Abstract
We validate a method of calcium scoring on CT coronary angiography (CTCA) and propose an algorithm for the assessment of patients with stable chest pain. 503 consecutive patients undergoing coronary artery calcium score (CACS) and CTCA were included. A 0.1 cm2 region of interest was used to determine the mean contrast density on CTCA images either in the left main stem (LM) or right coronary artery. Axial 3 mm CTCA images were scored for calcium using conventional software with a modified threshold: mean LM contrast density (HU) + 2SD. A conversion factor (CF) for predicting CACS from raw CTCA scores (rCTCAS) was determined using a multivariable regression model adjusted for model over-optimism (1,000 bootstrap samples). Accuracy of this method was determined using weighted kappa for NICE recommended CACS groupings (0, 1–400, >400) and Bland–Altman analysis for absolute score. With the CF applied: CACS = (1.183 × rCTCAS) + (0.002 × rCTCAS × threshold), there was excellent agreement between methods for absolute score (mean difference 5.44 [95 % limits of agreement −207.0 to 217.8]). The method discriminated between high (>400) and low risk (<400) calcium scores with a sensitivity and specificity of 85 and 99 %, and a PPV and NPV of 92 and 98 %, respectively, and led to a significant reduction in radiation exposure (6.9 [5.1–10.2] vs. 5.2 [6.3–8.7] mSv; p < 0.0001). Our proposed method allows a comprehensive assessment of coronary artery pathology through the use of an individualised, semi-automated approach. If incorporated into stable chest pain guidelines the need for further functional testing or invasive angiography could be determined from CTCA alone, supporting a change to the current guidelines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery calcium scoring (CACS) allows identification and quantification of calcified coronary plaque that can be used to categorise patients into age and gender normalised centiles [1]. This provides prognostic information about future cardiovascular events over and above traditional clinical risk factors used in multivariate models. More recently CT coronary angiography (CTCA) has emerged as an imaging modality to non-invasively delineate coronary anatomy with high spatial and temporal resolution. Its advantage over CACS lies in its ability to provide additional information about non-calcified plaque, plaque morphology (whether calcified or non-calcified), and stenosis severity.
The current UK NICE guideline is often referred to as a way in which to evaluate patients with stable chest pain. It recommends an initial CACS scan as a first line investigation in the assessment of patients with stable chest pain with a low pre-test probability of underlying coronary artery disease (CAD), with subsequent evaluation of the coronary tree with CTCA if the CACS lies between 1 and 400 [2]. However, this currently requires a separate acquisition and whilst improvements in CT technology (prospective gating and rapid acquisition platforms) and software advances (such as iterative reconstruction algorithms) have facilitated significant decreases in the radiation exposure for CTCA image acquisition, CACS doses have remained largely static over time (≈1 mSv) [3]. This has led to considerable interest in developing methods to derive calcium scores and its prognostic information on CTCA, thus potentially negating the need for separate image acquisition and theoretically reducing total patient radiation exposure by up to 50 %.
Methods published to date that attempt to quantify coronary calcium on contrast-enhanced images show good agreement with traditional Agatston scores, but are limited in clinical practice due to either the requirement for extensive manual correction [4], differences in scan protocols including dual energy techniques [5], or underestimation at high scores [6].
Aim
The aim of this study was to validate a method of coronary calcium quantification on CTCA scans taking into account the confounding factors of previous studies. Specifically we address the implications such a method would have on current clinical practice, and propose an algorithm for the assessment of patients with stable chest pain.
Methods
Study population
We prospectively assessed 520 consecutive clinical patients referred for investigation of suspected CAD according to NICE guidance undergoing both traditional CACS and CTCA on a 128-slice dual-source multi-detector CT (DSCT) (Siemens FLASH, Forchheim, Germany). The following patients were excluded: those with a permanent pacemaker (PPM) (n = 12), with coronary stents (n = 3), or complex congenital coronary abnormalities (n = 2). This left 503 consecutive cases that were used in the final analysis.
