Abstract
Purpose of Review
To summarize current artificial intelligence (AI)-based applications for coronary artery calcium scoring (CACS) and their potential clinical impact.
Recent Findings
Recent evolution of AI-based technologies in medical imaging has accelerated progress in CACS performed in diverse types of CT examinations, providing promising results for future clinical application in this field.
Summary
CACS plays a key role in risk stratification of coronary artery disease (CAD) and patient management. Recent emergence of AI algorithms, particularly deep learning (DL)-based applications, have provided considerable progress in CACS. Many investigations have focused on the clinical role of DL models in CACS and showed excellent agreement between those algorithms and manual scoring, not only in dedicated coronary calcium CT but also in coronary CT angiography (CCTA), low-dose chest CT, and standard chest CT. Therefore, the potential of AI-based CACS may become more influential in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the main cause of death worldwide [1, 2]. Considering the burden of CAD to patients and the healthcare system, early detection of disease and prediction of patients’ risk of developing adverse cardiovascular events have become crucial to advancements in the medical field, leading to breakthroughs in disease treatment and patient care. Such risk stratification also plays an important role in defining when to initiate preventive therapies or change treatment strategies [2,3,4].
Coronary artery calcium scoring (CACS) is a non-invasive CT technique for the quantification of coronary artery calcium (CAC), used to determine the presence and extent of calcified atherosclerotic plaque and overall plaque burden. The use of CT imaging for direct visualization and characterization of plaque burden enables a more individualized approach for risk assessment than traditional risk factors [2, 4], providing insight into disease stages and response to treatment [5, 6]. Currently, CACS is most commonly performed in asymptomatic individuals, particularly those at intermediate cardiovascular risk. The test is used to estimate cardiovascular risk and predict future cardiac events by providing incremental risk information beyond mere risk factor-based approaches (e.g., Framingham Risk Score) [2].
Quantification of CAC usually needs human input to identify and mark calcified lesions in each image section [3, 7]. This is a time-consuming approach that requires a moderate level of expertise. Therefore, the development of an automated, precise postprocessing method is desired to reduce the need for human observer interaction [7, 8]. Recent advances in artificial intelligence (AI) modeling, including deep learning (DL) with convolutional neural networks (CNN), have provided promising applications in numerous industries, including medical imaging [9,10,11]. Currently, there has been growing interest in the potential of AI to improve various steps of the medical imaging workflow, especially in automatic detection and characterization of radiology findings [9,10,11,12,13,14,15]. Accordingly, many investigations have focused on the potential role of DL in CACS and shown promising results for clinical application in this field with the potential to increase demand [7, 8, 16].
This article reviews current applications of AI-based algorithms for CACS with their recent achievements, challenges, and potential clinical impact. This review also provides a brief summary of AI including important terms for a basic understanding of the technique.
Basics of AI: Terms and Concepts
The application of AI in medical imaging is becoming an integral part of clinical medicine [10, 12]. AI describes any computational program performing tasks that are typical of human intelligence. It incorporates a system that can make automatous decisions based on input data, without immediate human control [9,10,11,12,13].
The concept of ML was introduced early in 1959 and is a subfield of AI that provides computers with the ability to learn rules and extract patterns from data [10, 13, 17]. One subset of ML includes computational models and algorithms inspired by the complex neuronal connections in the human brain and is called an “artificial neural network (ANN).” An ANN is structured in layers composed of interconnected nodes: one input layer, which receives input data; one or more hidden layer, which extracts the pattern of data; and one output layer, which produces the results [9, 10]. In general, train-test systems are used to develop AI models. These consist of three sets: training, validation, and testing [9•]. The training set is needed for the algorithm to learn from example by fitting the model. For validation, a separate data set is used to evaluate different model fits and to adjust the model parameters for optimizing the initial algorithm. Then, the trained model is tested on a new data set to assess how well the algorithm performs under prespecified conditions [9, 13].
Compared with ML, however, AI incorporates a broader scope of intelligent functions generated by computer systems such as pattern recognition, planning, problem solving, recognizing objects, and understanding language. By definition, however, ML algorithms have the ability to “improve by learning” through experience without explicit rules [9,10,11,12,13, 18, 19]. Therefore, rule-based algorithms such as computer-aided detection/diagnosis fall within the category of AI and are not considered as belonging to ML. When used as a broad term, however, computer-aided detection/diagnosis may encompass ML approaches [9•].
