Abstract
Purpose of Review
Elucidating the mechanisms that contribute to adverse cardiovascular (CV) outcomes and reduce quality of life among patients with cancer is paramount. Cancer, certain cancer drugs, radiation therapy, cancer-associated lifestyle disturbances, and cancer-independent comorbidities combine to predispose oncology patients to autonomic dysfunction (AD). This review will explore the assessment, etiology, and clinical implications of AD in cancer patients and will speculate on therapeutic and research opportunities.
Recent Findings
AD is particularly prevalent among patients with advanced cancer, but studies suggest increased prevalence across the entire continuum of cancer survivors compared to cancer-free controls. Data on cancer therapy-induced injury to the autonomic nervous system are limited to small studies. AD has been reported after cranial, neck, and mediastinal radiation therapy. Although AD has been shown to confer increased risk of adverse CV outcomes in cancer-free patients, the prognostic relevance of AD in oncology patients is less well investigated. Markers of AD including elevated resting heart rate (HR), reduced HR variability, and abnormal HR recovery have been associated with shorter survival times in various cancer cohorts. Furthermore, AD has been implicated in the etiology of cancer-related fatigue and exercise limitation.
Summary
Multiple risk factors predispose oncology patients to AD, which is associated with adverse outcomes, including increased mortality, exercise limitation, and fatigue among this cohort. The contribution of AD to overall morbidity and mortality in cancer survivors has largely been overlooked to date. Further investigation is necessary to better understand cancer-treatment specific autonomic injury and to evaluate the role of various pharmacological and non-pharmacological interventions with potential to tackle the sympathovagal imbalance observed in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in cancer care have improved 5-year survival for all cancer sites from 49% in 1980 to 69% currently, such that there are over 14 million people living with cancer in the USA today [1]. However, improvements in oncology outcomes are undermined by increased cardiovascular (CV) morbidity and mortality among survivors. Multiple factors contribute to increased CV risk in oncology patients, including cardiotoxicity as a consequence of various cancer therapies. Injury to myocardium, pericardium, valves, coronary arteries, and large vessels is well recognized in the aftermath of cancer treatments such as radiation therapy, anthracyclines, and targeted cancer therapies. Although the autonomic nervous system (ANS) is also susceptible to toxicity, autonomic dysfunction (AD) associated with cancer therapies and its contribution to overall morbidity and mortality in cancer cohorts is frequently overlooked. This review will explore the assessment, etiology, and clinical implications of AD in patients with cancer and will highlight therapeutic and research opportunities.
Regulation of Cardiovascular Autonomic Function
The CV ANS can be divided into intrinsic and extrinsic components [2]. The extrinsic cardiac ANS comprises fibers that mediate connections between the heart and the nervous system facilitating delicate modulation of baseline cardiac function. The intrinsic cardiac ANS consists of autonomic nerve fibers within the pericardial sac serving to integrate the signals with the underlying conduction system [3]. The extrinsic cardiac ANS comprises two limbs: the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) [4]. Serving a predominant cardio-acceleratory function, activation of the SNS is associated with positive chronotropic and inotropic effects, a reduction of venous capacitance and constriction of resistance vessels [5]. In counterbalance, the PNS serves a predominant cardio-inhibitory function associated with negative chronotropic and inotropic effects, reduced arterial stiffness and increased venous capacitance [6•]. The dynamic interactions between these two limbs together with the integration of additional physiological inputs and reflexes serve to modulate the baseline hemodynamic and electrical functions of the heart and the vasculature [6•].
The sympathetic input to the heart is primarily derived from the major autonomic ganglia, the superior cervical and the thoracic ganglia, located adjacent to the cervical and thoracic spinal cord [7]. These ganglia house the cell bodies of most post-ganglionic neurons whose axons converge to form the superior, middle and inferior cardiac nerves that terminate on the surface of the heart. The parasympathetic input is derived from the nucleus ambiguus of the medulla oblongata, and its preganglionic fibers are carried almost entirely within the vagus nerve. The vagus nerve and its branches converge at a distinct fat pad between the superior vena cava and the aorta, the third fat pad, before continuing its course to interact with the sinoatrial and atrioventricular nodes. The extracardiac inputs of the SNS and PNS further interact with a complex network of intrinsic cardiac neurons known as the epicardial neuron plexus [2]. This provides an additional layer of regulation of autonomic function, and it is increasingly apparent that dysregulation of this layer is implicated in heart rhythm disturbances [3, 8].