Coronary artery calcium scoring and CT-coronary angiography
The protocols used for CACS and CTCA image acquisition are shown in Table 1. CACS images were reconstructed as contiguous 3 mm slices using a medium smooth (B35f) kernel. Axial images were analysed using the standard Agatston algorithm [1] on a clinical workstation (iNtuition, Terarecon, Foster City, CA, USA). Each vessel (RCA, LCX, LMA, and LAD) was scored separately and summed to give a total Agatston score used to place patients into NICE recommended risk groupings (0, 1–400 and >400).
For CTCA, images were reconstructed as 0.75 mm slices every 0.6 mm using a smoother (B26f) kernel and intravenous beta-blockade was administered where appropriate, aiming for a heart rate below 60 bpm. Patients were scanned using either a high pitch protocol (HR <60 bpm), prospective gating in diastole (HR 60–70 bpm) or systole (HR >70 bpm). All patients were scanned at 100 kV unless their weight exceeded 90 kg when a 120 kV protocol was used. All 503 eligible patients were scored by three experienced cardiac CT practitioners.
Deriving coronary calcium on CTCA
Contrast enhanced axial slices were reconstructed as contiguous 3.0 mm slices, with the same kernel reconstruction as the CACS (B35f). This method has previously been described by our group [7, 8], but briefly a patient-specific attenuation threshold was determined from the contrast density in a region of interest (ROI) in the left main coronary artery (LM) unless (1) the LM was heavily calcified or (2) the RCA was clearly a more dominant vessel with a larger calibre than the LM based on visual assessment. In this case the ROI was placed in the RCA at whichever location had the largest cross-sectional area. The patient-specific threshold was calculated based on the mean and standard deviation of the contrast density within the ROI:
Each vessel was subsequently scored using the same CACS software with the new threshold applied (rather than the standard 130 HU threshold) to give a CTCA calcium score Fig. 1.
Radiation exposure
Dose-length product (DLP; mGycm) was recorded for both non-contrast and contrast enhanced scans, and converted to effective dose (mSv) by multiplying the DLP by the region-specific normalised effective dose for cardiac CT (0.028) [9].
Internal validation: inter-observer and intra-observer variability
Each patient had a traditional calcium score and CTCA-derived calcium score calculated by two independent readers to avoid bias. 75 randomly selected cases were used to assess both interobserver and intraobserver reproducibility. Interobserver was defined as two observers blindly scoring the same set of images and intra-observer as the same observer blindly re-scoring the same set of images. Image analysis was separated by 12 months and in both cases absolute score and correct risk stratification were used to determine agreement.
Predictive model development and statistical analysis
A conversion factor (CF) for estimating traditionally derived Agatston CACS from calcium scores on CTCA was determined by modelling the relationship between the two scores using linear regression. As the relationship between scores depended on the attenuation threshold used for CTCA-derived CACS, an interaction term for the threshold value was included in the model in addition to variables representing scanning protocol (high-pitch vs. prospective scanning), ROI position (LM or RCA) and tube voltage (kV). The performance of the model was evaluated in terms of calibration (agreement between scores) and discrimination (ability to distinguish between low and high risk patients). Agreement between the two scores was evaluated by measuring the difference between observed values (traditional CACS scores) and predicted values (CTCA-derived CACS with CF) plotted against the mean CACS score as a Bland–Altman plot. Discrimination was evaluated by measuring the ability of CTCA-derived CACS to classify patients into NICE risk groups (Agatston scores of 0, 1–400 or >400) using the weighted kappa statistic. Since a large proportion of CACS values were 0 (259/503; 51 %), the kappa statistic was also derived for NICE risk groups 1–400 and >400 only.