Deep learning (DL) is a subset of ML and a special type of ANN that has multiple hidden processing layers, which characterize the depth of the network. Multiple processing layers enable mathematical calculations before producing outputs, thus allowing the DL model to learn the representation of data with high-level abstractions [9, 13, 20]. Among the different DL models, CNN has gained much popularity in computer vision and medical image analysis, especially for extraction of visual features from images. These convolutional DL algorithms have exhibited robust performance similar to human level performance in various areas, including medical imaging [13, 21, 22]. Recent success in DL applications in medical imaging are possible because of a combination of accelerated computing power, advances in hardware technologies such as graphic processing units, increased available datasets, and user-friendly software needed to analyze the data [9, 10, 20]. Currently, DL has the potential to improve multiple steps of workflow in medical imaging, such as patient scheduling, image acquisition, automated detection and interpretation of findings, and reporting and analysis. Automated detection and characterization of abnormalities on medical imaging is a domain where AI has exhibited significant progress and thus may have an immediate and positive impact [15, 21].
Application of AI in Detection and Quantification of Calcified Plaques
CACS is typically performed by using non-contrast-enhanced, ECG-triggered calcium scoring CT. The use of beta blockers is not generally required but may be administered with a small benefit in accuracy in cases of high heart rates. The scan parameters include a tube voltage of 120 kVp and variable tube currents according to patient size. Typically, using a prospectively ECG-triggered sequential scan, images are obtained during diastole and reconstructed in 3 mm section thickness for CACS. Coronary calcification is defined as a lesion of at least 3 consecutive pixels (or 1 mm2) with an attenuation of ≥ 130 HU. Using dedicated software, calcifications in coronary arteries are manually selected and quantified with the Agatston score, which is the weighted sum of each area of calcified plaque multiplied by a factor (between 1 and 4) related to corresponding CT density [2,3,4].
Currently, CACS is performed not only in dedicated calcium scoring CT, but also in the context of other types of CT studies, such as coronary CT angiography (CTA) or non-gated chest CT for lung cancer screening [23,24,25,26]. However, quantification of CAS requires slice-by-slice expert level detection and annotation of calcified lesions. Since this manual approach requires a certain degree of clinical experience and is a monotonous and time-consuming process, a more automated method is highly desirable, especially in large screening populations or in settings where it is not primarily intended but may be clinically useful [8, 27, 28].
Automated CACS
To overcome these drawbacks, several automated methods have been developed, from rule-based methods [29, 30] to ML and more recent DL approaches. The major obstacle in CACS is of differentiation of CAC from other structures with similar attenuation, such as mitral anulus calcifications [27].
ML-based methods for quantification of CAC on ECG-gated, non-contrast-enhanced cardiac CT were used prior to the introduction of DL. These approaches are based on identification of CAC among a large set of candidate lesions. Those images are designated with morphologic features such as size, shape, texture, and location to discriminate CAC from other neighboring candidates such as mitral annulus, aortic and pulmonary calcifications [6•]. Among these features, location features are of particular importance, which are constructed from anatomy-based approaches (heart coordinate system or spatial relation) [31,32,33]. Later, atlas-based localization of the coronary tree was introduced by creating a probabilistic CAC map [34]. The map determined the individual positions of the 3 major epicardial coronary arteries by using independent CTA-based atlases to compute the location estimate for each individual artery [35].
Automated CACS may also be performed on standard contrast enhanced cardiac CTA scans. An initial segmentation step is performed of the contrast-filled coronary artery tree. Because calcifications are usually higher in attenuation than contrast enhanced vascular lumen, calcifications are easily detected by searching for structures with high attenuation along the segmented coronary tree. Since contrast-filled luminal attenuation varies depending on injection protocols, from 250 to 600 HU, a threshold for calcium detection is recommended to be set at 2 standard deviations or 120–150% of mean vascular attenuation, in lieu of a fixed value [36,37,38].
Current Status of DL-Based CACS
Most recent approaches for automated CACS adopt DL algorithms using CNNs, well known for their capabilities of automatic extraction of visual features. In contrast to ML, DL-based methods typically classify individual voxels in place of candidate lesions [6•]. In their early study, Lessmann et al. [39] used a single CNN that classified CAC lesions in lung cancer screening chest CT. Wolterink et al. [7], however, used a pair of CNNs to classify all voxels to identify CAC in coronary CTA. First, a CNN identified voxels of potential CAC and discarded the majority of non-candidate voxels and then a second CNN further discriminated between CAC and neighboring similar negatives. In both studies [7, 39], to simplify the classification by reducing the volume of interest, a bounding box was created to localize the heart using a combination of three additional CNNs, where each detects the heart in an orthogonal plane.