The ANS is a dynamic system consisting of an intricate and interactive set of neuronal and humoral feedback mechanisms which act to modulate CV function [9]. Stress-sensitive baroreceptors are located in high-pressure systems (aortic arch and carotid sinus) and low-pressure systems (e.g., systemic veins). Stimulation of these baroreceptors in response to increases in blood pressure (BP) triggers afferent signals to the dorsal medulla of the brainstem with subsequent reduction in sympathetic tone and increase in vagal tone [10, 11]. Stimulation of chemoreceptors within the carotid body by changes in arterial levels of oxygen and carbon dioxide is associated with augmentation of sympathetic tone [6•]. The ANS is further modulated by its bidirectional feedback with the renin-angiotensin-aldosterone system (RAAS) [6•].
Assessment of Cardiovascular Autonomic Function
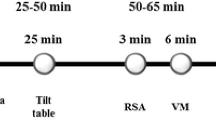
The complexity of the ANS is reflected by the lack of any single measure that can comprehensively measure autonomic function. Many tests for assessing autonomic function evaluate CV reflexes in response to provocative maneuvers such as regular exercise, isometric exercise, orthostatic testing, deep breathing, and the Valsalva maneuver. BP responses to orthostatic testing and Valsalva maneuver largely reflect sympathetic activity, whereas changes in HR during these stimuli as well as during deep breathing largely reflect parasympathetic modulation [12]. In addition, HR variability (HRV) measures how the RR intervals change over time, and is generally assessed by time domain or frequency domain analysis. A summary of key noninvasive tests for cardiac autonomic function are presented in Table 1. Comprehensive assessment of cardiovascular autonomic function includes incorporating data from a combination of these tests rather than relying on any one technique in isolation.
Etiology of Cardiovascular Autonomic Dysfunction Among Oncology Patients
Multiple factors frequently coexist in oncology patients which predispose to AD (Fig. 1). Direct injury to one or more components of the ANS can occur as a consequence of malignancy itself (e.g. direct tumor invasion or compression) or as a complication of cancer therapies including chemotherapy, surgery, and radiation therapy. Multiple small studies report a prevalence of AD as high as ~ 80% among patients with advanced cancer, and so AD at least in part reflects severity of illness in these patients [20,21,22,23]. In addition, AD can also reflect the burden of cancer-related or cancer-independent comorbidities, as it correlates with risk factors including diabetes and obstructive sleep apnea. Various cancer-associated lifestyle perturbations including emotional stress, sleep disturbances, weight gain, and low cardiorespiratory fitness likely contribute [24•]. Therefore, AD in cancer patients is likely a consequence of an additive or synergistic interaction between one and more of these factors. AD associated with various chemotherapies and radiation therapy is discussed further below.
Multifactorial etiology and clinical implications of cardiovascular autonomic dysfunction in patients with cancer, and therapeutic opportunities that merit consideration. CV = cardiovascular, HR = heart rate, HRR = heart rate recovery, HRV = heart rate variability, RAAS = renin angiotensin aldosterone system
Chemotherapy-Induced Autonomic Dysfunction
Neurotoxicity is a well-established adverse effect of specific cancer therapies, such as platinum compounds, vinca alkaloids, taxanes, lenalidomide, thalidomide, and bortezomib. In particular, chemotherapy-induced peripheral neuropathy is a common cause of permanent symptoms and disability in cancer survivors [25]. While AD can frequently accompany and may precede motor and sensory manifestations of peripheral neuropathy [26], systematic evaluation for autonomic involvement in oncology patients has been deficient.
