Abstract
Although elevated resting heart rate (RHR) has been shown to be associated with mortality in the general population and patients with certain diseases, no study has examined this association in patients with breast cancer. A total of 4786 patients with stage I–III breast cancer were retrospectively selected from the Severance hospital breast cancer registry in Seoul, Korea. RHR was measured at baseline and the mean follow-up time for all patients was 5.0 ± 2.5 years. Hazard ratios (HRs) with 95 % confidence intervals (CIs) were calculated using Cox regression models. After adjustment for prognostic factors, patients in the highest quintile of RHR (≥85 beat per minute (bpm)) had a significantly higher risk of all-cause mortality (HR: 1.57; 95 %CI 1.05–2.35), breast cancer-specific mortality (HR: 1.69; 95 %CI 1.07–2.68), and cancer recurrence (HR: 1.49; 95 %CI 0.99–2.25), compared to those in the lowest quintile (≤67 bpm). Moreover, every 10 bpm increase in RHR was associated with 15, 22, and 6 % increased risk of all-cause mortality, breast cancer-specific mortality, and cancer recurrence, respectively. However, the association between RHR and cancer recurrence was not statistically significant (p = 0.26). Elevated RHR was associated with an increased risk of mortality in patients with breast cancer. The findings from this study suggest that RHR may be used as a prognostic factor for patients with breast cancer in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resting heart rate (RHR) has been suggested to be an important predictor of overall health. Epidemiological studies have consistently shown that elevated RHR is associated with increased risk of all-cause mortality and cardiovascular disease (CVD) mortality in the general population [3, 7, 11, 13, 16, 17, 19, 20, 22, 24, 26, 27, 31] and patients with CVD [2, 4, 6, 9, 10, 23]. Moreover, several studies have demonstrated a significant association between RHR and mortality in patients with other common diseases such as hypertension [12, 21, 30] and diabetes [1, 15, 28]. However, little is known about the relationship between RHR and cancer. There are only a few studies that have specifically examined the association between RHR and cancer mortality in the general population [18, 22, 24]. Moreover, to our knowledge, no study to date has explored the prognostic value of RHR in relation to mortality among patients with major cancer, particularly breast cancer.
Lack of research in this area is surprising for several reasons. First, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death among women worldwide [8]. Moreover, the number of breast cancer survivors is rapidly increasing and they are at high risk of cancer recurrence and death due to breast cancer or other diseases. Second, RHR is a simple, objective, and safe monitoring method that could be widely used to predict the prognosis of patients in clinical settings. However, research on identifying potential prognostic value of RHR among patients with breast cancer has been very limited.
Therefore, the purpose of this study was to investigate the association between RHR and the prognostic outcomes, including all-cause and breast cancer-specific mortality and cancer recurrence, in a large population of patients with breast cancer.
Materials and methods
Patient selection
A total of 4786 patients with stage I–III breast cancer were retrospectively selected from the Severance hospital breast cancer registry, Department of Surgery, Yonsei University College of Medicine, Seoul, Republic of Korea. All patients underwent definitive surgery for primary breast cancer between January 2005 and December 2013. Exclusion criteria were as follows: patients with in situ carcinoma, stage IV disease at the time of diagnosis, occult breast cancer, or non-epithelial origin malignancy. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea (IRB No. 4-2016-0040). Written informed consent was waived and patient information was deidentified prior to analysis.
Clinicopathological characteristics
Clinicopathological information, including RHR and expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), was obtained through medical records and registry database. The database collected personal past history, clinicopathological parameters, treatment patterns, and follow-up outcomes related with breast cancer. After definitive surgery for breast and axilla, adjuvant treatments were administered if the patient was able to tolerate it. Clinical follow-up included history taking, physical examination, laboratory tests, and radiological imaging tests every 6–12 months for detection of relapse.