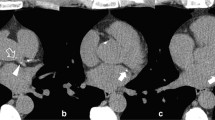
More recently, without such localization methods, Lessmann et al. [40] used sequential CNNs to classify CAC as well as valve and aortic calcifications on chest CT images. With the reinforced capabilities of feature extractions and a large receptive field, the first CNN, was used to identify and label potential calcification voxels according to their anatomical locations. Subsequently, a second CNN refined the output of the first by identifying true calcifications among the candidates with similar shapes and locations. Even though their approach was challenging for training and evaluation, mostly due to low-dose protocol without ECG synchronization, this new strategy achieved good performance (F1 value of 0.68–0.90) in calcium detection and strong agreement (75–91%) with manual reference standard in cardiovascular risk categorization. Another study [8], a DL-based automated calcium scoring method for non-contrast ECG-triggered cardiac CT, showed high accuracy when compared to manually obtained reference scores in 511 patients. This calcium scoring application relied on a combination of multiple CNNs for understanding the context of the CT image (Fig. 1). The calcium scoring model was trained using an annotated dataset of 2000 coronary calcium CT scans to determine the probability of a voxel being a coronary calcification. This model showed that 93.2% of patients were classified into the same risk category as by the human observers.
The combination of a convolutional neural network for the image features and a fully connected network for the spatial coordinate features. A pre-computed coronary territory map is also used as an additional input to specify the likelihood of different voxels belonging to coronary arteries (a). An example case with automated detection of calcifications in coronary arteries overlayed and color coded in red (b)
Although several DL-based CACS methods have been published and achieved excellent performance, they have been specialized to a specific type of CT examination and used two steps similar to traditional CACS method, based on identification of calcium first and quantification thereafter on a specific type of CT examination. De Vos et al. [27], however, proposed a method for direct quantification of calcium score in input image sections without calcium segmentation using data from non-enhanced, ECG-synchronized cardiac CT and non-enhanced chest CT for lung cancer screening. By adopting the workflow for direct regression of CACS, this method successfully achieved accurate prediction of CACS in different types of CT examinations nearly on a real-time basis, showing an intra-class correlation coefficient (ICC) between automatic and manual CACS of 0.98 for both cardiac and chest CT.
In this regard, a recent multicenter study was performed to validate the performance of a DL algorithm in previously unexposed types of CT examinations [41••]. The results showed that the method trained for a lung-screening low-dose chest CT (baseline) adapted also well to several CT study types in a variety of population groups, yielding ICCs of 0.79–0.97 between automatically and manually obtaining scores. When a few representative cases of the respective CT type were supplemented to the baseline for performing protocol-specific training, ICCs between DL-based and manual CACS in that specific type of CT examinations improved to 0.84–0.99. Furthermore, a combined DL model trained with all available CT protocols (combined training) also improved the DL performance for CACS, with ICCs ranging from 0.85 to 0.99. Although there would be shifting in absolute score by using scan protocols other than that of CACS (e.g., tube voltage of 100 kVp), nevertheless, this study showed promising results that suggest extensibility of DL—i.e., applying one DL model trained for a specific CT type to diverse types of CT examinations.
Advantages and Limitations of AI in CACS
While various automated methods have been examined for years, the recent emergence of ML followed by a DL approach has provided considerable progress in the field. Use of DL in CACS has the potential to reduce human interaction for performing time-consuming and monotonous tasks in the clinical setting [6,7,8,9,10]. The re-direction of a clinician’s role to more value-added tasks has the potential to reduce costs and increase quality of healthcare [6, 8]. In addition, DL in CACS could be used as an additional screening option to CT studies including the heart without significant additional effort in terms of human interaction and radiation [6, 8, 27]. Furthermore, recent DL-based approaches achieved similar performance compared to manual reference but generated calcium score even hundred times faster, reaching in almost real-time basis with less than a few seconds [8, 13, 27]. For wide-spread use of AI in CACS, such fast computation is highly desirable and particularly important to screen large populations.
However, the major obstacles for development and adoption of AI in clinical practice are the need for a large collection of high-quality ground truth databases as well as standardization of diagnostic techniques [9, 42]. Historically, obtaining qualified training datasets has been very challenging due to few case numbers at an early stage of algorithm development [6, 9] or confounders such as motion or partial volume artifacts, which are generated through a diversity of CT acquisition and reconstruction techniques—i.e., low dose protocol, non-ECG synchronization, or thick slice reconstruction [43, 44]. Fortunately, for the development of DL-based CACS, large annotated datasets are readily available and image quality continues to improve thanks to constant advances in CT technology and reconstruction techniques [41, 45, 46]. Furthermore, CACS is a fairly obvious task that does not require a high level of expertise and the quantification method is highly standardized by adopting the Agatston score. In this respect, CACS became an excellent candidate for automated approaches and currently, several types of early solutions are commercially available for clinical use [16].
