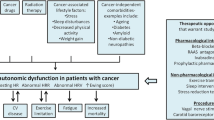
Abstract
Purpose of Review
Cardiovascular autonomic dysfunction (AD) among cancer survivors is increasingly being recognized. However, the mechanisms and incidence are poorly understood. In this review, the clinical features, diagnostic modalities, proposed mechanisms, and currently available treatments of cardiovascular AD in cancer survivors are described.
Recent Findings
Much of our current understanding of cardiovascular AD is based on disease states such as diabetes, multisystem atrophy, and Parkinson’s disease. Several non-invasive tests, measurements, and scoring systems have been developed as surrogates for autonomic function, with some even demonstrating associations with all-cause mortality. The mechanism of cardiovascular AD specifically in the cancer population, however, has not been directly studied. The etiology of cardiovascular AD in cancer survivors is likely multifactorial, and proposed mechanisms include direct nerve damage by chemoradiation, the pro-inflammatory state associated with malignancy, and paraneoplastic syndromes. It may also be that cardiovascular AD is an early marker of global cardiomyopathy rather than its own condition. Current pharmacologic options for cardiovascular AD are extrapolated from how it has been treated in other disease processes, and these agents have not been studied in the cancer population or compared head-to-head.
Summary
Cardiovascular AD in cancer survivors can cause significant debilitation and may be associated with all-cause mortality. Current diagnostic modalities have several limitations, such as standardization and validity. However, given the nonspecific nature of cardiovascular AD, these tools provide an objective marker for diagnosis and tracking treatment response. While the mechanism of cardiovascular AD in cancer survivors has not been directly studied, it may be useful to evoke mechanisms of cardiovascular AD in other disease states such as diabetes, Parkinson’s disease, and multisystem atrophy in addition to identifying unique conditions associated with malignancy like a pro-inflammatory state. Until further studies are performed, management of cardiovascular AD as seen in other disease states may serve as a guide for symptom management in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With improvements in chemoradiation therapies, the lifespan of patients living with cancer and those in remission have significantly increased. Based on data from the Surveillance, Epidemiology, and End Results (SEER) Program Cancer Statistics Review, 5-year survival from cancer of all sites from 1950 to 1954 compared to 2008–2014 has improved from 35 to 69.7%, and overall mortality from 1950 to 2015 has decreased by 18.8% [1]. As such, cancer survivorship has emerged as a type of chronic condition in and of itself, in part due to short- and long-term adverse effects of the treatment [2,3,4,5].
Both radiation therapy (RT) and many chemotherapeutic agents have been well-documented to be associated with an increased risk for cardiovascular disease [6]. These studies tend to focus primarily on left ventricular dysfunction and cardiomyopathy as clinical endpoints for cardiotoxicity. Additionally, many cancer-directed treatments are known to cause peripheral neuropathy and generalized autonomic dysfunction (AD) such as gastrointestinal and genitourinary dysfunction [7,8,9,10]. More recently, cardiovascular AD among cancer survivors is increasingly being recognized as its own distinct entity [11,12,13,14,15]. However, the mechanisms underlying cardiovascular AD in this patient population are less well understood.
Cardiovascular AD can arise from several etiologies including, but not limited to, hereditary, metabolic, immunologic, infectious, inflammatory, neurodegenerative, and toxic causes [16, 17••]. It is challenging to identify a causative link between cardiovascular AD and cancer therapies, as there are many confounding variables and potential etiologies. Cardiovascular AD in cancer patients could be related to pre-existing neuropathy, paraneoplastic effect, tumor invasion or compression of the autonomic nervous system (ANS), cancer-related deconditioning, or an underlying autoimmune etiology. Likewise, it can take decades after treatment for symptoms to develop, at which point it can be difficult to discern age-related cardiovascular AD from cancer-related cardiovascular AD [15, 18]. Furthermore, the heterogeneity of cancer treatment regimens and types of cancers make the study of cardiovascular AD in cancer challenging. Current methods of diagnosing cardiovascular AD are difficult to access, perform, and interpret, and their validity and normative values are subject to debate. As such, little is known regarding the incidence and precise mechanisms by which cardiovascular AD ensues in patients with cancer.
In this review, the clinical features of cardiovascular AD are described, along with methods in which to quantify and diagnose cardiovascular AD. Potential mechanisms for cardiovascular AD associated with chemotherapy and radiotherapy, with suggested treatment options of symptoms, are proposed in this unique population. The following two patient cases demonstrate the challenges in the diagnosis and treatment of cancer-related cardiovascular AD.
Case 1
A 65-year-old man with prior history of hypertension, hyperlipidemia, chronic kidney disease, and multiple myeloma was referred to cardio-oncology clinic for evaluation of dizziness and atrial flutter. The patient had been diagnosed with multiple myeloma 4 months prior to evaluation and had undergone five cycles of lenalidomide, bortezomib, and dexamethasone in addition to autologous stem cell transplant after conditioning with melphalan followed by maintenance lenalidomide. Bone marrow biopsy 100 days after this treatment course was negative for plasma cells.
His post-transplant course was complicated by one episode of new-onset atrial flutter that self-resolved following correction of electrolyte abnormalities. Additionally, approximately 2 weeks after his bone marrow transplant, he began experiencing lightheadedness with exertion and orthostatic positional changes with documented vital signs consistent with orthostatic hypotension (OH) in oncology clinic. These symptoms did not improve with conservative measures including routine intravenous fluid resuscitation, prompting referral to cardio-oncology clinic.
On evaluation, he endorsed palpitations associated with fatigue and generalized weakness for several weeks lasting a few seconds at a time. He denied any chest pain or symptoms of decompensated heart failure. His hematologic medications consisted of lenalidomide 15 mg every other day. His prior anti-hypertensive medications had been held in the setting of low blood pressure and aspirin had been held in the setting of chemotherapy-induced thrombocytopenia.