RHR was checked at admission to hospital one day before surgery. Tumor-node-metastasis (TNM) stage was based on the American Joint Committee on Cancer 6th edition criteria. Tumor with ≥1 % nuclear-stained cells was categorized as positive expression for ER and PR in accordance with the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [14]. HER2 was stained by Hercep Test (Dako, Glostrup, Denmark) and interpreted as 0, 1± , 2± , or 3± according to ASCO/CAP guidelines [32]. In cases with HER2 2 ± results, fluorescence in situ hybridization (FISH) was performed using a PathVysion HER2 DNA Probe Kit (Vysis, Downer Grove, IL, USA). HER2 gene amplification was defined as a HER2 gene/chromosome 17 copy number ratio ≥2.0 or a case with HER2 gene/chromosome 17 copy number ratio <2.0 but with average HER2 copy number ≥6.0 signals/cell according to ASCO/CAP guidelines [32]. HER2 was considered positive in cases with an immunohistochemistry score of 3 + or gene amplification by FISH.
Breast cancer subtypes were categorized by hormone receptors (HRs) and HER2 expression as follows: HRs +/HER2-, ER-positive or PR-positive, and HER2-negative; HRs +/HER2 +, ER-positive or PR-positive, and HER2-positive; HRs-/HER2 +, ER-negative, PR-negative, and HER2-positive; HRs-/HER2-, ER-negative, PR-negative, and HER2-negative, a subtype also known as triple-negative breast cancer (TNBC).
Locoregional recurrence was defined as tumor recurrence in ipsilateral breast, chest wall, or regional lymph node. Any recurrence at a distant site including contralateral axillary or supraclavicular lymph nodes was considered as a distant metastasis. Cancer recurrence was measured from the date of the first curative surgery to the date of the first locoregional or distant recurrence. All-cause mortality was calculated from the date of the first surgery to the date of the last follow-up or death from any cause. For breast cancer-specific mortality, cause of death was collected according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) from the review of medical records.
Statistical methods
For the analyses, patients were divided into quintiles based on their RHR (beat per minute, bpm). For the comparison of baseline characteristics, ANOVA was conducted for continuous variables and χ2 test for categorical variables. The Kaplan–Meier curves were computed for overall and breast cancer-specific survival. The log-rank test was used to test for differences in the survival curves across RHR quintiles.
Cox proportional hazard models were used to adjust for confounders and to estimate the hazard ratios (HRs) and 95 % confidence intervals (CIs) of all-cause and breast cancer-specific mortality and cancer recurrence across the quintiles of RHR. Proportional hazard assumption was checked by including interaction terms for each covariate and time in the model. Test for trend was conducted using the median value of each quintile as a continuous variable in the cox regression models. For subgroup analyses, we conducted cox proportional hazard models stratified by potential effect modifiers. All models were adjusted for potential confounders including age, body mass index (BMI), menopause status, age at menarche, age at first birth, hormone replacement therapy, diabetes, hypertension, CVD, respiratory disease, liver disease, other disease, histology, histologic grade, molecular subtypes, operation type, radiation therapy, hormone therapy, chemotherapy, and TNM stage.
To check for the robustness of the findings, several sensitivity analyses were performed. First, all main analyses were conducted after excluding patients with any comorbid conditions. Moreover, additional analyses were done after excluding patients who have died in the early follow-up period of the study (i.e., 3 and 6 months) to see whether the initial findings were influenced by potential bias.
All tests were two sided and considered to be significant at P-value <0.05. Statistical analyses were conducted using the SAS 9.4 (SAS institute, Cary, NC).
Results
Baseline characteristics
After exclusion, a total of 4786 breast cancer patients remained in the final analyses. During a 9-year period, we documented 278 cases of all-cause death, 212 cases of breast cancer-specific death, and 339 cases of cancer recurrence. Baseline characteristics of 4786 participants by quintiles of RHR are presented in Table 1. Overall, patients had a mean age of 50.7 ± 10.6 years and a mean BMI of 23.4 ± 3.2. The average follow-up time for all patients was 5.0 ± 2.5 years. Among quintiles of RHR (i.e., ≤67, 68–72, 73–78, 79–84, and ≥85), there were statistically significant differences in age, menopausal status, age at menarche, age at birth, comorbidity conditions including diabetes, hypertension and CVD, operation type, radiation therapy, hormone therapy, chemotherapy, and TNM stage.