Finally, to date, most AI algorithms for CACS have been developed for specific CT protocols and validated mostly in single center studies. Therefore, despite of early promising results for AI-application, their performance requires further validation in clinical practice using diverse sets of CT protocols in larger populations in accordance with ongoing technical improvement in AI.
Conclusion
Since detection and quantification of coronary calcium plays a key role in risk stratification of CAD and management of patients, automatic CACS may provide diagnostic aids to physicians in clinical practice. Although still under development and refinement, the current rapid progress in AI technology in this field may allow for future routine application of automated CACS in clinical practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. https://doi.org/10.1161/circoutcomes.118.005375.
Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8(5):579–96. https://doi.org/10.1016/j.jcmg.2015.02.006.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. https://doi.org/10.1016/0735-1097(90)90282-t.
Divakaran S, Cheezum MK, Hulten EA, Bittencourt MS, Silverman MG, Nasir K, et al. Use of cardiac CT and calcium scoring for detecting coronary plaque: implications on prognosis and patient management. Br J Radiol. 2015;88(1046):20140594. https://doi.org/10.1259/bjr.20140594.
Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122(1 Suppl):S3–s14. https://doi.org/10.1016/j.amjmed.2008.10.013.
• Hampe N, Wolterink JM, van Velzen SGM, Leiner T, Isgum I. Machine learning for assessment of coronary artery disease in cardiac CT: a survey. Front Cardiovasc Med. 2019;6:172. https://doi.org/10.3389/fcvm.2019.00172. This article provides an overview of AI methods for detection, quantification, and characterization of atherosclerotic plaque.
Wolterink JM, Leiner T, de Vos BD, van Hamersvelt RW, Viergever MA, Isgum I. Automatic coronary artery calcium scoring in cardiac CT angiography using paired convolutional neural networks. Med Image Anal. 2016;34:123–36. https://doi.org/10.1016/j.media.2016.04.004.
Martin SS, van Assen M, Rapaka S, Hudson HT Jr, Fischer AM, Varga-Szemes A, et al. Evaluation of a deep learning-based automated CT coronary artery calcium scoring algorithm. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):524–6. https://doi.org/10.1016/j.jcmg.2019.09.015.
• Choy G, Khalilzadeh O, Michalski M, Do S, Samir AE, Pianykh OS, et al. Current applications and future impact of machine learning in radiology. Radiology. 2018;288(2):318-28. https://doi.org/10.1148/radiol.2018171820. This article provides basic definitions of terms commonly used in AI applications and discuss how AI techniques can be developed and applied to medical imaging workflow.
Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. https://doi.org/10.1186/s41747-018-0061-6.
Sharma P, Suehling M, Flohr T, Comaniciu D. Artificial intelligence in diagnostic imaging: status quo, challenges, and future opportunities. J Thorac Imaging. 2020;35:S11–6. https://doi.org/10.1097/rti.0000000000000499.
Siegersma KR, Leiner T, Chew DP, Appelman Y, Hofstra L, Verjans JW. Artificial intelligence in cardiovascular imaging: state of the art and implications for the imaging cardiologist. Neth Heart J. 2019;27(9):403–13. https://doi.org/10.1007/s12471-019-01311-1.
Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317–35. https://doi.org/10.1016/j.jacc.2018.12.054.
Monti CB, Codari M, van Assen M, De Cecco CN, Vliegenthart R. Machine learning and deep neural networks applications in computed tomography for coronary artery disease and myocardial perfusion. J Thorac Imaging. 2020;35:S58–65. https://doi.org/10.1097/rti.0000000000000490.
Retson TA, Besser AH, Sall S, Golden D, Hsiao A. Machine learning and deep neural networks in thoracic and cardiovascular imaging. J Thorac Imaging. 2019;34(3):192–201. https://doi.org/10.1097/rti.0000000000000385.
Sandstedt M, Henriksson L, Janzon M, Nyberg G, Engvall J, De Geer J, et al. Evaluation of an AI-based, automatic coronary artery calcium scoring software. Eur Radiol. 2020;30(3):1671–8. https://doi.org/10.1007/s00330-019-06489-x.