Platinum Compounds
The platinum compounds (cisplatin, oxaliplatin, and carboplatin) play an important role in the treatment of many solid tumors, but they have been associated with dose-dependent peripheral neurotoxicity observed in almost half of cisplatin-treated patients and almost all patients receiving oxaliplatin to some extent [27]. However, this neurotoxicity is selective for large sensory neurons, with relative sparing of autonomic neurons [28, 29]. This sparing of the ANS is supported by some small studies that have assessed CV autonomic function. For example, baroreflex sensitivity did not differ when 90 patients with testicular cancer treated with cisplatin-based chemotherapy were compared to 44 patients with testicular cancer managed with orchiectomy alone, and 47 healthy male controls [30]. Similarly, in a study of 16 males with testicular cancer treated with cisplatin, etoposide, and bleomycin, autonomic function as assessed by HR response to both deep breathing at a rate of 6 breaths/min and to Valsalva maneuver was preserved even after high (> 400 mg/m2) doses of cisplatin [28]. In contrast, a small study that included Valsalva maneuvers before and during cisplatin treatment in 11 patients with ovarian adenocarcinoma reported a decrease in the Valsalva ratio to < 1.3 in 2 patients, suggesting occasional involvement of autonomic nerves [31]. PNS dysfunction as suggested by abnormalities in HR response to (i)Valsalva maneuver, (ii)standing up, and/or (iii)deep breathing was also observed in 10 of 28 patients treated with cisplatin, vincristine, and bleomycin for metastatic germ cell cancer, but postural hypotension to implicate sympathetic dysfunction was not seen in any of these patients [32]. However, postural hypotension was observed in 5 of 71 (7%) patients treated with cisplatin-based combination chemotherapy for metastatic germ cell cancer [33]. The values of the 30/15 ratio, a marker of parasympathetic heart innervation, were significantly reduced compared to baseline at 3–4 and 6–8 months after initiation of oxaliplatin-based chemotherapy in 36 patients with colorectal cancer, and diastolic orthostatic hypotension was more frequently observed at 6–8 months [34]. Thus, evidence for AD as a complication of exposure to platinum compounds is mixed and limited to small studies, emphasizing the need for more systematic evaluation.
Vinca Alkaloids
While vincristine is mainly associated with a dose-dependent primary sensory neuropathy, more than one-third of patients manifest signs of ANS injury characterized by orthostatic hypotension, as well as gastrointestinal and genitourinary dysfunction [29]. Small studies have reported vinca alkaloid-induced abnormal variation in BP and HR response to standing, hand grip, deep breathing, and tilt-testing [35,36,37]. Onset of autonomic injury is early following exposure, and recovery is expected following cessation of the drug. This timescale was emphasized in a study of heart rate variability (HRV) in nine children receiving vincristine treatment for acute lymphoblastic leukemia, where HRV was markedly reduced during both induction and reinduction phases, with subsequent recovery after vincristine discontinuation [13]. However, recovery time can vary for vincristine-related neuropathy from months to years [25].
Taxanes
Taxanes (paclitaxel and docetaxel) are associated with peripheral neuropathy, but the association of taxanes with autonomic phenomena is less well understood. Data are conflicting and limited to only a few small studies. In a study of 31 patients with ovarian cancer treated with paclitaxel and carboplatin, orthostatic hypotension was observed in 19% of patients at 3–4 months, which had resolved by the time of repeat assessment at 6–8 months. This study also demonstrated sustained abnormalities in alterations in cardiac rhythm in response to changes from supine to erect position, which is an assessment of parasympathetic heart innervation [38]. Impairment of HRV was observed when 24-h ambulatory ECG monitoring was performed before and 1 day after the second course of paclitaxel treatment in 14 women with breast or ovarian carcinoma, but subsequent recovery was not evaluated [39]. In contrast, docetaxel treatment did not influence parasympathetic cardiac control in 9 anthracycline-treated women with metastatic breast cancer, but abnormalities of sympathetic vascular control were observed in this small study [40]. Ekholm et al. also reported that paclitaxel altered sympathetic control of BP but did not impair vagally mediated HR responses or HRV in 18 women with ovarian or breast cancer who underwent autonomic testing before treatment and again after the second course of paclitaxel [41].
Anthracylines
Anthracyclines can exert toxic effects on the CV autonomic system in addition to toxicity at the myocyte level [42]. Autonomic testing performed in 31 women with stage II/III breast cancer treated with anthracycline-containing chemotherapy regimens and 24 women with similar stage disease treated with CMF (cyclophosphamide, methotrexate, and fluorouracil) revealed that anthracycline-treated patients exhibit a significantly higher number of abnormal tests for CV autonomic function [42]. HRV was assessed for time domain and frequency domain parameters in 20 asymptomatic breast cancer patients with normal left ventricular systolic function, who had been treated with high-dose anthracycline-based chemotherapy and radiation therapy, and abnormalities were found in 85%, particularly with parasympathetic indices [43]. Relatively low doses of epirubicin used in 40 breast cancer patients did not produce persistent alterations in HRV [44]; this may suggest that a dose-response relationship may underlie any anthracycline-mediated autonomic injury similar to that seen in myocardial injury.