Bootstrapping was used as a statistical method to correct for over-optimistic model fitting as the model was developed and tested in the same sample. Bootstrapping involves repeatedly sampling from the original data, a process that simulates sampling from the underlying source population from which the original data were drawn. Each bootstrap sample includes the same number of patients as in the original sample, but some are excluded, others included once, twice, etc. producing a sample similar but not identical to the original sample. In the context of predictive models the bootstrap procedure provides a ‘shrinkage’ factor, which is used to adjust model coefficients for optimism or over-fitting [10]. The shrinkage factor is estimated from the model calibration slope, i.e. the regression coefficient β in a model with the predicted value as the only covariate: CACS = α + β × CTCA-derived CACS. Well-calibrated models have a slope of 1, but many predictive models have a slope less than 1, indicating that predictions for new subjects are often too extreme. The shrinkage factor in this study was obtained by averaging the calibration slopes when applying the original model in 1,000 bootstrap samples. The model coefficients were multiplied by the shrinkage factor to provide a final CF, adjusted for over-fitting.
Sensitivity, specificity, positive and negative predictive values (PPV and NPV) were estimated for each risk group. Normally distributed data are expressed as mean (SD); otherwise median (IQR). Paired data were analysed using the Wilcoxon test. The independent student’s T test was used to compare the means of two independent, normally distributed data sets. Correlation between variables was assessed using the Pearson’s product moment correlation coefficient. All statistical analysis was carried out using Stata. A two tailed p ≤ 0.05 was considered statistically significant.
Results
503 eligible consecutive patients (median age 57 ± 12.2; 63 % male) underwent both standard CACS and CTCA. Patient demographics and scan characteristics are shown in Table 2.
Conversion factor determination
Across the dataset raw CTCA calcium scores systematically underestimated traditional CACS [median scores (IQR) 0 (0.0–42) vs. 0 (0.0–87.6); p = 0.01)]. The raw CTCA and traditional calcium scores were modelled to produce a CF for predicting calcium scores from raw CTCA scores. Multivariable analysis showed that neither scanning protocol (high-pitch versus prospective; p = 0.22), tube voltage (kV; p = 0.77) nor ROI position (LM or RCA; p = 0.28) affected the relationship between CACS and raw CTCA scores. However, the attenuation threshold did have a significant effect (p < 0.0001). The final CF, adjusted for model over-optimism with a shrinkage factor of 0.999 as estimated from the average calibration slope in 1,000 bootstrap samples was of the form CACS = (1.183 × raw CTCA score) + (0.002 × raw CTCA score × attenuation threshold). This was used as the final CF and applied to all raw CTCA calcium scores
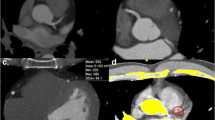
Calcium quantification on CTCA. Axial views of non-contrast cardiac CT images at the level of the left main artery with semi-automated detection of calcified lesions highlighted in red at a threshold of 130 HU (a); and contrast-enhanced CTCA images with the ROI in the left main for calculation of the patient-specific threshold (b), open in the traditional CACS software at a threshold of 130 HU with contrast falsely identified as calcium (yellow) (c), and correct lesion identification at a threshold of 366 HU (d). Traditional CACS was 220 and CTCA calcium score with a CF applied was 207
Agreement between scores and risk groupings
With the CF applied there was excellent correlation between CACS and CTCA-derived CACS across a range of scores (r = 0.95; p < 0.001) with better agreement between methods for calcium scores <500. The mean difference between scores was 5.44 (95 % limits of agreement −207.0 to 217.8; Fig. 2). Using the derived scores patient categorisation into NICE risk groupings using CTCA-derived calcium scores was very good with a weighted kappa of 0.83 and an overall accuracy of 85 % (Table 3). When excluding the CACS = zero group, the weighted kappa was 0.84. Furthermore, there was excellent discrimination between high (>400) and low risk (<400) calcium scores with a sensitivity and specificity of 85 and 99 %, and a PPV and NPV of 92 and 98 %, respectively. The trend for absolute score to be underestimated on CTCA was mirrored by a tendency for patients to be classified in a lower-risk group (8/52). Importantly of those patients falsely categorised as having a score of ‘0’ on CTCA image analysis, the true median Agatston CACS score was 8.2 (IQR 6.8–37.7) with 65 % of cases having a CACS <10.