Main analyses
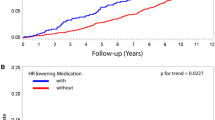
The Kaplan–Meier curves for overall and breast cancer-specific survival according to quintiles of RHR are presented in Fig. 1. Across all the quintiles of RHR, there were statistically significant differences in both overall and breast cancer-specific survival (log-rank test, p < .001).
Table 2 presents the age-adjusted and multivariable-adjusted hazard ratios of prognostic outcomes associated with RHR among patients with breast cancer. Compared to patients with lowest RHR (≤ 67 bpm), patients with highest RHR (≥85 bpm) had 1.57 (95 %CI 1.05–2.35) times higher risk of all-cause mortality after fully adjusting for potential confounders. Moreover, patients with highest RHR had 1.69 (95 %CI 1.07–2.68) times higher risk of breast cancer-specific mortality when compared to those with lowest RHR. Elevated RHR was marginally significantly associated with 1.49 (95 %CI 0.99–2.25) times higher risk of cancer recurrence compared to the reference group. When TNM stage was not adjusted in the multivariable models, the aforementioned associations substantially increased. Overall, there was a significant linear trend between RHR and prognostic outcomes.
When RHR was used as a continuous variable, an increase in 10 bpm of RHR was associated with 15, 22, and 6 % increased risk of all-cause mortality, breast cancer-specific mortality, and cancer recurrence, respectively, after fully adjusting for the same confounders. However, the association between RHR and cancer recurrence was not statistically significant.
Subgroup analyses
Table 3 shows the multivariable-adjusted hazard ratios of prognostic outcomes associated with 10 bpm increase in RHR, stratified by age, BMI, menopausal status, hypertension, histologic grade, molecular subtype, operation type, cancer therapy, and TNM stage. RHR was used as a continuous variable for subgroup analyses to increase the statistical power and also because there was a linear relationship between RHR and prognostic outcomes. The association of RHR with all-cause and breast cancer mortality appeared to be stronger among patients who were under 60 years old or had a hormone therapy. Moreover, the association between RHR and cancer recurrence was pronounced among post-menopausal patients. Other than those, there was no statistically significant effect modifier in the association between RHR and prognostic outcomes. Although statistically not significant, the positive association tended to be stronger for patients with BMI <25 kg/m2, post-menopause status, hypertension, histologic grade I/II, HRs + subtypes, TM, no radiation therapy, hormone therapy, or TNM stage II/III.
Sensitivity analyses
Overall, sensitivity analyses have supported the robustness of our findings. After excluding patients with any comorbidity conditions, there were no noticeable changes in all estimates and our findings remained statistically significant. Moreover, excluding patients who have died in the early follow-up of 3 months (n = 8) and 6 months (n = 15) did not change our results (data not shown).
Discussion
In this large study of 4786 breast cancer patients, we found elevated RHR to be significantly associated with increased risk of overall prognostic outcomes. Compared to patients with ≤67 bpm of RHR, those with ≥85 bpm of RHR had 1.57, 1.69, and 1.49 times higher risk of all-cause mortality, breast cause-specific mortality, and cancer recurrence, respectively. Moreover, there was a strong linear relationship between RHR and prognostic outcomes; an increase in RHR by every 10 bpm was associated with approximately 6–22 % increased risk of overall prognostic outcomes in patients with breast cancer. Interestingly, we found that TNM stage, which is probably the most important prognostic factor, could mediate the causal pathway between RHR and prognostic outcomes. That is, there may be a stage difference by RHR even before the follow-up is initiated. These findings were robust even after adjusting for potentially important confounders (e.g., major comorbid conditions, breast cancer risk factors, tumor characteristics and treatment) and excluding deaths occurred in the early follow-up, which reduce the possibility of confounding and reverse causation by measured or unmeasured pre-existing diseases.