Other Cancer Drugs
Bortezomib has been reported to cause an autonomic neuropathy [45, 46] in addition to the well-established major side effect of peripheral neuropathy, but the epidemiology of any bortezomib-associated autonomic injury is poorly understood. Similarly, whether autonomic injury is a feature of the neurotoxicity observed with the immunomodulatory agents, thalidomide and lenalidomide, warrants investigation.
Summary
Data on cancer therapy-induced injury to the ANS are limited to small studies, and the extent of any autonomic involvement in neurotoxicity observed with certain cancer therapies requires more systematic investigation.
Radiation-Induced Autonomic Dysfunction
Radiation-mediated injury to the myocardium, pericardium, valves, coronary arteries, and large vessels is well described; therefore, it is logical that components of the ANS included in the radiation field should also be vulnerable to injury. AD has been reported after cranial, neck, and mediastinal radiation therapy (RT) [47, 48, 49•, 50].
Cranial irradiation is associated with impaired autonomic nervous regulation of the heart (higher LF:HF ratio and diminished orthostatic LF and HF power responses to orthostatic stress; see Table 1) in a study of 29 patients treated for acute lymphoblastic leukemia in childhood who were in remission and at least 20 months following cessation of therapy [47]. Neck irradiation can cause acute, subacute, or chronic baroreflex failure as a consequence of inflammation and fibrosis of carotid arterial walls which can lead to splinting of the carotid sinus baroreceptors, attenuating the arterial baroreflex in response to changes in BP. Failure of this reflex undermines BP homeostasis and can result in labile BP, labile HR, and orthostatic intolerance [48]. Compared to matched controls, survivors of mantle radiation had an almost 4 times higher likelihood of elevated resting HR and over a 5 times higher likelihood of abnormal HR recovery following cessation of exercise, in adjusted analyses [49•]. Prevalence of these autonomic abnormalities increases with time from radiation therapy [49•]. Similarly, exercise treadmill testing and 24-h ECG monitoring in 48 Hodgkin lymphoma survivors who were a median of 14.3 year post diagnosis and who all received mediastinal irradiation, indicate AD as suggested by a monotonous HR in 57%, persistent tachycardia in 31%, and blunted hemodynamic response to exercise in 27% [50]. There was no difference in resting HR or HR recovery in a comparison of cardiorespiratory fitness in 180 early stage breast cancer patients and 180 non-cancer controls [51]. However, in a larger study that compared 448 breast cancer survivors to age- and sex-matched controls, elevated resting HR (23.7 vs 17%, p = 0.013) and abnormal HR recovery (25.9 vs 20.3%, p = 0.048) were observed with higher frequencies in the breast cancer cohort. Two-thirds of these breast cancer survivors had prior radiation therapy, and RT was associated with a higher likelihood of elevated resting HR (adjusted odds ratio 1.54 (95% confidence interval (CI) 1.09, 2.17); p = 0.02) but not abnormal HR recovery (adjusted odds ratio 1.12 (95% CI 0.79, 1.58); p = 0.53) in adjusted analyses [52].
Clinical Significance of Autonomic Dysfunction
Markers of AD such as elevated resting HR, abnormal HR recovery, and reduced HRV are robust predictors of all-cause mortality in many non-cancer populations [14, 15, 53,54,55,56,57]. AD has been shown to confer increased risk of CV morbidity and mortality in cancer-free patients [58]. However, the prognostic relevance of AD in oncology patients is less well investigated. Guo et al. compared HRV in 651 patients with cancer who had undergone 24-h Holter monitoring and observed shorter survival times among those patients with a standard deviation of normal RR intervals of 24-h recording SDNN < 70 msec, independent of age, cancer stage, and performance status [59]. In a study of 4786 patients with stage I–III breast cancer, patients with resting HR in the highest quintile (≥ 85 bpm) had a significantly higher risk of all-cause mortality compared to those in the lowest quintile (≤ 67 bpm) after adjusting for other prognostic factors (hazard ratio 1.57; 95 %CI 1.05–2.35) [60]. Abnormal HR recovery predicted a fourfold increased risk of all-cause mortality in a cohort of 263 Hodgkin lymphoma survivors who were clinically referred for exercise treadmill testing after a median interval from mediastinal radiation of 19 (12, 26) years (age-adjusted hazard ratio 4.60 (95% CI, 1.62–13.02)) [49•]. In a study of 47 male patients with advanced cancer, a Ewing test score (see Table 1) of > 2 was associated with shorter survival [23].