His vital signs revealed a blood pressure of 115/65 mmHg and pulse of 91 beats per minute (BPM) with lying, blood pressure of 84/62 mmHg and pulse of 113 BPM after standing for 2 min. His exam was notable for inability to sit up due to dizziness but was otherwise unremarkable. Relevant laboratory abnormalities included hemoglobin of 9.9 g/dL, platelet count of 65 × 103 cells/μL, and creatinine of 3.0 mg/dL. His electrocardiogram (ECG) demonstrated normal sinus rhythm at 82 BPM. TTE was notable for severe septal hypertrophy, delayed basal septal wall contraction, left ventricular ejection fraction (LVEF) of 55–60%, normal left ventricular diastolic function, abnormal global longitudinal strain of − 15.0%, mildly reduced right ventricular systolic function, normal pulmonary artery systolic pressure (PASP), and mild to moderate mitral valve regurgitation.
Fourteen-day ambulatory rhythm monitoring demonstrated sinus rhythm with an average heart rate of 83 BPM, range of 51–140 BPM, and no arrhythmias or reported symptoms.
The patient subsequently underwent autonomic reflex testing. Tilt-table test demonstrated a significant systolic blood pressure (SBP) drop of 53 mmHg and tachycardic response of 43 BPM, with reported symptoms of dizziness (Fig. 1). Valsalva maneuver demonstrated failure of late phase 2 to return to baseline and absent phase 4 overshoot (Fig. 2). Sudomotor testing was notable for abnormal sweat response in the forearm, proximal leg, distal leg, and foot. Heart rate response to deep breathing was normal. Given these findings, the patient was diagnosed with severe OH. Additionally, these studies indicated severe generalized autonomic impairment, with severe adrenergic and sudomotor impairment and mild cardiovagal impairment in the adrenergic and sudomotor systems.
Tilt-table test results for case 1. The top graph demonstrates the patient’s heart rate over time throughout the tilt maneuver. The bottom graph demonstrates the patient’s systolic, mean, and diastolic blood pressures over time throughout the tilt maneuver. The green arrows indicate the start and end of the tilt maneuver. The black arrow points to the lowest systolic blood pressure (111.4 mmHg) during the maneuver. The patient had a decrease in their systolic blood pressure of 53 mmHg and an increase in their heart rate of 43 BPM. This is consistent with a diagnosis of orthostatic tachycardia
Valsalva maneuver test results for case 1. The yellow line demonstrates the patient’s expiratory pressure, which is used as a marker to indicate when the patient is performing the Valsalva maneuver. The first and second purple arrows represent the start and stop of the Valsalva maneuver, respectively. The blue line demonstrates the patient’s systolic blood pressure throughout the Valsalva maneuver. The patient’s systolic blood pressure tracing throughout the maneuver was notable for late phase 2 blood pressure not returning to baseline and an absence of phase 4 overshooting the baseline blood pressure, which are abnormal findings. The patient’s Valsalva ratio, which is defined as the maximum heart rate during the maneuver divided by the lowest heart rate during the maneuver, was within the 5th–95th percentile range
Due to his debilitating symptoms and diagnosis of OH, the patient was started on midodrine 7.5 mg three times daily. With this regimen, the patient had almost complete resolution of his orthostatic lightheadedness. He was able to ambulate without a walker and no longer needed routine intravenous hydration.
Case 2
A 22-year-old woman was referred to a cardio-oncology clinic for evaluation of persistent tachycardia and postural-related symptoms of dizziness. Two years prior to evaluation, she was diagnosed with primary mediastinal diffuse large B cell lymphoma (DLBCL). Over the next year and a half, the patient achieved complete response after an allogeneic stem cell transplant, approximately 40 Gy of radiation to the mediastinum, and multiple regimens of chemotherapy, including alkylating agents, anthracyclines (total cumulative dosing of doxorubicin equivalent of 253 mg/m2), anti-metabolites, DNA replication inhibitors, platinum-based agents, monoclonal antibodies, steroids, and vinca alkaloids.
She was noted throughout routine medical visits to have tachycardia beginning shortly after her bone marrow transplant. Six months after the tachycardia was noted, she began to feel intermittent palpitations. The patient developed graft-versus-host disease of the heart complicated by pericardial effusion and cardiac tamponade, for which she received a pericardial window.
The patient was then referred to a cardio-oncology clinic. She described symptoms of dizziness with positional changes, fatigue, dyspnea on exertion with walking more than 30 min, and a 50-pound weight gain over the previous year. Her other medical history was notable for well-controlled type 1 diabetes, adrenal insufficiency, and peripheral small fiber sensory neuropathy attributed to cyclosporine and diabetes. Despite being on metoprolol succinate 100 mg daily, her pulse at her initial cardio-oncology visit was 105 BPM with a blood pressure of 142/96 mmHg. Her physical exam was notable for tachycardia without evidence of volume overload. Labs were notable for a chronic anemia with hemoglobin of 11.8 g/dL and hemoglobin A1c of 6.0%. Thyroid studies and brain natriuretic peptide levels were within normal limits. Her electrocardiogram (ECG) demonstrated sinus tachycardia at 103 BPM. Transthoracic echocardiography (TTE) demonstrated a low normal left ventricular ejection fraction of 50–55% and normal global longitudinal strain.
