage-derived TNF-α significantly reduced albuminuria, histopathologic changes and kidney macrophage recruitment compared to control mice [51••].
MCP-1
MCP-1 (CCL2) is one of the key chemokines involved in regulating the migration and infiltration of monocytes, macrophages, T cells and dendritic cells to the sites of inflammation [52, 53]. In animal models of diabetes-induced kidney damage, levels of MCP-1 in the kidney have been shown to increase progressively [12, 19]. In addition, experimental models of both type 1 and type 2 diabetes have shown that renal injury is attenuated in MCP-1−/− animals [7, 12]. Clinically, increased urinary excretion of MCP-1 is seen in patients with microalbuminuria and macroalbuminuria and correlate with urine albumin/creatinine ratio and decline in renal function [54•]. In diabetic patients with macroalbuminuria, urinary MCP-1 levels are a predictor of progression of nephropathy [55]. The pathogenic role of MCP-1 is further supported by the fact that inhibition of the MCP-1 receptor (CCR-2) with a selective antagonist ameliorated glomerulosclerosis in an experimental model of diabetes [56].
A number of other studies have provided indirect evidence for the role of MCP-1 in the pathogenesis of DKD Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs) and the aldosterone-receptor antagonist spironolactone have all been shown to reduce MCP-1 expression, renal macrophage infiltration and renal injury [57,58,59,60]. Similarly, the immunosuppressant MMF has been shown to block increases in urinary AER and glomerular damage, along with reducing kidney macrophage accumulation and the severity of renal injury in experimental models of DKD. This has been shown to be associated with decreased expression of MCP-1 and ICAM-1 [61, 62]. A clinical trial to evaluate the effects of MMF on proteinuria and progression of DKD is currently underway [63].These studies provide strong evidence for a key role for MCP-1 in the progression of DKD. They have demonstrated that decreased MCP-1 expression is associated with decreased macrophage infiltration into the kidney and importantly with reductions in diabetic kidney damage. These data suggest that specific targeting of MCP-1 would potentially be beneficial in the treatment of DKD. NOXXON Pharma have developed an anti-MCP-1 Spiegelmer NOX-E36 (an anti-MCP-1-enantiomeric RNA aptamer) and have sponsored a number of phase I and phase II clinical trials to investigate the potential therapeutic benefit in DKD [64]. NOX-E36 was well tolerated in these trials and promising results were seen. MCP-1 inhibition attenuated albuminuria and restored glomerular endothelial glycocalyx and barrier function. While overall numbers of macrophages in the kidney were not altered, NOX-E36 altered cytokine and cell-surface marker expression of these macrophages in favour of an anti-inflammatory phenotype [65••].
Other Cytokines
Increased serum levels of IL-6 have been reported in patients with overt nephropathy when compared to diabetic patients with normal renal function [66]. Serum IL-6 has been found to correlate with urinary AER and glomerular basement membrane thickening [67]. Additionally, patients with type 2 diabetes carrying certain polymorphisms in the IL-6 gene have increased risk of developing DKD [68,69,70].
Macrophage migration inhibitory factor (MIF) plays a critical role in inflammation and has been shown to be elevated in the kidney in experimental models of DKD where it precedes the onset of microalbuminuria [19, 71]. Elevated MIF levels have also been reported in the serum of patients with type 2 diabetes [72]. The MIF receptor CD74 has also been shown to be upregulated in both experimental and clinical DKD [73]. Wang et al. have reported that treatment with the MIF inhibitor ISO-1 led to decreased blood glucose, albuminuria, extracellular matrix accumulation, epithelial-mesenchymal transition (EMT) and macrophage activation in the kidneys of db/db mice [74•]. Thus, MIF inhibition may be a potential therapeutic strategy for DKD attributable to its inhibitory effect on macrophage activation in the diabetic kidney.
Adhesion Molecules
ICAM-1 and VCAM-1 are cell-surface glycoproteins which mediate the adhesion of leukocytes to vascular endothelium. Increased levels of both have been reported in the rat model of STZ-induced diabetes [75]. Clinical studies have also described increased levels of soluble forms of ICAM-1 and VCAM-1 in the plasma of patients with DKD compared to diabetic patients without nephropathy [76,77,78,79]. More recently, Polat et al. have demonstrated that serum ICAM-1 and VCAM-1 levels are increased in patients with microalbuminuria compared to those with normalbuminuria and that serum VCAM-1 correlated with urinary AER, suggesting that it might be useful as a predictive marker for development and progression of DKD [80].