In addition to prognostic relevance, AD appears to have significant functional implications for cancer patients (Fig. 1), having been implicated in the etiology of cancer-related fatigue which is common in patients undergoing cancer treatment and can persist for many years. Breast cancer survivors with reduced HRV report higher levels of fatigue [61, 62]. AD also likely contributes to exercise limitation which can undermine quality of life in cancer survivors. Elevated resting HR and abnormal HR recovery were each associated with mean reductions of ~ 1 metabolic equivalent in exercise capacity during exercise treadmill testing in Hodgkin lymphoma survivors treated with mediastinal radiation after adjusting for potential confounders [49•]. Breast cancer survivors with these abnormalities demonstrate similar reductions in exercise capacity [52].
Therapeutic Opportunities
The ANS warrants consideration as a potential therapeutic target to improve outcomes in cancer patients. However, clinical trials to inform practice are lacking. Randomized controlled trials of agents such as vitamin E, calcium and magnesium infusions, n-acetylcysteine, and glutathione have demonstrated variable efficacy in the prevention of chemotherapy-induced peripheral neuropathy [63], but specific strategies to attenuate injury to the ANS have not been described. Pretreatment with fullerenol prevented doxorubicin-induced alterations in HRV in a rat model of colorectal cancer [64], but such prophylaxis studies in humans are awaited. Other pharmacological interventions that should be explored include beta blockers, RAAS antagonists, and ivabradine. Non-pharmacological interventions with potential to improve sympathovagal imbalance in cancer patients include sleep interventions, stress reduction techniques, and exercise training (Fig. 1). In particular, exercise training for prophylaxis or treatment of AD is very appealing, and there are data supporting the use of exercise training to improve autonomic function in non-cancer patients [65, 66]. For select patients with particularly advanced and symptomatic AD, interventions such as vagal nerve stimulation and carotid baroreceptor stimulation can be considered on a case by case basis [6•].
Knowledge Gaps and Research Opportunities
Risk factors that predispose to chemotherapy-induced peripheral neuropathy include age, dose intensity, cumulative dose, duration of therapy, combination with other neurotoxic therapies, and pre-existing conditions such as diabetes, alcohol abuse, and inherited neuropathies [29, 63]. Whether such factors also influence the likelihood of chemotherapy-induced AD requires investigation. AD has been described in the acute, subacute, and delayed setting in oncology patients; however, the time course, endurance, and reversibility of AD associated with various cancer therapies are poorly understood. It is uncertain if AD can identify a cohort of cancer patients at increased CV risk who warrant increased CV surveillance or risk-mitigating interventions. For example, is AD an early marker of cardiotoxicity that precedes overt myocardial dysfunction? The prognostic and functional implications of AD observed in specific cancer cohorts need to be defined, and whether interventions that target AD can improve outcomes warrant study. Finally, the extent to which AD in any given patient is a marker or mediator of CV disease is difficult to ascertain.
Conclusion
As survivorship continues to improve, understanding the mechanisms that contribute to adverse CV outcomes and reduced quality of life among patients with cancer is paramount. Multiple risk factors predispose oncology patients to AD, which is associated with adverse outcomes, including increased mortality, exercise limitation, and fatigue. The contribution of AD to overall morbidity and mortality in cancer survivors has largely been overlooked to date. There is a need for studies to better understand cancer-treatment specific autonomic injury and to evaluate the role of various pharmacological and non-pharmacological interventions with potential to tackle sympathovagal imbalance in the growing continuum of cancer survivors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER cancer statistics review, 1975–2014, National Cancer Institute 2016.
Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 2010;7:994–6.
Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–21.
Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–7.
Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–22.
• Chatterjee NA, Singh JP. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail. 2015;3:786–802. Excellent review of anatomy of the autonomic nervous system as well as metrics of its assessment. There is discussion on contemporary non-pharmacological autonomic nervous system modulation therapies, and on related novel technologies and strategies on the horizon.
Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl). 2005;209:425–38.
Schwartz PJ. Cutting nerves and saving lives. Heart Rhythm. 2009;6:760–3.