Further evaluation of her symptoms and tachycardia included an exercise stress echocardiogram, 48-h ambulatory rhythm monitoring, and autonomic reflex testing. Stress echocardiography demonstrated a submaximal cardiac workload of 4.6 metabolic equivalent of task (MET), maximum heart rate 142 BPM (72% of maximum predicted heart rate). Cardiac monitoring revealed heart rates ranging from 63 to 124 BPM (average 97 BPM) and no arrhythmias or reported symptoms. Tilt-table test demonstrated maximal SBP drop of 18 mmHg with tachycardia response of 30 BPM. Valsalva maneuver demonstrated a Valsalva ratio of 1.4 (age-normative value 1.46–2.73), failure of late phase 2 to return to baseline, and prolonged pressure recovery time. Heart rate deep breathing test demonstrated mean heart rate difference of 7.3 BPM, below the age-normative lower limit of 14.0 (Fig. 3). Sudomotor testing was normal. Her tilt-table test demonstrated a cardio-inhibitory hemodynamic pattern, which was considered indeterminate for an orthostatic pattern, but was clinically suspicious for postural orthostatic tachycardia syndrome (POTS). Overall, these studies indicated moderate generalized autonomic impairment with severe adrenergic and mild cardiovagal impairment.
Heart rate deep breathing test results for case 2. The green line demonstrates the patient’s heart rate over time throughout the heart rate deep breathing test. The blue line demonstrates the patient’s respirations over time. The first and second green arrows represent the start and stop of the deep breathing maneuvers, respectively. The heart rate deep breathing test demonstrated a mean heart rate difference of 7.3 BPM, which is below the age-normative lower limit of 14.0 BPM
For these findings and her symptoms, she was started on ivabradine 5 mg twice daily. Her resting heart rates improved to approximately 60–70 BPM, and she reported significant improvement in her symptoms. However, ivabradine was discontinued due to the patient reporting luminous phenomena, a known side effect of this medication. After discontinuing ivabradine, her symptoms recurred, and she had a syncopal episode in the context of positional changes. She was then switched to short acting diltiazem 30 mg twice a day with improvement of symptoms and continues to do well.
Clinical Features, Identifying, and Quantifying Cardiovascular Autonomic Dysfunction
Clinical Features
The sympathetic and parasympathetic limbs of the ANS act in a coordinated fashion via multiple reflex loops to regulate cardiovascular function, including heart rate, blood pressure, and cardiopulmonary integration [19,20,21]. This interplay allows the circulatory system to maintain relatively consistent and appropriately compensated hemodynamics despite fluctuations in variables such as position, exertion, and fluid status. While the sympathetic nervous system releases norepinephrine that is responsible for increasing heart rate and contractility and vasoconstricting peripheral vasculature, the parasympathetic nervous system counteracts the sympathetic output and results in the opposite effects. The dynamic interplay between these two systems is mediated partially by responses to stretch of pressure baroreceptors in the carotid sinus and aortic arch (Fig. 4) and volume baroreceptors in the heart and pulmonary vasculature [22, 23].
Dysfunction in this system can result in symptoms of lightheadedness, dizziness, blurred vision, palpitations, fatigue, headache, syncope, and cognitive impairment. Given the nonspecific nature of these symptoms, it can be challenging to attribute them to cardiovascular AD. This underscores the utility of a reliable, objective assessment tool not only to diagnose but also to characterize the type and severity of cardiovascular AD. Unfortunately, there is currently no gold standard diagnostic strategy with regard to hemodynamic criteria nor is there a comprehensive classification system that captures the spectrum of diseases seen. There are a handful of non-invasive tests and measurements that can be performed in an attempt to quantify and diagnose cardiovascular AD, but many are complex, burdensome, and inadequate as isolated tests necessitating use in combination with other techniques.
Diagnostic Maneuvers and Measurements
Some objective data can be obtained using physical exam techniques and routine studies that can readily be done in the outpatient setting [24, 25]. Perhaps the most useful and convenient physical exam technique, though nonspecific, is measurement of OH and POTS, which can be performed by measuring blood pressure supine and then 3‑5 min after standing. OH is defined as a reduction of the SBP of at least 20 mmHg and/or a reduction in diastolic blood pressure (DBP) of 10 mmHg. In patients with baseline SBP > 160 mmHg at rest, a decrease in SBP of 30 mmHg or more is diagnostic of OH [22]. POTS is defined as an increase in heart rate by at least 30 BPM in the absence of a reduction in blood pressure that would be consistent with OH [26••]. One limitation of diagnosing OH by physical exam is that this method does not distinguish between hypovolemic, anemic, polypharmacy, and neurogenic orthostasis. However, Norcliffe-Kaufmann et al. demonstrated that using the ratio of the change in heart rate over change in systolic blood pressure (ΔHR/ΔSBP) can help distinguish between neurogenic and other causes. A blunted heart rate increase during hypotension, defined as a ΔHR/ΔSBP < 0.49 BPM/mmHg, diagnosed neurogenic OH with 91.3% sensitivity and 88.4% specificity [27].
Another physical exam maneuver that reflects sympathetic function involves assessing blood pressure response to sustained handgrip. It requires a handgrip dynamometer and having the patient maintain handgrip at 30% of their maximum strength for as long as possible for up to 5 min. A reflexive increase of at least 16 mmHg in DBP is considered normal [28].
Exercise testing can also be helpful in determining the blood pressure, chronotropic, and rate-pressure product responses to exertion. Abnormalities in these markers are associated with cardiovascular AD [24, 25]. Blunted (≥ 10 mmHg fall in SBP) or exaggerated blood pressure response (≥ 190/105 mmHg in women or ≥ 210/105 mmHg in men) to exercise can be indicative of cardiovascular AD [29, 30]. Groarke et al. measured heart rate recovery (HRR) in patients with a history of Hodgkin’s lymphoma by comparing their heart rates at peak exercise and after 1 min of recovery [24]. An abnormal recovery was constituted as ≤ 12 BPM decrease after 1 min of recovery in active cool-down, or ≤ 18 BPM decrease in passive recovery. What was particularly concerning was the finding that abnormal HRR was associated with increased all-cause mortality in this cohort (age-adjusted hazard ratio of 4.6, 95% CI: 1.62–13.02).