ICAM-1−/− mice have been found to be resistant to renal injury in terms of albuminuria, glomerular hypertrophy and tubular damage, in experimental models of diabetes. This is associated with a decrease in the infiltration of CD4+ cells and macrophages into the kidneys of these mice [6, 81]. As such, modulation of the activity of these adhesion molecules may be a potential target for therapeutic intervention in DKD. ICAM-1 antagonists are currently under development [82].
NLRP3 Inflammasome—IL-1β and IL-18
A number of features of DKD including hyperglycaemia, formation of AGEs and mitochondrial oxidative stress are known to activate the NLRP3 inflammasome resulting in the production of IL-1β and IL-18 [83••, 84] (Fig. 2). Studies in both patients and animal models of DKD associated with both type 1 and type 2 diabetes have demonstrated increased renal levels of components of the inflammasome including caspase 1, mature IL-1β and IL-18 that were associated with podocyte injury, albuminuria and mesangial matrix accumulation [83••, 85, 86]. In addition, Nlrp3 and caspase-1 deficient mice are protected against DKD [82, 85]. Loss of these genes in myeloid cells alone did not confer protection against DKD, indicating that NLRP3 inflammasome activity in other cell types plays a role in the development of DKD [83••].
Inflammasome activation. Diabetes-associated products including hyperglycaemia and AGEs have been shown to activate the NLRP3 inflammasome via the production of mitochondrial ROS. This involves the oligomerization of inactive NLRP3, ASC and pro-caspase 1 leading to the activation of caspase 1 and subsequent production and secretion of mature pro-inflammatory cytokines IL-1β and IL-18. AGEs, advanced glycation end products; ASC, apoptosis-associated speck-like protein containing a carboxy-terminal CARD; IL, interleukin; ROS, reactive oxygen species
Blockade of hyperglycaemia-induced mitochondrial ROS with the superoxide dismutase mimetic MitoTempo has been shown to block DKD in diabetic mice and also to block glomerular NLRP3 activation [83••, 85]. Treatment of diabetic mice with an IL-1 receptor antagonist also reduces renal damage [8].
Several NLRP3 antagonists have been shown to have therapeutic potential in animal models of inflammatory diseases [87]. These have not been tested in models of DKD but based on the above evidence there is potential for these agents to have a beneficial effect.
NF-κB
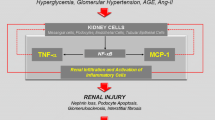
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor acts as a master regulator of the expression of pro-inflammatory genes including cytokines, chemokines, adhesion molecules and immune receptors. There is significant experimental evidence from both in vivo models and human studies to support a critical role for NF-κB in the pathogenesis of renal inflammation. NF-kB activation has been shown in the STZ model of type 1 diabetes along with increased macrophage infiltration into the kidney and increased production of pro-inflammatory cytokines including TNF-α and IL-1β [88]. Similarly, nuclear translocation and activation of NF-κB has been demonstrated in tubular epithelial cells from DKD patients. This was found to correlate with proteinuria and interstitial cell infiltration in these patients [89, 90]. Increased NF-κB protein and mRNA expression have been reported in peripheral blood mononuclear cells from patients with diabetes in comparison to healthy control individuals and to correlate with the severity of DKD [91].
Although NF-κB inhibitors are not currently in use for the treatment of renal disease, many drugs currently in clinical use have been shown to block NF-κB along with their other actions. These include statins, steroids and vitamin D receptor agonists [92,93,94]. Similarly, curcumin (an anti-inflammatory plant polyphenolic compound) has been shown to protect against the development of DKD in STZ rats by reducing macrophage infiltration through the inhibition of NF-κB activation [88]. Also using the STZ model, the antioxidant tocotrienol has been shown to suppress NF-κB activation, reduce TNF-α and transforming growth factor (TGF)-β1 levels and reverse renal dysfunction [95, 96].
Also in STZ rats, administration of pyrrolidine dithiocarbamate (an antioxidant inhibitor of NF-κB activity) or BAY-110782 (an inhibitor of IκB phosphorylation) for 4 weeks after the onset of diabetes led to a reduction in NF-κB activation along with decreased renal macrophage infiltration and production of inflammatory cytokines including MCP-1, TNF-α, IL-1β and IL-6 [97, 98].