Method comparison. Bland–Altman plot showing the mean calcium score against the difference in scores derived from contrast-enhanced and non-enhanced CT after applying the conversion factor, adjusted for model optimism using bootstrapping, with mean difference (dotted line) and 95 % limits of agreement (dashed lines). a Constant relationship between difference in scores and amount of calcium; b allowing difference to increase with larger calcium scores
Radiation exposure
The median radiation dose from the traditional non-enhanced and contrast-enhanced scans was 1.15(0.8–1.6) and 5.3(4.0–8.7) mSv, respectively. There was a statistically significant reduction in radiation exposure with exclusive use of CTCA-derived CACS both overall (6.9 [5.1–10.2] vs. 5.2 [6.3–8.7] mSv; p < 0.0001), and with high pitch single heart-beat acquisition (3.3 [2.8–5.3] vs. 2.0 [1.8–3.8] mSv; p < 0.0001). This represented a reduction of 24.6 ± 12.5 and 39.4 ± 8.2 %, respectively.
Intra- and inter-observer variability
Both inter and intra-observer variability were excellent with no significant difference in median CTCA-derived CACS for all risk groups between observers (0 [0–25.9] vs. 0 [0–33.0]; p = 0.49, weighted kappa = 0.90) and (0 [0–24] vs. 0 [0–27]; p = 0.68), weighted kappa = 0.94), respectively.
Discussion
Calcium scoring is routinely performed in clinical practice for prognostic purposes and is often combined with CTCA, but it is unable to assess plaque morphology or coronary artery stenoses. UK NICE guidance recommends the use of a CACS to initially risk stratify patients with stable chest pain with a low (10–29 %) pre-test probability of underlying CAD. If the CACS is between 1 and 400, then it is recommended that the patient should proceed to CTCA. The present study demonstrates that coronary calcium can be accurately derived from a CTCA scan, obviating the need for a dedicated acquisition and potentially reducing the necessary radiation exposure. This allows both the prognostic information of calcium scoring and the detailed anatomical information provided by CTCA to be ascertained using one scan. We propose a novel algorithm for assessing stable chest pain patient, given that a more complete anatomical and prognostic assessment can now be made on a single CTCA, often acquired at a comparable dose to a CACS.
Model performance
CTCA-derived calcium scores systematically underestimated traditional calcium scores hence linear regression was used to derive a CF to apply to all CTCA-derived scores. Previously a split-sample approach was used with a derivation and validation cohort [4, 7, 8] though this method is disadvantageous in that model coefficients can be unstable and data is “lost” as only a subset of the data can be used for model derivation. The shrinkage factor obtained in the present study used to correct the conversion factor for model over-optimism was close to 1 indicating close agreement between observed and predicted values.
Method accuracy
The present method allowed accurate derivation of coronary calcium from CTCA across a wide range of values with agreement particularly close with scores <500. A limitation of all the published techniques deriving CACS from CTCA, including ours, is that patients with low Agatston scores (between 1 and 10 Agatston Units) are classified as 0 using contrast techniques [4–6]. This underestimation is likely due to automated removal by the calcium software module of small areas of calcium that are less dense than the new patient-specific attenuation threshold. In our study, those patients incorrectly classified as scoring zero had a median Agatston score of 8.2; however it should be noted that clinically there is no statistical difference in event-free survival between CACS of 0 and 1–10 [10]. However, these minor levels of plaque formation and associated coronary artery calcification are likely to be detected on review of the CTCA images in clinical practice and appropriate comment can then be made on the extent and distribution. Additionally the detection of any flow limiting stenoses on CTCA will ensure patients are not wrongly labelled as having unobstructed coronary arteries.
Whist agreement was less robust with scores >500, it is practically less clinically important since all patients with scores >400 are deemed to be at the higher end of the cardiovascular risk profile and a high calcium burden significantly impairs the ability to determine true lumenography on CTCA due to the associated blooming artefact. Additionally, routine clinical practice usually utilises CTCA as a tool to rule out significant CAD meaning the vast majority of patients will have a low pre-test probability of having CAD and therefore lower scores, making this technique clinically applicable.