AL S. Some studies in machine learning using the game of checkers. IBM J Res Dev 1959;3:210–229.
Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. 2015;349(6245):255–60. https://doi.org/10.1126/science.aaa8415.
Kohli M, Prevedello LM, Filice RW, Geis JR. Implementing machine learning in radiology practice and research. AJR Am J Roentgenol. 2017;208(4):754–60. https://doi.org/10.2214/ajr.16.17224.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. https://doi.org/10.1038/nature14539.
Lee JG, Jun S, Cho YW, Lee H, Kim GB, Seo JB, et al. Deep learning in medical imaging: general overview. Korean J Radiol. 2017;18(4):570–84. https://doi.org/10.3348/kjr.2017.18.4.570.
Schlett CL, Nattenmuller J, Tsuchiya N, Vogel-Claussen J, Kauczor HU, Levin D, et al. Noncontrast chest computed tomographic imaging of obesity and the metabolic syndrome: part I cardiovascular findings. J Thorac Imaging. 2019;34(2):116–25. https://doi.org/10.1097/rti.0000000000000391.
Pavitt CW, Harron K, Lindsay AC, Ray R, Zielke S, Gordon D, et al. Deriving coronary artery calcium scores from CT coronary angiography: a proposed algorithm for evaluating stable chest pain. Int J Cardiovasc Imaging. 2014;30(6):1135–43. https://doi.org/10.1007/s10554-014-0439-3.
Fischer AM, Eid M, De Cecco CN, Gulsun MA, van Assen M, Nance JW, et al. Accuracy of an artificial intelligence deep learning algorithm implementing a recurrent neural network with long short-term memory for the automated detection of calcified plaques from coronary computed tomography angiography. J Thorac Imaging. 2020;35:S49–57. https://doi.org/10.1097/rti.0000000000000491.
Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276(1):82–90. https://doi.org/10.1148/radiol.15142062.
Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. Journal of cardiovascular computed tomography. 2017;11(1):74–84. https://doi.org/10.1016/j.jcct.2016.11.003.
de Vos BD, Wolterink JM, Leiner T, de Jong PA, Lessmann N, Isgum I. Direct automatic coronary calcium scoring in cardiac and chest CT. IEEE Trans Med Imaging. 2019;38(9):2127–38. https://doi.org/10.1109/tmi.2019.2899534.
Celeng C, Takx RAP, Lessmann N, Maurovich-Horvat P, Leiner T, Isgum I, et al. The association between marital status, coronary computed tomography imaging biomarkers, and mortality in a lung Cancer screening population. J Thorac Imaging. 2020;35(3):204–9. https://doi.org/10.1097/rti.0000000000000457.
Gonzalez G, Washko GR, Estepar RS. Automated Agatston score computation in a large dataset of non ECG-gated CHEST computed tomography. Proc IEEE Int Symp Biomed Imaging. 2016;2016:53–7. https://doi.org/10.1109/isbi.2016.7493209.
Xie Y, Liu S, Miller A, Miller J, Markowitz S, Akhund A, et al. Coronary artery calcification identification and labeling in low-dose chest CT images. SPIE Medical Imaging: SPIE; 2017.
Kurkure U, Chittajallu DR, Brunner G, Le YH, Kakadiaris IA. A supervised classification-based method for coronary calcium detection in non-contrast CT. Int J Cardiovasc Imaging. 2010;26(7):817–28. https://doi.org/10.1007/s10554-010-9607-2.
Brunner G, Chittajallu DR, Kurkure U, Kakadiaris IA. Toward the automatic detection of coronary artery calcification in non-contrast computed tomography data. Int J Cardiovasc Imaging. 2010;26(7):829–38. https://doi.org/10.1007/s10554-010-9608-1.
Sanchez CI, Niemeijer M, Isgum I, Dumitrescu A, Suttorp-Schulten MS, Abramoff MD, et al. Contextual computer-aided detection: improving bright lesion detection in retinal images and coronary calcification identification in CT scans. Med Image Anal. 2012;16(1):50–62. https://doi.org/10.1016/j.media.2011.05.004.
Isgum I, Prokop M, Niemeijer M, Viergever MA, van Ginneken B. Automatic coronary calcium scoring in low-dose chest computed tomography. IEEE Trans Med Imaging. 2012;31(12):2322–34. https://doi.org/10.1109/tmi.2012.2216889.