To the best of our knowledge, this is the first study to examine the relationship between RHR and mortality among cancer patients. Epidemiological studies examining this association in the general population [3, 7, 11, 13, 16, 17, 19, 20, 22, 24, 26, 27, 31] and patients with other diseases [1, 2, 4, 6, 9, 10, 12, 15, 21, 23, 28, 30] exist. However, data to investigate the relationship between RHR and cancer risk or the prognostic value of RHR in patients with cancer are sparse. The potential positive relationship between RHR and cancer mortality was first hypothesized from observational studies among middle-aged men in 1981 [25]. Subsequently, a number of studies have been conducted to explore RHR in relation to cancer mortality in the general population. Some studies have shown a strong and graded relationship between high RHR and all-cancer mortality [18, 29], while a few studies have reported either weak [13] or null [22, 24] results, but the latter were limited by small number of cancer deaths. The significant relationships between heart rate and all-cancer mortality found in prior studies were comparable to the results shown in many studies that had the endpoint outcome of CVD mortality. Despite the suggestive role of RHR for predicting cancer mortality in the general population, there have been very limited studies that investigated the prognostic importance of RHR in cancer patients. Of note, the endpoint of cancer mortality in the general population incorporates both incidence and survival, so any association may not necessarily be due to prognosis.
In contrast to patients with cancer, there has been a greater effort to examine the prognostic value of RHR among patients with other major diseases such as CVD and diabetes. The findings on the relationship between RHR and prognostic outcomes in patients with CVD were confirmed in relatively recent years. Among patients with suspected or proven coronary artery disease (CAD), those with RHR ≥83 bpm at baseline had 32 and 31 % increased risk of total mortality and cardiovascular mortality, respectively, when compared to those with RHR <63 bpm [6]. In similar comparison groups of RHR, stronger results were observed in patients after ischemic stroke [4] and patients with chronic heart failure, reaching 74–86 % increased risk [5]. Moreover, for every 5 bpm increase of RHR, there was an approximately 7–9 % increased risk of all-cause and cardiovascular-related death among patients with CAD and left-ventricular systolic dysfunction [10], stable chronic CVD [23], or any vascular disease [2]. These findings provide evidence that RHR is an important prognostic marker, which needs to be assessed in clinical settings to guide optimal treatments for patients with CVD. More recently, a few observational studies have been published to examine the association between RHR and mortality in patients with diabetes. One study that followed 523 diabetic patients showed that each 10 bpm increase of RHR was associated with 31 and 43 % increased risk of all-cause and CVD mortality in patients with type 2 diabetes, but not type 1 diabetes [28]. A subsequent study with a larger sample size of patients with type 2 diabetes found consistent results, suggesting a potential prognostic value of RHR for patients with type 2 diabetes [15].
Cancer is one of the major global burden in public health. Among all cancers, breast cancer has overwhelmingly the highest incidence and mortality in women worldwide. More importantly, the cumulative number of breast cancer survivors is continuing to grow rapidly and managing breast cancer survivors to improve their prognosis is becoming more important. RHR is a simple, objective, and non-intrusive method which could be easily measured in clinical setting for cancer patients. Considering the consistent and emerging evidence from patients with CVD and non-CVD, our study extends the understanding of relationship between RHR and mortality outcomes to a population of patients with breast cancer. Interestingly, we observed a strong linear positive association between RHR and all-cause mortality that was comparable, in terms of direction and magnitude, to the results of previous studies done in other patient populations. Furthermore, we found a stronger association between RHR and breast cancer-specific mortality, but the association was weaker for the outcome of cancer recurrence. This result indicates that RHR may be a better predictor of mortality than cancer recurrence among cancer patients. Although we additionally conducted subgroup analyses by clinically important covariates, including tumor characteristics and treatments, we did not find statistically meaningful results, except for age, hormone therapy, and menopausal status, but the lack of power in the subgroup analyses may have masked significant findings in the study. Future studies with larger sample size may be able to detect important subpopulations of breast cancer patients who can benefit more by managing RHR.