The mean difference in CTCA-derived scores and traditional CACS on Bland–Altman analysis was small and whilst the repeatability limits appear wide these were derived from the entire range of scores from 0 to 2,599 and is therefore skewed by the greater difference in scores observed at higher values of CACS (a well described phenomenon [11]). It is likely that the close agreement between non-contrast and CTCA-derived scores is in part due to the DSCT technology that with its low temporal resolution (approximately 80 ms), reduces motion artefact compared with previous single-source scanners without the need for beta-blockade [12].
Critically, the CTCA-derived CACS (with CF) was within acceptable limits of expected variation based on previous MESA study data [13]. For example, the calculated 95 % repeatability limits for CACS at a score of 500 are ±198 in the MESA study, compared to the values found when comparing our novel scoring method to traditional CACS (±approximately 207). The observed difference in calcium scores between the two methods in our study can therefore in a large part be explained by the inherent measurement error when performing calcium scoring.
Clinical decision making
Our described method would support a change to clinical practice by allowing a single CTCA acquisition to be performed to allow both prognostic and anatomical data to be derived. We have shown this technique can discriminate between those at high (CACS >400 AU) and low (CACS <400 AU) cardiovascular risk, and given that CTCA provides information on luminal stenosis and plaque morphology, our method would also identify individuals with potentially significant non-calcified plaque that would potentially be missed if using traditional CACS as a gateway to CTCA [14, 15].
A recent review of the role of non-contrast CT for CACS in the era of CTCA [16] calculated that in patients with CACS = 0 the number needed to scan with contrast CT in order to identify one patient who will reach the end-point of revascularisation, MI or death would be 338, 948 and 1,579, respectively. Their conclusion from this data was that CTCA was neither economically viable for patients with a score of zero, nor could the additional patient radiation exposure be justified. However, given that in the UK there are 600,000 admissions with chest pain per annum, the dose of CTCA using high-pitch protocols may approximate the dose of the non-contrast study, especially with high-pitch single heart-beat acquisition protocols, and coupled with the high accuracy of the present method in identifying patients at high and low risk, we suggest that CTCA could be used as the initial investigation in patients with suspected CAD (Fig. 3). Additionally, it has been shown that CTCA is a robust technique for excluding significant coronary artery disease in both low risk and intermediate risk (up to 70 % pre-test probability) [17]. Furthermore given the potential radiation doses associated with SPECT, we would argue that CTCA may have a place in the assessment of patients with a pre-test probability of 31–70 %, if appropriate expertise is available locally.
Score of ‘0’
Patients who undergo a CACS currently and have no coronary calcium are deemed low-risk by current guidelines and require no further investigation for CAD due to the high negative predictive value of CACS. However, in a sub-study of the CORE-64 trial 20 % of those who had one stenosis of >50 % at invasive angiography had a CACS = 0 [18], whilst in a larger meta-analysis of 10,000 patients the value who had significant CAD was 7 % [19]. Our method has a high negative predictive value and would similarly identify low risk patients but would additionally identify those with non-calcified plaque that would otherwise not have been identified under current guidelines.
Score of ‘1–400’
The current recommendation is for patients that fall within this range to undergo CTCA to guide further investigation whether a functional test, or invasive angiography, depending on the degree of lesions stenosis and flow limitation. Patients would already have had the CTCA under our new recommendation but without a dedicated CACS thus reducing overall radiation exposure. Additionally this would significantly enhance throughput on CT scanners as all patients would be prepared for a CTCA at the outset, and no patients would need to return for a separate CTCA, a practice that is not uncommon in the UK.
Score of ‘>400’
Currently these high risk patients are investigated with invasive angiography or, if more appropriate, with a functional test. Our method identifies these high risk patients and allows further investigative strategies to be decided by characterising plaque and any flow limitation.. The ability to more formally assess the type and distribution of both calcified and non-calcified lesions on the CTCA would likely reduce the number of patients who are referred for further investigation (as the actual luminal narrowing can be assessed accurately in many cases), potentially reducing the overall radiation and invasive procedural risk to the patient from subsequent investigation, as well as significantly reducing the overall cost of investigating the chest pain.