Several hemodynamic surrogates for autonomic function and diagnostic procedures have been developed to specifically study autonomic impairment (Table 1). These tests allow for more robust measurements of type and severity of dysfunction [31, 32]. Some of the most accepted surrogates for autonomic function include heart rate variability (HRV), pre-ejection period (PEP), and baroreflex sensitivity (BRS) [33, 34]. In addition, tests such as tilt-table testing, sudomotor testing, and blood pressure and heart rate changes during the Valsalva maneuver can be used to assess the integrity of the autonomic reflex [35]. These different markers are tested in response to various stressors, such as deep breathing, Valsalva, volume loads, and posture change to assess the integrity of the autonomic reflex response.
Tilt-table testing can be used to diagnose and better characterize postural syndromes such as OH. It is more sensitive and specific than orthostatic blood pressure maneuvers. It is indicated in patients with symptoms of orthostasis despite normal vital signs or patients who have physical limitations to undergoing orthostatic measurements [36]. Tilt-table testing can also help assess response to treatment and distinguish subtypes of orthostasis such as vasodepressor syncope, cardio-inhibitory syncope, subtypes of OH, and POTS [37].
The Valsalva maneuver can be performed while measuring blood pressure with a continuous non-invasive monitor to assess sympathetic function. The normal physiological response to Valsalva involves four phases with distinct hemodynamic effects on cardiac physiology (Fig. 5) [32, 35]. A larger drop in the blood pressure during phase 2, lack of the blood pressure returning to baseline at the end of late phase 2 recovery period, absence of the expected phase 4 overshoot, and prolonged pressure recovery time are markers of sympathetic dysfunction.
The Valsalva maneuver. The Valsalva maneuver is normally composed of 4 phases: 1, the initial pressure rise in blood pressure during inhalation at the onset of Valsalva strain; 2, initial drop in blood pressure due to decreased venous return followed by compensatory vasoconstriction and rise in heart rate; 3, pressure release of Valsalva causing slight fall in pressure but concomitant rise in venous return; and 4, return of cardiac output and blood pressure to baseline. Adapted from Mariorenzi MC, Matson A, Sonne C (2015) Dynamic auscultation. In: Taylor A. (eds) Learning cardiac auscultation. Springer, London
HRV can help assess parasympathetic and sympathetic function. It is measured in both frequency and time domains [38]. These domains can be tested at rest or with provocative maneuvers such as change in position or with Valsalva.
Using spectral analysis, the frequency of heart rate modulation is separated into high (HF) and low frequency (LF) domains, denoting parasympathetic and sympathetic activity respectively [13]. In comparing the ratios between LF and HF, a larger ratio (> 3.0 in a 35-year-old female, for instance) indicates greater sympathetic activity, although this is not without controversy [19, 20, 39].
The time domain of HRV considers the time interval between heart beats and how this interval varies between successive beats, which can differentiate and characterize sympathetic and parasympathetic modulation of the heart rate. An example of the time domain is the standard deviation of beat to beat intervals (SDNN), which measured over a 24-h interval that can provide information about both the parasympathetic and sympathetic nervous system. Additionally, heart rate variability in response to deep breathing isolates a vagally mediated reflex, thereby acting as a surrogate for parasympathetic function. Heart rate variability in response to deep breathing represents the ratio between the mean of the maximum and minimum instantaneous heart rates while subjects breathe deeply at a set rate for a set number of cycles [40]. The Valsalva ratio measures the ratio of mean electrocardiographic RR interval after a strain compared to during the strain, with a higher ratio representing autonomic adjustment to altering hemodynamics, and suggests intact autonomic function [41].
Baroreflex sensitivity (BRS) has been developed as a means to quantify arterial baroreflexes. Under normal circumstances, baroreflexes serve to trigger sympathetic and parasympathetic responses based on changes in blood pressure to maintain appropriate hemodynamics [42]. An increase in BP should result in reflexive slowing of the heart rate via decreased sympathetic tone and increased parasympathetic activity. BRS measures the change in the inter-beat interval divided by the change in blood pressure. This marker can be obtained non-invasively by comparing HRV and blood pressure variability [43, 44].
Postganglionic sympathetic cholinergic function can be assessed using sudomotor testing. Capsules filled with acetylcholine are placed on the patient’s skin at multiple sites along the extremities. The capsules are stimulated with an electrical current. The amount of sweat output over time is recorded. The degree of AD can be assessed based on the latency, volume, and persistence of sweat response [35].
Pre-ejection period (PEP) is considered to be an indirect measurement of myocardial contractility. It demarcates the period from the onset of ventricular depolarization until the point at which the aortic valve opens [45]. Since contractility is controlled by autonomic input, a decrease in PEP corresponds with increased sympathetic activity or withdrawal of parasympathetic activity.
Scoring Systems
A variety of scoring systems have been developed to characterize the type and severity of autonomic impairment using objective measures. This is helpful not only in determining treatment choices, but also in assessing response to treatment. The Composite Autonomic Severity Score (CASS) takes into account heart rate variability in response to deep breathing, tilt-table testing, Valsalva maneuver, and sudomotor testing to generate an aggregate score with cardiovagal (parasympathetic), adrenergic (sympathetic), and sudomotor (postganglionic sympathetic) subgroups [35] (Table 2). The Ewing scoring system uses the Valsalva ratio, heart rate variability in response to deep breathing, blood pressure response to handgrip, immediate heart rate response to standing, and blood pressure response to standing [28] (Table 3).