Immune Complex Deposition and Complement Activation
Immune Complex Deposition
Immune complex deposition has been reported along the glomerular and tubular basement membranes in DKD patients [99]. Greater intensity of this staining is associated with worse outcomes in diabetic patients [100]. A number of modified proteins which develop in diabetes including Ox-LDL and AGEs are potentially immunogenic and can result in the formation of immune complexes [101, 102]. A number of studies have shown increased serum concentration of Ox-LDL immune complexes in patients with DKD which are associated with the development of albuminuria [103, 104]. In mesangial cells in vitro, anti-Ox-LDL immune complexes have been shown to promote production of MCP-1 and colony-stimulating factor (CSF)-1 and also to stimulate mesangial expansion by inducing collagen production [105, 106]. Anti-Ox-LDL complexes have also been shown to activate the classical complement pathway and the production of inflammatory cytokines including TNF-α, IL-1 and IL-6 in human macrophages [107]. These responses occur through the binding of immune complexes to Fcγ receptors on the surface of mesangial cells and macrophages [106]. Mice genetically deficient in Fcγ receptors are protected from inflammation, glomerular damage and albuminuria in DKD [108].
Complement Activation
The complement system is a component of the innate immune system that enhances the ability of antibodies and phagocytic cells to clear microbes and damaged cells and also promotes inflammation. Growing evidence suggests that activation of the complement cascade may contribute to DKD [109,110,111]. Both hyperglycemia-induced ROS and immune complex deposition have been shown to activate complement [107, 109]. Glomerular deposition of complement component C3 is a feature of animal models of both type 1 and type 2 diabetes-associated DKD [19, 37, 112]. In addition, analysis of renal biopsies has found that 50–60% of DKD patients have glomerular deposition of complement component C3 which correlates with the severity of glomerulosclerosis [113]. C3 can act as both a potent chemoattractant and activator of mast cells.
An inhibitor of the complement cascade (K-76 COONa) has been shown to significantly reduce proteinuria and mesangial expansion in a rat model of diabetes, and this was associated with decreased glomerular immune complex deposition [114]. Similarly, blockade of the complement C3a receptor reduces kidney inflammation, albuminuria, renal fibrosis and loss of renal function in diabetic rats [115].
Conclusions
The important role played by immune modulation in the development and progression of DKD is now indisputable. This modulation involves increased infiltration of immune cells to the kidney, pro-inflammatory cytokine production, chemokine production, immune complex formation, complement activation and ultimately renal tissue damage. Macrophages are the primary leukocytes recruited to the diabetic kidney. Consequently compounds which reduce macrophage infiltration to the kidney, including those which block MCP-1 and CCR2, have proven beneficial in experimental models of DKD and as adjunct therapies in clinical trials. Studies have demonstrated that the macrophages located within the diabetic kidney are predominantly M1 phenotype and pre-clinical studies suggest that reprogramming of these macrophages to a pro-resolution M2 phenotype may attenuate renal damage. As such, much ongoing research is investigating the specific targeting of macrophages as potential therapeutic strategy in DKD.
Another emerging area of interest is the role of the inflammasome in the progression of DKD. In experimental models, hyperglycaemia and other products of diabetes have been shown to activate the NLRP3 inflammasome via induction of mitochondrial ROS. This provides two potential therapeutic targets—blockade of mitochondrial ROS production and inhibition of inflammasome activation. Compounds targeting both of these pathways have proved effective in experimental models of DKD and should be investigated in clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7. e1-420
Perlman AS, Chevalier JM, Wilkinson P, Liu H, Parker T, Levine DM, et al. Serum inflammatory and immune mediators are elevated in early stage diabetic nephropathy. Ann Clin Lab Sci. 2015;45(3):256–63.
Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton). 2006;11(3):226–31.
•• Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2017;32(8):1322–9. This study investigated the presence and phenotype of glomerular and interstitial macrophages.
Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49(3):466–75.
Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16(6):1711–22.
Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50(2):471–80.
Webster L, Abordo EA, Thornalley PJ, Limb GA. Induction of TNF alpha and IL-1 beta mRNA in monocytes by methylglyoxal- and advanced glycated endproduct-modified human serum albumin. Biochem Soc Trans. 1997;25(2):250S.
Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108(9):1070–7.
Tesch GH. Role of macrophages in complications of type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34(10):1016–9.
You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol. 2013;305(12):F1719–27.
Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69(1):73–80.
Sayyed SG, Ryu M, Kulkarni OP, Schmid H, Lichtnekert J, Grüner S, et al. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80(1):68–78.
Seok SJ, Lee ES, Kim GT, Hyun M, Lee JH, Chen S, et al. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant. 2013;28(7):1700–10.
Sullivan T, Miao Z, Dairaghi DJ, Krasinski A, Wang Y, Zhao BN, et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol. 2013;305(9):F1288–97.
• de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–96. This clinical trial indicated that inhibition of CCR2 has renoprotective effects in patients with type 2 diabetes.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35.
Kennedy A, Fearon U, Veale DJ, Godson C. Macrophages in synovial inflammation. Front Immunol. 2011;2:52.
Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116–28.
Wang X, Yao B, Wang Y, Fan X, Wang S, Niu A, et al. Macrophage cyclooxygenase-2 protects against development of diabetic nephropathy. Diabetes. 2017;66(2):494–504.
Zheng D, Wang Y, Cao Q, Lee VW, Zheng G, Sun Y, et al. Transfused macrophages ameliorate pancreatic and renal injury in murine diabetes mellitus. Nephron Exp Nephrol. 2011;118(4):e87–99.
•• Sun H, Tian J, Xian W, Xie T, Yang X. Pentraxin-3 attenuates renal damage in diabetic nephropathy by promoting M2 macrophage differentiation. Inflammation. 2015;38(5):1739–47. This study in a mouse model of hyperglycaemia-induced nephropathy nicely demonstrated that pentraxin-3attenuated renal damage by promoting M2 macrophage differentiation.
Decker Y, McBean G, Godson C. Lipoxin A4 inhibits IL-1beta-induced IL-8 and ICAM-1 expression in 1321N1 human astrocytoma cells. Am J Physiol Cell Physiol. 2009;296(6):C1420–7.
Baker N, O’Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182(6):3819–26.
Börgeson E, Godson C. Molecular circuits of resolution in renal disease. S ScientificWorldJournal. 2010;10:1370–85.
Börgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, et al. Lipoxin A4 and benzo-lipoxin A4 attenuate experimental renal fibrosis. FASEB J. 2011;25(9):2967–79.
Moon JY, Jeong KH, Lee TW, Ihm CG, Lim SJ, Lee SH. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol. 2012;35(2):164–74.
Lim AK, Ma FY, Nikolic-Paterson DJ, Kitching AR, Thomas MC, Tesch GH. Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia. 2010;53(8):1772–82.
Herrera M, Söderberg M, Sabirsh A, Valastro B, Mölne J, Santamaria B, et al. Inhibition of T-cell activation by the CTLA4-Fc Abatacept is sufficient to ameliorate proteinuric kidney disease. Am J Physiol Renal Physiol. 2017;312(4):F748–F59.
Norlin J, Nielsen Fink L, Helding Kvist P, Douglas Galsgaard E, Coppieters K. Abatacept treatment does not preserve renal function in the streptozocin-induced model of diabetic nephropathy. PLoS One. 2016;11(4):e0152315.
Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–62.
Wu CC, Sytwu HK, Lu KC, Lin YF. Role of T cells in type 2 diabetic nephropathy. Exp Diabetes Res. 2011;2011:514738.
• Zhang C, Xiao C, Wang P, Xu W, Zhang A, Li Q, et al. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Hum Immunol. 2014;75(4):289–96. This study demonstrated that alterations in the proportions of Th1/Th2/Th17/Treg cells are skewed towards Th1 and Th17 in patients with type 2 diabetes.
Kim SM, Lee SH, Lee A, Kim DJ, Kim YG, Kim SY, et al. Targeting T helper 17 by mycophenolate mofetil attenuates diabetic nephropathy progression. Transl Res. 2015;166(4):375–83.
Mohamed R, Jayakumar C, Chen F, Fulton D, Stepp D, Gansevoort RT, et al. Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J Am Soc Nephrol. 2016;27(3):745–65.
Kuo HL, Huang CC, Lin TY, Lin CY. IL-17 and CD40 ligand synergistically stimulate the chronicity of diabetic nephropathy. Nephrol Dial Transplant. 2018;33:248–256.