Underlying mechanism between RHR and prognostic outcomes, especially related to cancer prognosis, is unknown. Moreover, it is yet to be determined whether RHR is a direct independent predictor of prognosis or merely an indirect factor that is associated with other factors known to be associated with poor prognosis in patients with breast cancer. However, it appears more likely that RHR is a good general marker of health-related factors, including behavioral and lifestyle factors, for patients with breast cancer. Therefore, further studies are warranted to not only verify the potential causal relationship and underlying mechanism between RHR and prognostic outcomes, but also determine how RHR or associated factors can be modified to reduce the risk of mortality and cancer progression in patients with breast cancer.
There are several potential limitations of the current study. First, although we thoroughly adjusted for potential confounders, we could not fully adjust for severity of comorbid condition, detailed medications, and undetected pre-existing diseases. However, our sensitivity analyses showed robust results after excluding patients with any comorbid conditions and those who died in the early follow-up period possibly due to pre-existing diseases. Second, we cannot entirely rule out the potential confounding by lifestyle factors, such as physical activity/physical fitness, smoking, and alcohol. RHR could be affected by lifestyle factors, especially physical activity/physical fitness. Though elevated RHR has been shown to be an independent prognostic factor of mortality in patients with CVD after adjusting for the aforementioned lifestyle factors, further studies are needed to clarify whether RHR is an independent prognostic factor in patients with breast cancer. Third, a single measure of RHR near the time of operation is prone to misclassification due to within-person variation and the timing of exposure measurement. However, misclassification is likely to be random with regard to the outcomes and RHR measured prior to the operation is less likely to be affected by the diagnosis of breast cancer because the average time of operation after breast cancer diagnosis was less than a week. Lastly, our study participants included breast cancer patients in South Korea only, and thus our findings may not be generalizable to other populations with different race/ethnicity.
In conclusion, high RHR was associated with all-cause and breast cancer-specific mortality in patients with breast cancer. This study suggests the potential use of RHR as a prognostic factor for patients with breast cancer in clinical settings. Future studies are needed to further verify the usefulness of RHR in diverse cancer patients and to determine feasible and efficient strategies to modify RHR to improve cancer progression and overall survival in patients with cancer.
References
Anselmino M, Öhrvik J, Rydén L (2010) Resting heart rate in patients with stable coronary artery disease and diabetes: a report from the euro heart survey on diabetes and the heart. Eur Heart J: ehq368
Bemelmans RH, van der Graaf Y, Nathoe HM, Wassink AM, Vernooij JW, Spiering W, Visseren FL, Group SS (2013) The risk of resting heart rate on vascular events and mortality in vascular patients. Int J Cardiol 168:1410–1415
Benetos A, Rudnichi A, Thomas F, Safar M, Guize L (1999) Influence of heart rate on mortality in a French population role of age, gender, and blood pressure. Hypertension 33:44–52
Böhm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, Bath PM, Yusuf S, Diener H-C (2012) Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J 33:2804–2812
Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators S (2010) Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 376:886–894
Diaz A, Bourassa MG, Guertin MC, Tardif JC (2005) Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J 26:967–974. doi:10.1093/eurheartj/ehi190
Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, BERKSON DM, LEPPER M, SCHOENBERGER JA, LINDBERG HA (1980) Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 112:736–749
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Fox K, Bousser M-G, Amarenco P, Chamorro A, Fisher M, Ford I, Hennerici MG, Mattle HP, Rothwell PM, Investigators PS (2013) Heart rate is a prognostic risk factor for myocardial infarction: a post hoc analysis in the PERFORM (Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack) study population. Int J Cardiol 168:3500–3505
Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R (2008) Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 372:817–821
Fujiura Y, Adachi H, Tsuruta M, Jacobs DR, Hirai Y, Imaizumi T (2001) Heart rate and mortality in a Japanese general population: an 18-year follow-up study. J Clin Epidemiol 54:495–500
Gillman MW, Kannel WB, Belanger A, D’Agostino RB (1993) Influence of heart rate on mortality among persons with hypertension: the framingham study. Am Heart J 125:1148–1154
Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J (1999) Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the chicago heart association detection project in industry. Am J Epidemiol 149:853–862
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J clin oncol 28:2784–2795. doi:10.1200/jco.2009.25.6529
Hillis G, Woodward M, Rodgers A, Chow C, Li Q, Zoungas S, Patel A, Webster R, Batty G, Ninomiya T (2012) Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia 55:1283–1290
Jensen MT, Marott JL, Allin KH, Nordestgaard BG, Jensen GB (2012) Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: the copenhagen city heart study. Eur J of preventive cardiol 19:102–108
Jensen MT, Suadicani P, Hein HO, Gyntelberg F (2013) Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the copenhagen male study. Heart 99:882–887
Jouven X, Escolano S, Celermajer D, Empana JP, Bingham A, Hermine O, Desnos M, Perier MC, Marijon E, Ducimetiere P (2011) Heart rate and risk of cancer death in healthy men. PLoS ONE 6:e21310. doi:10.1371/journal.pone.0021310
Jouven X, Zureik M, Desnos M, Guérot C, Ducimetière P (2001) Resting heart rate as a predictive risk factor for sudden death in middle-aged men. Cardiovasc Res 50:373–378
Kannel WB, Kannel C, Paffenbarger RS, Cupples LA (1987) Heart rate and cardiovascular mortality: the framingham study. Am Heart J 113:1489–1494
Kolloch R, Legler UF, Champion A, Cooper-DeHoff RM, Handberg E, Zhou Q, Pepine CJ (2008) Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J
Kristal-Boneh E, Silber H, Harari G, Froom P (2000) The association of resting heart rate with cardiovascular, cancer and all-cause mortality. eight year follow-up of 3527 male israeli employees (the CORDIS Study). Eur Heart J 21:116–124. doi:10.1053/euhj.1999.1741
Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, Yusuf S, Böhm M (2014) Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol 103:149–159
Mensink G, Hoffmeister H (1997) The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J 18:1404–1410
Perskly V, Dyer AR, Leonas J, Stamler J, Berkson DM, Lindberg HA, Paul O, Shekelle RB, Lepper MH, Schoenberger JA (1981) Heart rate: a risk factor for cancer? Am J Epidemiol 114:477–487
Reunanen A, Karjalainen J, Ristola P, Heliövaara M, Knekt P, Aromaa A (2000) Heart rate and mortality. J Intern Med 247:231–239
Saxena A, Minton D, Lee D-c, Sui X, Fayad R, Lavie CJ, Blair SN (2013) Protective role of resting heart rate on all-cause and cardiovascular disease mortality. In: Mayo Clinic Proceedings. Elsevier, p 1420–1426
Stettler C, Bearth A, Allemann S, Zwahlen M, Zanchin L, Deplazes M, Christ E, Teuscher A, Diem P (2007) QTc interval and resting heart rate as long-term predictors of mortality in type 1 and type 2 diabetes mellitus: a 23-year follow-up. Diabetologia 50:186–194
Thomas F, Guize L, Bean K, Benetos A (2001) Pulse pressure and heart rate: independent risk factors for cancer? J Clin Epidemiol 54:735–740
Thomas F, Rudnichi A, Bacri A-M, Bean K, Guize L, Benetos A (2001) Cardiovascular mortality in hypertensive men according to presence of associated risk factors. Hypertension 37:1256–1261
Wang A, Chen S, Wang C, Zhou Y, Wu Y, Xing A, Luo Y, Huang Z, Liu X, Guo X (2014) Resting heart rate and risk of cardiovascular diseases and all-cause death: the Kailuan study
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline update. J clin oncol 31:3997–4013. doi:10.1200/jco.2013.50.9984
Funding
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2015S1A5B8036349).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None declared.
Additional information
Dong Hoon Lee and Seho Park have equally contributed to this study.
Rights and permissions
About this article
Cite this article
Lee, D.H., Park, S., Lim, S.M. et al. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res Treat 159, 375–384 (2016). https://doi.org/10.1007/s10549-016-3938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3938-1