Decrease in radiation
The median radiation exposure for CACS and CTCA in our study was in keeping with previous published studies [20]. Whilst the absolute dose for CACS is low (1 mSv), reductions of between one-quarter and over one-third could be achieved depending on the CTCA acquisition protocol used. Furthermore we utilized a regions-specific CF of 0.028 for effective dose calculation according to recent data suggesting that using a chest CF of 0.017 underestimated the true dose when performing cardiac CT [9]. This strengthens the argument that further dose-reduction strategies need to be developed in cardiac CT. Lastly the potential reduction in radiation is dependent on the CT scanner, since omitting the non-contrast calcium scoring component when using older platforms with single-source 64-detector technology will have proportionally less effect on total radiation burden. Despite evidence to support using the non-contrast calcium scan to reduce CTCA scan-length and radiation [21], this benefit is likely to diminish significantly when using the latest generation of CT technology with a median radiation dose of the order of 5 mSv for CTCA using a tissue-specific weighted factor of 0.028, as in the present study.
Previously published methods
The agreement between the traditional and CTCA scores observed in our study is similar to that seen in previously published studies that attempt to quantify coronary calcium using CTCA images. However, these previous published techniques are significantly more complex and labour intensive requiring differing calibration factors (based on arbitrary cut-off values) to be calculated and applied based on each individuals intra-aortic contrast density [5], or require luminal centre-line extraction which is known to be challenging with heavy calcification and luminal obliteration [22]. Furthermore, there were differences in slice thickness and reconstruction kernels between non-enhanced and CTCA scans (3.0 and 0.75 mm slice thickness with overlap) that has previously been shown to over-estimate coronary calcium scores [23]. Our estimation of coronary calcium using a conversion factor based on two variables (the raw calcium score on CTCA and attenuation threshold) was quick to perform with a short learning curve and takes into account these confounding factors.
Limitations
This was single-centre study using a single CT and software platform and requires external validation. It is also acknowledged that the use of contrast based studies for all studies raises the population risk of contrast reactions, a small, but real clinical risk.
Conclusion
Our proposed method allows a comprehensive assessment of coronary artery pathology and its prognostic implications through the use of a simple, semi-automated approach that allows CACS to be derived from CTCA. This negates the requirement for a separate unenhanced CACS component in coronary CT assessment. If applied clinically, this protocol could lead to significant reductions in radiation exposure, whilst maintaining the prognostic accuracy of cardiac CT, and we suggest this approach is incorporated into future guidelines for investigation of stable chest pain.
Abbreviations
- CACS:
-
Coronary artery calcium score
- CT:
-
Computed tomography
- CTCA:
-
CT coronary angiography
- CAD:
-
Coronary artery disease
- DSCT:
-
Dual source multi-detector CT
- PPM:
-
Permanent pacemaker
- ROI:
-
Region of interest
- RCA:
-
Right coronary artery
- LCX:
-
Left circumflex artery
- LMA:
-
Left main coronary artery
- LAD:
-
Left anterior descending artery
- DLP:
-
Dose-length product
- MESA:
-
Multi-Ethnic Study Atherosclerosis
References
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15(4):827–832
National Institute for Heath and Clinical Excellence (NICE) (2010) CT calcium scoring factsheet. NICE guidelines [Internet]. NICE Guidelines. [Cited 2013 Nov 17]. http://www.nice.org.uk/nicemedia/live/12947/47987/47987.pdf
Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K et al (2010) Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 31(3):340–346
Otton JM, Lønborg JT, Boshell D, Feneley M, Hayen A, Sammel N et al (2012) A method for coronary artery calcium scoring using contrast-enhanced computed tomography. J Cardiovasc Comput Tomogr 6(1):37–44