Shahzad R, van Walsum T, Schaap M, Rossi A, Klein S, Weustink AC, et al. Vessel specific coronary artery calcium scoring: an automatic system. Acad Radiol. 2013;20(1):1–9. https://doi.org/10.1016/j.acra.2012.07.018.
Wesarg S, Khan MF, Firle EA. Localizing calcifications in cardiac CT data sets using a new vessel segmentation approach. J Digit Imaging. 2006;19(3):249–57. https://doi.org/10.1007/s10278-006-9947-6.
Eilot D, Goldenberg R. Fully automatic model-based calcium segmentation and scoring in coronary CT angiography. Int J Comput Assist Radiol Surg. 2014;9(4):595–608. https://doi.org/10.1007/s11548-013-0955-y.
Ahmed W, de Graaf MA, Broersen A, Kitslaar PH, Oost E, Dijkstra J, et al. Automatic detection and quantification of the Agatston coronary artery calcium score on contrast computed tomography angiography. Int J Cardiovasc Imaging. 2015;31(1):151–61. https://doi.org/10.1007/s10554-014-0519-4.
Lessmann N, Išgum I, Setio AA, de Vos B, Ciompi F, de Jong P, et al. Deep convolutional neural networks for automatic coronary calcium scoring in a screening study with low-dose chest CT. SPIE Medical Imaging: SPIE; 2016.
Lessmann N, van Ginneken B, Zreik M, de Jong PA, de Vos BD, Viergever MA, et al. Automatic calcium scoring in low-dose chest CT using deep neural networks with dilated convolutions. IEEE Trans Med Imaging. 2018;37(2):615–25. https://doi.org/10.1109/tmi.2017.2769839.
•• van Velzen SGM, Lessmann N, Velthuis BK, Bank IEM, van den Bongard D, Leiner T, et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology. 2020:191621. https://doi.org/10.1148/radiol.2020191621. This study showed promising results that suggest extensibility of AI algorithms in CACS, applying one DL model trained for a specific CT type to diverse types of CT examinations.
Zitzelsberger T, Scholz A, Hetterich H, Lorbeer R, Bamberg F, Auweter SD, et al. Magnetic resonance-based assessment of myocardial 2-dimensional strain using feature tracking: association with cardiovascular risk factors in a population-based cohort free of cardiovascular disease. J Thorac Imaging. 2020;35(1):49–55. https://doi.org/10.1097/rti.0000000000000380.
Arcadi T, Maffei E, Sverzellati N, Mantini C, Guaricci AI, Tedeschi C, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World Journal of Radiology. 2014;6(6):381–7. https://doi.org/10.4329/wjr.v6.i6.381.
Christensen JL, Sharma E, Gorvitovskaia AY, Watts JP Jr, Assali M, Neverson J, et al. Impact of slice thickness on the predictive value of lung cancer screening computed tomography in the evaluation of coronary artery calcification. J Am Heart Assoc. 2019;8(1):e010110. https://doi.org/10.1161/jaha.118.010110.
Hou KY, Tsujioka K, Yang CC. Optimization of HU threshold for coronary artery calcium scans reconstructed at 0.5-mm slice thickness using iterative reconstruction. Journal of applied clinical medical physics. 2020;21(2):111–20. https://doi.org/10.1002/acm2.12806.
Caruso D, De Santis D, Biondi T, Panvini N, Zerunian M, Rivosecchi F, et al. Half-dose coronary artery calcium scoring: impact of iterative reconstruction. J Thorac Imaging. 2019;34(1):18–25. https://doi.org/10.1097/rti.0000000000000340.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Heon Lee, Simon Martin, Jeremy R. Burt, Hunter N. Gray, and Tyler J. Leonard declare that they have no conflict of interest.
Pooyan Sahbee Bagherzadeh is a research collaboration manager.
Saikiran Rapka reports personal fees and is an employee of Siemens Healthcare GmbH.
Chris Schwemmer reports personal fees and is an employee and stockholder of Siemens Healthcare GmbH.
U. Joseph Schoepf has received institutional research support and/or honoraria for speaking and consulting from Astellas, Bayer, Bracco, Elucid BioImaging, General Electric, Guerbet, HeartFlow Inc., and Siemens Healthineers.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac PET, CT, and MRI
Rights and permissions
About this article
Cite this article
Lee, H., Martin, S., Burt, J.R. et al. Machine Learning and Coronary Artery Calcium Scoring. Curr Cardiol Rep 22, 90 (2020). https://doi.org/10.1007/s11886-020-01337-7
Published:
DOI: https://doi.org/10.1007/s11886-020-01337-7