Limitations
Overall, these markers are not perfect, nor are they standardized. For example, the Valsalva ratio declines inversely with age and may be difficult to perform correctly in elderly and frail patients [34, 35]. Heart rate variability in response to deep breathing can decrease inversely to percentage of body fat [46]. PEP can be affected by changes in preload and afterload. HRV can have limited usefulness in certain cardiac arrhythmias such as atrial fibrillation or in patients with pacemakers. The high frequency domain of HRV can be influenced by respiratory variation [35, 43]. Thus, the diagnosis of cardiovascular AD remains a challenge.
Potential Mechanisms for Chemotherapy and Radiation-Associated Dysautonomia
Autonomic Nerve Dysfunction/Damage
Peripheral somatic neuropathies are well studied and are common among patients with diabetes, certain chemotherapy exposure, alcoholism, and other metabolic derangements. Somatic sensory fibers are preferentially affected due to the fact that they are surrounded by little or no myelination compared to motor fibers [47, 48]. Similarly, postganglionic autonomic fibers are unmyelinated and preganglionic fibers are lightly myelinated. It is likely that autonomic neurons are susceptible to many of the same mechanisms by which peripheral nerves are damaged in these other conditions. It is plausible that similar processes play key roles in the development of nerve damage in cardiovascular AD associated with radiation and chemotherapy [4] (Fig. 6).
For example, the themes contributing to peripheral somatic neuropathy in the context of diabetes likely overlap with the mechanisms responsible for chemotherapy-associated cardiovascular AD [49]. In diabetes, hyperglycemia results in the activation of several metabolic pathways that increase oxidative stress due to increased mitochondrial activity. As a result, there is an increase in reactive oxygen species (ROS) and reactive nitrogen species that trigger an inflammatory cascade that further results in demyelination, neuronal apoptosis, and axonal atrophy. Although specific mechanisms vary among chemotherapy agents, inflammatory pathways, damage to neurovascular supply, and oxidative stress all contribute [50] (Table 4). Platinum-based agents, taxanes, and proteasome inhibitors cause mitochondrial damage and the release of pro-inflammatory cytokines, which results in oxidative stress and the formation of ROS [51•]. Thalidomides may also cause the formation of ROS through dysregulation of neurotrophins [51•, 52, 53].
As studied outside of oncology, injury to different components of sensory neurons can result in neuropathy. Injury to microtubules within a neuron would alter axonal transport. Taxanes, vinca alkaloids, and epothilones, such as ixabepilone, cause microtubule injury by various mechanisms [54]. Vinca alkaloids, including vincristine and vinblastine, prevent polymerization of dimers to form microtubules, which play an important role in axonal length, arrangement, and orientation. Taxanes, by comparison, reconfigure axonal microtubules, cause direct axonal damage, and impair organelle function [11, 12].
Effects on ion channels can alter signaling and result in neuronal apoptosis [12, 55]. Proteasome inhibitors, such as bortezomib, alter cell signaling pathways through sphingolipid dysregulation, which can cause increases in neuroinflammatory signaling pathways and inappropriate neurotransmitter release [51•]. Moreover, taxanes disrupt intracellular signaling through alterations in calcium homeostasis. Platinum-based agents are also known to alter cell signaling pathways [51•]. These mechanisms that affect the cell signaling of somatic sensory neurons are also likely to play a role in damage to the nerves of the autonomic system.
Thalidomides cause peripheral nerve damage through ischemia and hypoxia of nerve fibers and dysregulation of neurotrophins, which accelerates neuronal cell death [51•, 52]. Platinum-based agents and taxanes also cause voltage-gated ion channel dysfunction in somatic nerve cells. It is plausible that these compounds also cause cardiovascular AD through similar mechanisms.
Furthermore, the autonomic system is composed of parasympathetic and sympathetic components, and understanding which arms of the system are dysregulated could lead to refinement of treatment methods. A number of case reports have described AD associated with chemotherapy, and the etiology has been attributed to parasympathetic nerve damage. One early study identified autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. In this case series, autonomic neuropathy was attributed to damage to the small fibers of parasympathetic nerves, similar to peripheral sensory nerves [14]. Hironven et al. evaluated cytotoxic treatment with vincristine in acute lymphoblastic leukemia patients and found that transient autonomic cardiotoxicity occurred, as reflected by reduced HRV. The authors postulated that reduced HRV represented reduced parasympathetic activity, as the HRV abnormality could be improved with atropine and not beta blockade. Furthermore, they identified similar effects on HRV in diabetic cardioneuropathy, suggesting both chemotherapy and diabetic AD may be reflective of shared mechanisms of damage to the vagal nerve [56].
Direct nerve damage has also been proposed as a mechanism by which radiation contributes to RT-associated cardiovascular AD. Direct radiation to the regions in which the carotid and vagus nerve reside can cause inflammation and ultimately fibrosis, which results in injury to the vagal nerve and the baroreflexes of the carotid sinus [57,58,59,60]. A recent case‑control trial showed that long-term survivors of Hodgkin lymphoma who underwent thoracic irradiation had higher resting heart rates (defined as above 80 BPM; OR of 6.22 in women, 1.90 in men) and more frequent abnormal HRR (31.9% compared to 9.3%) compared to Hodgkin lymphoma survivors who did not receive radiation [24]. Adams et al similarly demonstrated that Hodgkin’s survivors who underwent mediastinal radiation developed persistent tachycardia and impaired blood pressure and HRR to exercise [18]. Radiation to the skull has also been shown to be associated with cardiovascular AD [15, 61].
Thus, we propose chemotherapy and RT can cause cardiovascular AD via similar mechanisms by which somatic sensory nerves are damaged by chemotherapy agents and other disease processes as well as by direct damage to the nerves by radiation.