Xiao X, Ma B, Dong B, Zhao P, Tai N, Chen L, et al. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J Autoimmun. 2009;32(2):85–93.
Lopes-Virella MF, Carter RE, Baker NL, Lachin J, Virella G, Group DER. High levels of oxidized LDL in circulating immune complexes are associated with increased odds of developing abnormal albuminuria in type 1 diabetes. Nephrol Dial Transplant. 2012;27(4):1416–23.
Lopes-Virella MF, Hunt KJ, Baker NL, Virella G, Investigators VGo. High levels of AGE-LDL, and of IgG antibodies reacting with MDA-lysine epitopes expressed by oxLDL and MDA-LDL in circulating immune complexes predict macroalbuminuria in patients with type 2 diabetes. J Diabetes Complicat. 2016;30(4):693–9.
Okoń K, Stachura J. Increased mast cell density in renal interstitium is correlated with relative interstitial volume, serum creatinine and urea especially in diabetic nephropathy but also in primary glomerulonephritis. Pol J Pathol. 2007;58(3):193–7.
Zheng JM, Yao GH, Cheng Z, Wang R, Liu ZH. Pathogenic role of mast cells in the development of diabetic nephropathy: a study of patients at different stages of the disease. Diabetologia. 2012;55(3):801–11.
Jones SE, Gilbert RE, Kelly DJ. Tranilast reduces mesenteric vascular collagen deposition and chymase-positive mast cells in experimental diabetes. J Diabetes Complicat. 2004;18(5):309–15.
Wu CC, Chen JS, Lu KC, Chen CC, Lin SH, Chu P, et al. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411(9–10):700–4.
Mensah-Brown EP, Obineche EN, Galadari S, Chandranath E, Shahin A, Ahmed I, et al. Streptozotocin-induced diabetic nephropathy in rats: the role of inflammatory cytokines. Cytokine. 2005;31(3):180–90.
Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52(5):605–8.
• Chen YL, Qiao YC, Xu Y, Ling W, Pan YH, Huang YC, et al. Serum TNF-α concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: a systematic review and meta-analysis. Immunol Lett. 2017;186:52–8. A systematic review of serum TNF-α in patients with type 2 diabetes with or without associated DKD.
Navarro JF, Mora C, Muros M, García J. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant. 2006;21(12):3428–34.
Koike N, Takamura T, Kaneko S. Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF-alpha stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 2007;80(18):1721–8.
Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141(8):2629–34.
Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, et al. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44(4):215–8.
•• Awad AS, You H, Gao T, Cooper TK, Nedospasov SA, Vacher J, et al. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88(4):722–33. This study demonstrated kidney protection in a mouse model of DKD by blockade of macrophage-derived TNF-α.
Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91(9):3652–6.
Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60(3):365–71.
• Shoukry A, Bdeer S-A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem. 2015;408(1–2):25–35. This study identified urinary MCP-1 as a potential novel biomarker for early detection of DKD.
Titan SM, Vieira JM, Dominguez WV, Moreira SR, Pereira AB, Barros RT, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complicat. 2012;26(6):546–53.
Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360(4):772–7.
Li C, Yang CW, Park CW, Ahn HJ, Kim WY, Yoon KH, et al. Long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. Kidney Int. 2003;63(2):454–63.
Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H. The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. J Cardiovasc Pharmacol. 2006;48(4):135–42.
Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26(8):2421–5.
Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17(5):1362–72.
Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R, et al. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63(1):209–16.
Wu YG, Lin H, Qian H, Zhao M, Qi XM, Wu GZ, et al. Renoprotective effects of combination of angiotensin converting enzyme inhibitor with mycophenolate mofetil in diabetic rats. Inflamm Res. 2006;55(5):192–9.
Hickey FB, Martin F. Diabetic kidney disease and immune modulation. Curr Opin Pharmacol. 2013;13(4):602–12.
Ninichuk V, Clauss S, Kulkarni O, Schmid H, Segerer S, Radomska E, et al. Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36-3’PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am J Pathol. 2008;172(3):628–37.
•• Boels MGS, Koudijs A, Avramut MC, Sol WMPJ, Wang G, van Oeveren-Rietdijk AM, et al. Systemic monocyte chemotactic protein-1 inhibition modifies renal macrophages and restores glomerular endothelial glycocalyx and barrier function in diabetic nephropathy. Am J Pathol. 2017:2430–40 This study nicely demonstrated the therapeutic potential of MCP-1 inhibition in a mouse model of DKD.