Bischoff B, Kantert C, Meyer T, Hadamitzky M, Martinoff S, Schömig A et al (2012) Cardiovascular risk assessment based on the quantification of coronary calcium in contrast-enhanced coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 13(6):468–475
Van der Bijl N, Joemai RMS, Geleijns J, Bax JJ, Schuijf JD, de Roos A et al (2010) Assessment of Agatston coronary artery calcium score using contrast-enhanced CT coronary angiography. AJR Am J Roentgenol 195(6):1299–1305
Pavitt CW, Zielke S, Ray R, Lindsay AC, Padley SP, Rubens MB, Nicol ED (2013) Deriving coronary artery calcium scores from CT coronary angiography: a potential change to UK NICE guidelines: abstracts of the ESC (European Society of Cardiology) Congress 2013. Amsterdam, Netherlands. August 31–September 4, 2013. Eur Heart J 34(Suppl 1):1–1218
Pavitt CW, Lindsay AC, Ray R, Zielke S, Padley SP, Rubens MB, Nicol ED (2013) Reducing patient radiation exposure: a novel method of coronary artery calcium scoring using images derived from CT angiography: abstracts of the 11th international conference of non-invasive cardiovascular imaging. J Nucl Cardiol 20(Suppl 1):S5–S108
Gosling O, Loader R, Venables P, Rowles N, Morgan-Hughes G, Roobottom C, Cardiac CT (2010) are we underestimating the dose? A radiation dose study utilizing the 2007 ICRP tissue weighting factors and a cardiac specific scan volume. Clin Radiol 65(12):1013–1017
Kwon SW, Kim YJ, Shim J, Sung JM, Han ME, Kang DW et al (2011) Coronary artery calcium scoring does not add prognostic value to standard 64-section CT angiography protocol in low-risk patients suspected of having coronary artery disease. Radiology 259(1):92–99
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2):135–160
Achenbach S, Ropers U, Kuettner A, Anders K, Pflederer T, Komatsu S et al (2008) Randomized comparison of 64-slice single- and dual-source computed tomography coronary angiography for the detection of coronary artery disease. JACC Cardiovasc Imaging 1(2):177–186
Chung H, McClelland RL, Katz R, Carr JJ, Budoff MJ (2008) Repeatability limits for measurement of coronary artery calcified plaque with cardiac CT in the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol 190(2):W87–W92
Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ et al (2011) Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 58(8):849–860
Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM et al (2007) ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 49(3):378–402
Joshi PH, Blaha MJ, Blumenthal RS, Blankstein R, Nasir K (2012) What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol 19(6):1226–1235
Meijboom WB, van Mieghem CAG, Mollet NR, Pugliese F, Weustink AC, van Pelt N et al (2007) 64-Slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol 50(15):1469–1475
Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L et al (2010) The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 55(7):627–634
Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U et al (2009) Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2(6):675–688
Bischoff B, Hein F, Meyer T, Krebs M, Hadamitzky M, Martinoff S et al (2010) Comparison of sequential and helical scanning for radiation dose and image quality: results of the Prospective Multicenter Study on Radiation Dose Estimates of Cardiac CT Angiography (PROTECTION) I Study. AJR Am J Roentgenol 194(6):1495–1499
Leschka S, Kim C-H, Baumueller S, Stolzmann P, Scheffel H, Marincek B et al (2010) Scan length adjustment of CT coronary angiography using the calcium scoring scan: effect on radiation dose. AJR Am J Roentgenol 194(3):W272–W277
Voros S, Qian Z (2012) Agatston score tried and true: by contrast, can we quantify calcium on CTA? J Cardiovasc Comput Tomogr 6(1):45–47
Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF (2002) Coronary artery calcium: absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology 223(2):474–480
Conflict of interest
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavitt, C.W., Harron, K., Lindsay, A.C. et al. Deriving coronary artery calcium scores from CT coronary angiography: a proposed algorithm for evaluating stable chest pain. Int J Cardiovasc Imaging 30, 1135–1143 (2014). https://doi.org/10.1007/s10554-014-0439-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-014-0439-3