Precursor to Heart Failure
An alternative explanation for cardiovascular AD associated with chemotherapy and RT is a more indirect relationship. Perhaps chemotherapy and RT cause left ventricular dysfunction, and the cardiovascular AD is related to LV dysfunction, rather than damage to the autonomic system itself. Multiple studies have shown that LV dysfunction is associated with increases in sympathetic drive and parasympathetic withdrawal leading to decreased HRV [62,63,64].
The mechanisms by which chemotherapeutic agents cause heart failure vary based on the type of chemotherapeutic agent. For instance, anthracyclines incite ROS that lead to lipid peroxidation and membrane damage as well as trigger apoptosis pathway in cardiomyocytes. Alkylating agents like cyclophosphamide can cause direct endothelial injury, releasing toxic metabolites that result in injury to cardiomyocytes. Taxanes like paclitaxel, as mentioned, target microtubules, which are also found in cardiac myocytes [65,66,67].
In patients who received anthracyclines, at a mean interval of 29 months from the last cycle of chemotherapy, Tjeerdsma et al. found that HRV was abnormal in 85% of those patients compared to diastolic dysfunction on echocardiography in only 50% of those patients. They concluded that HRV may be an early sign of diastolic dysfunction and a precursor to the downstream development of systolic dysfunction [68]. Ekholm et al. suggested that HRV decreased after paclitaxel use by a similar mechanism [13]. Thus, reduced HRV may not necessarily be direct sequelae of chemotherapy, but may serve as a predictor of incipient cardiac dysfunction.
The changes associated with direct radiation damage apply to not only the nerves, but also all structures within the radiation field. Fibrotic changes from radiation have also been associated with cardiomyopathies [69, 70]. Although difficult to quantify the epidemiology given the rapidly evolving techniques and decreasing radiation requirements, RT-induced cardiomyopathy has been seen in up to 24.8% of patients [71]. It is thought to be due to radiation-induced inflammation that causes interstitial fibrosis of myocardium and effacement of peri-myocyte endothelium. In turn, this leads to diastolic dysfunction and ultimately systolic dysfunction as time goes on. This would likely also manifest with reduced HRV [72,73,74].
Pro-inflammatory State
Another possible mechanism is that cardiovascular AD in cancer survivors is a result of modulation of the ANS due to a pro-inflammatory state without intrinsic nerve damage. Resting heart rates are under tonic inhibitory control by the vagus nerve. Pro-inflammatory states have been shown to dampen vagal activity by excess production of cytokines, including IL-1, IL-6, and TNF-alpha [20]. Therefore, decreased vagal activity will both increase resting heart rate and disrupt the balance of the two inputs of the autonomic system of the heart. In the setting of cancer in general, and even more so with initiation of a chemotherapeutic agent and radiation, pro-inflammatory states are created that may tip sympathovagal balance towards increased sympathetic neurotransmission. A growing body of research suggests that inflammation may be a unifying mechanism underlying a spectrum of cancer and cancer treatment-related symptoms, including autonomic dysfunction, peripheral neuropathy, pain, depression, cognitive dysfunction, and gastrointestinal symptoms. These symptoms are referred to as “sickness behavior,” and have been recreated in with the administration of pro-inflammatory cytokines, such as interleukin-1, tumor necrosis factor-alpha, interferon-alpha, and interleukin-2 in animal models [75, 76]. Conversely, anti-inflammatory agents have been shown to attenuate symptoms such as peripheral neuropathy [77]. Thayer et al. found that increased resting HR and reduced HRV were both independent predictors of leukocytosis and elevated CRP, reinforcing the effects of an inflammatory state [20].
Dobrek et al. examined the relationship between alkylating agents, including cyclophosphamide and ifosfamide, and overactive bladder, a clinical marker of autonomic dysfunction [78]. Patients with symptoms of overactive bladder were found to have decreased HF/LF ratio with respect to the frequency domain of HRV. This too was thought to be due to increased sympathetic drive due to inflammatory cytokine release in the setting of cyclophosphamide and ifosfamide.
Direct radiation to the neck and thorax induces inflammation and ultimately induces damage to the conduction system, coronary arteries, valves, myocardium, and the autonomic system [60, 70, 79,80,81,82]. Several case reports and series have identified atrioventricular blocks and bundle branch blocks in patients who received RT, which may contribute to syncopal and presyncopal events. Slama et al. observed six cases of atrioventricular block 10‑18 years following radiation. They concluded that this conduction disease was most likely due to radiation given the presence of concomitant pericardial involvement, other cardiac or mediastinal radiation-induced lesions, and development of other preceding conduction abnormalities [82]. Additionally, post-mortem autopsies performed on several patients who had undergone radiation for Hodgkin’s disease demonstrated significant fibrosis of the sinoatrial node, atrioventricular node, and the bundles of the conduction system [81]. Other case reports have demonstrated higher rates of lesions affecting the right ventricle and causing right bundle branch blocks [79]. Higher doses of radiation have also been shown to be associated with higher resting heart rates [24]. Combined, these findings suggest a direct relationship between radiation exposure, both with regard to proximity and dose, and the development of inflammation-induced fibrotic lesions. These lesions affect both the sensors and outputs of the autonomic system. Moreover, a latent period has also been observed, with clinical manifestations of arrhythmias and cardiomyopathies years to decades after the cancer and treatment have ceased. Cohen et al. postulated that perhaps initial capillary endothelial injury results in failure of microcirculation and ischemia that triggers a longer, chronic development of fibrosis [81]. Thus, cardiovascular AD in cancer survivors may be due to the overall pro-inflammatory milieu associated with cancer before, during, and after treatment.
Confounding Mechanisms
While each of the mechanisms described may play a part in chemotherapy and radiation-related cardiovascular dysautonomia, confounding mechanisms may also play a role that are difficult to differentiate from treatment-induced effects. One such mechanism is a paraneoplastic syndrome induced by the primary malignancy. Paraneoplastic neuropathies can present before clinical detection of a cancer or after a diagnosis has been made and treatment has been initiated [83]. It is challenging to differentiate a paraneoplastic neuropathy from any of the other concomitant processes including effects related to the primary cancer such as malnutrition, hyperviscosity, or other effects of cancer treatment.