Senthilkumar GP, Anithalekshmi MS, Yasir M, Parameswaran S, Packirisamy RM, Bobby Z. Role of omentin 1 and IL-6 in type 2 diabetes mellitus patients with diabetic nephropathy. Diabetes Metab Syndr. 2018;12:23–26.
Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2(2):72–9.
Ng DP, Nurbaya S, Ye SH, Krolewski AS. An IL-6 haplotype on human chromosome 7p21 confers risk for impaired renal function in type 2 diabetic patients. Kidney Int. 2008;74(4):521–7.
Papaoikonomou S, Tentolouris N, Tousoulis D, Papadodiannis D, Miliou A, Papageorgiou N, et al. The association of the 174G>C polymorphism of interleukin 6 gene with diabetic nephropathy in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27(6):576–9.
Chang WT, Huang MC, Chung HF, Chiu YF, Chen PS, Chen FP, et al. Interleukin-6 gene polymorphisms correlate with the progression of nephropathy in Chinese patients with type 2 diabetes: a prospective cohort study. Diabetes Res Clin Pract. 2016;120:15–23.
Watanabe T, Tomioka NH, Doshi M, Watanabe S, Tsuchiya M, Hosoyamada M. Macrophage migration inhibitory factor is a possible candidate for the induction of microalbuminuria in diabetic db/db mice. Biol Pharm Bull. 2013;36(5):741–7.
Herder C, Kolb H, Koenig W, Haastert B, Müller-Scholze S, Rathmann W, et al. Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg, Survey 4 (KORA S4). Diabetes Care. 2006;29(2):368–71.
Sanchez-Niño MD, Sanz AB, Ihalmo P, Lassila M, Holthofer H, Mezzano S, et al. The MIF receptor CD74 in diabetic podocyte injury. J Am Soc Nephrol. 2009;20(2):353–62.
• Wang Z, Wei M, Wang M, Chen L, Liu H, Ren Y, et al. Inhibition of macrophage migration inhibitory factor reduces diabetic nephropathy in type II diabetes mice. Inflammation. 2014;37(6):2020–9. This study highlights MIF inhibition as a potential therapeutic strategy in DKD.
Tang LQ, Ni WJ, Cai M, Ding HH, Liu S, Zhang ST. Renoprotective effects of berberine and its potential effect on the expression of β-arrestins and intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in streptozocin-diabetic nephropathy rats. J Diabetes. 2016;8(5):693–700.
Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17(9):644–9.
Güler S, Cakir B, Demirbas B, Yönem A, Odabasi E, Onde U, et al. Plasma soluble intercellular adhesion molecule 1 levels are increased in type 2 diabetic patients with nephropathy. Horm Res. 2002;58(2):67–70.
Wong CK, Ho AW, Tong PC, Yeung CY, Chan JC, Kong AP, et al. Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in type 2 diabetic patients with nephropathy. J Clin Immunol. 2008;28(1):36–43.
Rubio-Guerra AF, Vargas-Robles H, Lozano Nuevo JJ, Escalante-Acosta BA. Correlation between circulating adhesion molecule levels and albuminuria in type-2 diabetic hypertensive patients. Kidney Blood Press Res. 2009;32(2):106–9.
Polat SB, Ugurlu N, Aslan N, Cuhaci N, Ersoy R, Cakir B. Evaluation of biochemical and clinical markers of endothelial dysfunction and their correlation with urinary albumin excretion in patients with type 1 diabetes mellitus. Arch Endocrinol Metab. 2016;60(2):117–24.
Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52(10):2586–93.
Anderson ME, Siahaan TJ. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides. 2003;24(3):487–501.
•• Shahzad K, Bock F, Dong W, Wang H, Kopf S, Kohli S, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87(1):74–84. This study demonstrates renal inflammasome activation in an experimental DKD model and also in clinical samples and shows that IL-1R blockade prevents DKD.
Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26.
Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7(6):e38285.
Gao P, Meng XF, Su H, He FF, Chen S, Tang H, et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim Biophys Acta. 2014;1843(11):2448–60.
Anders HJ. Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol. 2016;27(9):2564–75.
Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond). 2011;8(1):35.
Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, et al. p38 MAPK phosphorylation and NF-kappa B activation in human crescentic glomerulonephritis. Nephrol Dial Transplant. 2002;17(6):998–1004.
Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55(11):2993–3003.
Yi B, Hu X, Zhang H, Huang J, Liu J, Hu J, et al. Nuclear NF-κB p65 in peripheral blood mononuclear cells correlates with urinary MCP-1, RANTES and the severity of type 2 diabetic nephropathy. PLoS One. 2014;9(6):e99633.
Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, et al. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-{kappa}B pathway. Am J Physiol Renal Physiol. 2009;296(5):F1212–8.
Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21(2):330–6.
Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol. 2008;19(9):1741–52.
Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of NFkB signaling pathway. Life Sci. 2009;84(9–10):296–301.
Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282(1):809–20.
Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, et al. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15(8):2139–51.
Kolati SR, Kasala ER, Bodduluru LN, Mahareddy JR, Uppulapu SK, Gogoi R, et al. BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway. Environ Toxicol Pharmacol. 2015;39(2):690–9.
Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy. Contrib Nephrol. 2011;170:36–47.
Mise K, Hoshino J, Ueno T, Sumida K, Hiramatsu R, Hasegawa E, et al. Clinical implications of linear immunofluorescent staining for immunoglobulin G in patients with diabetic nephropathy. Diabetes Res Clin Pract. 2014;106(3):522–30.
Mironova M, Virella G, Lopes-Virella MF. Isolation and characterization of human antioxidized LDL autoantibodies. Arterioscler Thromb Vasc Biol. 1996;16(2):222–9.
Lopes-Virella MF, Virella G. The role of immune and inflammatory processes in the development of macrovascular disease in diabetes. Front Biosci. 2003;8:s750–68.
Atchley DH, Lopes-Virella MF, Zheng D, Kenny D, Virella G. Oxidized LDL-anti-oxidized LDL immune complexes and diabetic nephropathy. Diabetologia. 2002;45(11):1562–71.
Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Lopes-Virella MF, et al. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol. 2008;127(3):394–400.
Hora K, Satriano JA, Santiago A, Mori T, Stanley ER, Shan Z, et al. Receptors for IgG complexes activate synthesis of monocyte chemoattractant peptide 1 and colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1992;89(5):1745–9.
Abdelsamie SA, Li Y, Huang Y, Lee MH, Klein RL, Virella G, et al. Oxidized LDL immune complexes stimulate collagen IV production in mesangial cells via Fc gamma receptors I and III. Clin Immunol. 2011;139(3):258–66.
Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47(9):1975–83.
Lopez-Parra V, Mallavia B, Lopez-Franco O, Ortiz-Muñoz G, Oguiza A, Recio C, et al. Fcγ receptor deficiency attenuates diabetic nephropathy. J Am Soc Nephrol. 2012;23(9):1518–27.
Østergaard J, Hansen TK, Thiel S, Flyvbjerg A. Complement activation and diabetic vascular complications. Clin Chim Acta. 2005;361(1–2):10–9.
Watanabe S, Tomino Y, Inoue W, Yagame M, Kaneshige H, Nomoto Y, et al. Detection of immunoglobulins and/or complement in kidney tissues from non-obese diabetic (NOD) mice. Tokai J Exp Clin Med. 1987;12(3):201–8.
Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13(5):311–8.
Kelly KJ, Liu Y, Zhang J, Dominguez JH. Renal C3 complement component: feed forward to diabetic kidney disease. Am J Nephrol. 2015;41(1):48–56.
Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60(9):2354–69.
Fujita T, Ohi H, Komatsu K, Endo M, Ohsawa I, Kanmatsuse K. Complement activation accelerates glomerular injury in diabetic rats. Nephron. 1999;81(2):208–14.
Li L, Yin Q, Tang X, Bai L, Zhang J, Gou S, et al. C3a receptor antagonist ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. PLoS One. 2014;9(11):e113639.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Fionnuala Hickey and Finian Martin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Microvascular Complications—Nephropathy
Rights and permissions
About this article
Cite this article
Hickey, F.B., Martin, F. Role of the Immune System in Diabetic Kidney Disease. Curr Diab Rep 18, 20 (2018). https://doi.org/10.1007/s11892-018-0984-6
Published:
DOI: https://doi.org/10.1007/s11892-018-0984-6