Goldsmith SR. Interactions between the sympathetic nervous system and the RAAS in heart failure. Curr Heart Fail Rep. 2004;1:45–50.
Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–26.
Biaggioni I, Whetsell WO, Jobe J, Nadeau JH. Baroreflex failure in a patient with central nervous system lesions involving the nucleus tractus solitarii. Hypertension. 1994;23:491–5.
Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11–8.
Hirvonen HE, Salmi TT, Heinonen E, Antila KJ, Valimaki IA. Vincristine treatment of acute lymphoblastic leukemia induces transient autonomic cardioneuropathy. Cancer. 1989;64:801–5.
Adabag AS, Grandits GA, Prineas RJ, Crow RS, Bloomfield HE, Neaton JD, et al. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am J Cardiol. 2008;101:1437–43.
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7.
Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–8.
Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–6.
Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–81.
Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed). 1982;285:916–8.
Walsh D, Nelson KA. Autonomic nervous system dysfunction in advanced cancer. Support Care Cancer. 2002;10:523–8.
Bruera E, Chadwick S, Fox R, Hanson J, MacDonald N. Study of cardiovascular autonomic insufficiency in advanced cancer patients. Cancer Treat Rep. 1986;70:1383–7.
Guo Y, Palmer JL, Strasser F, Yusuf SW, Bruera E. Heart rate variability as a measure of autonomic dysfunction in men with advanced cancer. Eur J Cancer Care (Engl). 2013;22:612–6.
Fadul N, Strasser F, Palmer JL, Yusuf SW, Guo Y, Li Z, et al. The association between autonomic dysfunction and survival in male patients with advanced cancer: a preliminary report. J Pain Symptom Manag. 2010;39:283–90.
• Lakoski SG, Jones LW, Krone RJ, Stein PK, Scott JM. Autonomic dysfunction in early breast cancer: incidence, clinical importance, and underlying mechanisms. Am Heart J. 2015;170:231–41. Excellent review of the clinical importance of autonomic dysfunction as a cardiovascular risk marker among breast cancer patients.
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–37.
McLeod JG, Tuck RR. Disorders of the autonomic nervous system: part 1. Pathophysiology and clinical features. Ann Neurol. 1987;21:419–30.
Screnci D, McKeage MJ. Platinum neurotoxicity: clinical profiles, experimental models and neuroprotective approaches. J Inorg Biochem. 1999;77:105–10.
Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rorth M, Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007;130:1076–88.
Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17.
Nuver J, Smit AJ, Sleijfer DT, van Gessel AI, van Roon AM, van der Meer J, et al. Left ventricular and cardiac autonomic function in survivors of testicular cancer. Eur J Clin Investig. 2005;35:99–103.
Boogerd W, ten Bokkel Huinink WW, Dalesio O, Hoppenbrouwers WJ, van der Sande JJ. Cisplatin induced neuropathy: central, peripheral and autonomic nerve involvement. J Neuro-Oncol. 1990;9:255–63.
Hansen SW. Autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. BMJ. 1990;300:511–2.
Richardson P, Cantwell BM. Autonomic neuropathy after cisplatin based chemotherapy. BMJ. 1990;300:1466–7.
Dermitzakis EV, Kimiskidis VK, Eleftheraki A, Lazaridis G, Konstantis A, Basdanis G, et al. The impact of oxaliplatin-based chemotherapy for colorectal cancer on the autonomous nervous system. Eur J Neurol. 2014;21:1471–7.
Nazir HF, AlFutaisi A, Zacharia M, Elshinawy M, Mevada ST, Alrawas A, Khater D, Jaju D and Wali Y. Vincristine-induced neuropathy in pediatric patients with acute lymphoblastic leukemia in Oman: frequent autonomic and more severe cranial nerve involvement. Pediatr Blood Cancer 2017;64.
Roca E, Bruera E, Politi PM, Barugel M, Cedaro L, Carraro S, et al. Vinca alkaloid-induced cardiovascular autonomic neuropathy. Cancer Treat Rep. 1985;69:149–51.
DiBella NJ. Vincristine-induced orthostatic hypotension: a prospective clinical study. Cancer Treat Rep. 1980;64:359–6.
Dermitzakis EV, Kimiskidis VK, Lazaridis G, Alexopoulou Z, Timotheadou E, Papanikolaou A, et al. The impact of paclitaxel and carboplatin chemotherapy on the autonomous nervous system of patients with ovarian cancer. BMC Neurol. 2016;16:190.