Likewise, in the setting of inflammation from the cancer and treatment, a previously latent underlying autoimmune condition may be unmasked. Although typically autoimmune autonomic ganglionopathy is not a subtle presentation with findings of diffuse autonomic failure characterized by OH, anhidrosis, sicca symptoms, and impaired GI motility, some studies suggest lower ganglionic acetylcholine receptor antibody levels may present with a limited form of dysautonomia. However, acetylcholine receptor antibodies, while highly specific, are only present in 50% of cases and more research is needed to better understand how to detect these autoimmune dysautonomias [84]. Anti-Hu, anti-CV2/CRMP-5, and antiganglionic acetylcholine receptor antibodies are associated with autonomic neuropathies and may be helpful in identifying autoimmune-associated autonomic dysfunction, but are limited in sensitivity and specificity [85].
Treatment of Autonomic Dysfunction
Currently there are few guidelines on how to address cardiac side effects as a result of chemotherapy and radiation [74]. Until further studies are performed, management of neurogenic AD as seen in Lewy body dementia, Parkinson’s disease, diabetes, and amyloidosis may serve as a guide for symptom management (Table 5). Recent guidelines for neurogenic autonomic dysfunction were created by the American Autonomic Society and the National Parkinson’s Foundation. Non-pharmacologic strategies include adequate oral hydration, increased salt consumption, low-intensity exercise, avoiding activities that increase core body temperature leading to vasodilation, compression stockings, and sleeping with a head-up position.
If lifestyle modifications are ineffective, initiation of pharmacologic agents can be considered. FDA-approved medications for neurogenic OH include midodrine, an alpha-1-adrenoreceptor agonist that increases vascular resistance, and droxidopa, a norepinephrine prodrug that is converted to norepinephrine centrally and peripherally [86, 87]. Off-label agents include pyridostigmine, an acetylcholinesterase inhibitor, and fludrocortisone, a mineralocorticoid which increases sodium and water reabsorption [88, 89]. General recommendations are to initiate one agent, uptitrate to maximum doses, and switch to an alternative agent if no improvement as opposed to combining agents. No studies thus far have performed head-to-head comparisons of these agents; thus, a personalized approach based on the suspected mechanism of AD should be tailored to each patient [90]. Other agents that have been trialed are atomoxetine, yohimbine, acarbose, recombinant erythropoietin, octreotide, and desmopressin, but the evidence is only in small or short-term studies [17••].
Conclusion
Cardiovascular AD in the setting of chemotherapy and RT can cause significant debilitation, exemplified by the unrelenting tachycardia with palpitations in the first case and paralyzing dizziness in the second case. The proper methods to identify, diagnose, and treat cardiovascular AD as a result of cancer and oncologic treatments remain largely under-studied. Identifying cardiovascular AD can be challenging given the nonspecific nature associated with its symptoms, particularly in cancer patients, where symptoms of presyncope, dizziness, fatigue, headaches, and cognitive impairment are common for multitudes of reasons, including hypovolemia from chemotherapy-induced nausea, vomiting and poor oral intake, anemias, deconditioning from prolonged bed rest, and anxiety [22]. Elevation in resting heart rate, impaired blood pressure response to exertion, particularly with symptoms—i.e., functional intolerance, palpitations, syncope, presyncope—should all trigger the provider to consider cardiovascular AD in the absence of other etiologies. Subsequent workup may include in office electrocardiogram and orthostatic measurements in addition to 24-h blood pressure and arrhythmia monitoring, exercise stress testing, and echocardiography.
The incidence and causative mechanisms of chemotherapy and RT-induced cardiovascular AD remain poorly understood and need further investigation to better understand chemotherapeutic agents and radiation fields that portend higher risk, with particular attention to the concentration and doses that precipitate cardiovascular AD. This is a challenging endeavor, as any individual patient has typically undergone chemotherapy and radiation with significant variation in regimens, techniques, and doses that makes it difficult to pinpoint culprit triggers.
A better understanding of the mechanisms may afford the possibility of tailoring treatment regimens based on adverse symptoms. For instance, if the mechanism is truly due to an inflammatory state causing neural dysfunction within the ANS, altering sympathovagal balance, coupling cancer treatment with targeted anti-inflammatory agents could minimize symptoms. Furthermore, perhaps early signs of autonomic dysfunction, including delayed OH where blood pressure drops after a longer period of standing or abnormal HRV, may serve as warning signals for impending heart failure and arrhythmias. Early identification of these findings may help prevent further, more permanent cardiac outcomes, including LV dysfunction and permanent fibrosis to baroreflexes and autonomic nerves. In the meantime, however, treatment is largely based on targeting symptoms once they have developed. Thus, delineating between parasympathetic versus sympathetic dysfunction and identifying hyperactivity versus blunted response may help tailor symptomatic treatments.
Data is even more limited regarding the effects of the newer biologic agents and the ANS, including monoclonal antibodies and small molecule inhibitors. One recent publication by Gu et al. described a case of sensorimotor and autonomic neuropathy in a patient with metastatic melanoma following initiation of immune checkpoint inhibitor therapy. They also identified 14 other case reports describing acute neuropathy following immunotherapy, ranging from hyporeflexia to Guillain-Barre syndrome [91]. With increasing use of these agents, there is a definite need for a better understanding of the prevalence and mechanism of their short and long-term adverse effects, including AD.