Ekholm EM, Salminen EK, Huikuri HV, Jalonen J, Antila KJ, Salmi TA, et al. Impairment of heart rate variability during paclitaxel therapy. Cancer. 2000;88:2149–53.
Ekholm E, Rantanen V, Bergman M, Vesalainen R, Antila K, Salminen E. Docetaxel and autonomic cardiovascular control in anthracycline treated breast cancer patients. Anticancer Res. 2000;20:2045–8.
Ekholm E, Rantanen V, Antila K, Salminen E. Paclitaxel changes sympathetic control of blood pressure. Eur J Cancer. 1997;33:1419–24.
Viniegra M, Marchetti M, Losso M, Navigante A, Litovska S, Senderowicz A, et al. Cardiovascular autonomic function in anthracycline-treated breast cancer patients. Cancer Chemother Pharmacol. 1990;26:227–31.
Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, Crijns HJ, et al. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart. 1999;81:419–23.
Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–53.
Stratogianni A, Tosch M, Schlemmer H, Weis J, Katona I, Isenmann S, et al. Bortezomib-induced severe autonomic neuropathy. Clin Auton Res. 2012;22:199–202.
Giannoccaro MP, Donadio V, Gomis Perez C, Borsini W, Di Stasi V, Liguori R. Somatic and autonomic small fiber neuropathy induced by bortezomib therapy: an immunofluorescence study. Neurol Sci. 2011;32:361–3.
Kamath MV, Halton J, Harvey A, Turner-Gomes S, McArthur A, Barr RD. Cardiac autonomic dysfunction in survivors of acute lymphoblastic leukemia in childhood. Int J Oncol. 1998;12:635–40.
Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–6.
• Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, et al. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–83. This cohort study reports an association between mediastinal radiation and markers of cardiac autonomic dysfunction, namely elevated resting heart rate and abnormal heart rate recovery. The authors demonstrate that these abnormalities are associated with reduced exercise capacity among radiation survivors, and that abnormal heart rate recovery is associated with higher risk of all-cause mortality during follow-up.
Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48.
Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, Defina LF, et al. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138:909–16.
Payne D, Mahmood S, Partridge AH, Nohria A, Groarke J. Cardiac autonomic dysfunction in breast cancer survivors. J Clin Oncol. 2017;35:10057.
Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–44.
Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–7.
Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–5.
Morshedi-Meibodi A, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am J Cardiol. 2002;90:848–52.
Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–21.
Lauer MS. Autonomic function and prognosis. Cleve Clin J Med. 2009;76(Suppl 2):S18–22.
Guo Y, Koshy S, Hui D, Palmer JL, Shin K, Bozkurt M, et al. Prognostic value of heart rate variability in patients with cancer. J Clin Neurophysiol. 2015;32:516–20.
Lee DH, Park S, Lim SM, Lee MK, Giovannucci EL, Kim JH, et al. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res Treat. 2016;159:375–84.
Crosswell AD, Lockwood KG, Ganz PA, Bower JE. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology. 2014;45:58–66.
Fagundes CP, Murray DM, Hwang BS, Gouin JP, Thayer JF, Sollers JJ 3rd, et al. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–47.
Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15.
Potocnik N, Perse M, Cerar A, Injac R, Finderle Z. Cardiac autonomic modulation induced by doxorubicin in a rodent model of colorectal cancer and the influence of fullerenol pretreatment. PLoS One. 2017;12:e0181632.
Snoek JA, van Berkel S, van Meeteren N, Backx FJ, Daanen HA. Effect of aerobic training on heart rate recovery in patients with established heart disease; a systematic review. PLoS One. 2013;8:e83907.
Scott JM, Jones LW, Hornsby WE, Koelwyn GJ, Khouri MG, Joy AA, et al. Cancer therapy-induced autonomic dysfunction in early breast cancer: implications for aerobic exercise training. Int J Cardiol. 2014;171:e50–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ben G.T. Coumbe and John D. Groarke declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
About this article
Cite this article
Coumbe, B.G.T., Groarke, J.D. Cardiovascular Autonomic Dysfunction in Patients with Cancer. Curr Cardiol Rep 20, 69 (2018). https://doi.org/10.1007/s11886-018-1010-y
Published:
DOI: https://doi.org/10.1007/s11886-018-1010-y