Clearly, further research is needed to parse out the indirect and direct mechanisms of cancer treatment-related autonomic dysfunction. Specifically, there is a paucity of information to understand the different mechanisms of cardiovascular AD specific to different classes of chemotherapy, doses of radiation, and now biologic agents [13, 92]. Additionally, new, more sensitive markers are needed to identify paraneoplastic and autoimmune confounders, and less cumbersome, more sensitive diagnostic tools must be developed to detect AD. Clinical trials are also needed to test the efficacy of treatments aimed at patients with confirmed AD in order to discover potential benefits in reducing morbidity, mortality, and improvement of quality of life metrics. Undoubtedly, improved understanding of these challenging sequelae of cancer patients can potentially lead to a substantial reduction in both short and long-term debilitating effects associated with cancer, its treatments, and associated cardiovascular AD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Noone A, Howlader N, Krapcho M, Miller D, Brest A, et al. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute; 2018.
Goodman M. Managing the side effects of chemotherapy. Semin Oncol Nurs. 1989;5:29–52.
Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol. 2006;18:24–8.
Mavrogenis AF, Papagelopoulos PJ, Romantini M, Angelini A, Ruggieri P. Side effects of chemotherapy in musculoskeletal oncology. J Long Term Eff Med Implants. 2010;20:1–12.
Perrino C, Schiattarella GG, Magliulo F, Ilardi F, Carotenuto G, et al. Cardiac side effects of chemotherapy: state of art and strategies for a correct management. Curr Vasc Pharmacol. 2014;12:106–16.
Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47.
Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. Journal of neurology. 2002;249:9–17.
Miltenburg N, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer treatment reviews. 2014;40:872–82.
Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15.
Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System. 2008;13:27–46.
Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77.
Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neuroscience letters. 2015;596:90–107.
Ekholm EM, Salminen EK, Huikuri HV, Jalonen J, Antila KJ, et al. Impairment of heart rate variability during paclitaxel therapy. Cancer. 2000;88:2149–53.
Hansen SW. Autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. Bmj. 1990;300:511–2.
Coumbe BGT, Groarke JD. Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep. 2018;20:69.
Terkelsen AJ, Hansen J, Klostergaard A, Otto M, Molgaard H, et al. 2018. [Neurogenic autonomic dysfunction in adults]. Ugeskr Laeger 180.
Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med. 2020;382:163–78 This paper is a fantastic summary of the mechanisms of baroreceptor dysfunction. Many of the mechanism of pathology described in this paper are likely induced by cancer-direct therapies.
Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48.
Shivkumar K, Ajijola OA, Anand I, Armor JA, Chen PS, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594:3911–54.
Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–42.
Ardell JL, Andresen MC, Armor JA, Billman GE, Chen PS, et al. Translational neurocardiology: preclinical models and cardioneural integrative aspects. The Journal of physiology. 2016;594:3877–909.
Munoz E, Marmadgkiolis K, Iliescu C. Miscellaneous syndromes (Takotsubo’s, orthostasis, and differentiation syndrome). In: Herrmann J, editor. Clinical cardio-oncology. Philadelphia: Elsevier; 2017. p. 291–310. Number of 291–310 pp.
Al-Khazraji BK, Shoemaker JK. The human cortical autonomic network and volitional exercise in health and disease. Appl Physiol Nutr Metab. 2018;43:1122–30.
Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, et al. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–83.
Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001;19:369–87.
Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, et al. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Canadian Journal of Cardiology. 2020;36:357–72 This paper used autonomic testing to help predict whether patients suffered from cardiovascular AD from neurodegenerative versus other causes. More refined autonomic testing can help determine the proper course of treatment.
Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Annals of neurology. 2018;83:522–31.
Ewing D, Clarke B. Diagnosis and management of diabetic autonomic neuropathy. British medical journal (Clinical research ed.). 1982;285:916.
Weston KS, Sacre JW, Jellis CL, Coombes JS. Contribution of autonomic dysfunction to abnormal exercise blood pressure in type 2 diabetes mellitus. J Sci Med Sport. 2013;16:8–12.
Low DA, da Nobrega AC, Mathias CJ. Exercise-induced hypotension in autonomic disorders. Auton Neurosci. 2012;171:66–78.
Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873–80.
Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748–52.
Jerian SM, Sarosy GA, Link CJ Jr, Fingert HJ, Reed E, Kohn EC. Incapacitating autonomic neuropathy precipitated by taxol. Gynecol Oncol. 1993;51:277–80.
Gelber DA, Pfeifer M, Dawson B, Schumer M. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst. 1997;62:40–4.
Novak P. 2011. Quantitative autonomic testing. JoVE (Journal of Visualized Experiments):e2502.
Faraji F, Kinsella LJ, Rutledge JC, Mikulec AA. The comparative usefulness of orthostatic testing and tilt table testing in the evaluation of autonomic-associated dizziness. Otol Neurotol. 2011;32:654–9.
Simova I. Role of tilt-table testing in syncope diagnosis and management. E-Journal of Cardiology Practice [Internet]. 2015.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65.
Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–61.
Ekholm E, Rantanen V, Antila K, Salminen E. Paclitaxel changes sympathetic control of blood pressure. Eur J Cancer. 1997;33:1419–24.
Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11–8.
Saint Martin M, Sforza E, Thomas-Anterion C, Barthelemy JC, Roche F. Baroreflex sensitivity, vascular risk factors, and cognitive function in a healthy elderly population: the PROOF cohort. J Am Geriatr Soc. 2013;61:2096–102.
Ottaviani CSD, Davydov DM, Goldstein IB. Autonomic stress response modes and ambulatory heart rate level and variability. Journal of Psychophysiology. 2008;22:28–40.
Swenne CA. Baroreflex sensitivity: mechanisms and measurement. Neth Heart J. 2013;21:58–60.
Newlin DB, Levenson RW. Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16:546–53.
Hrushesky WJ, Fader DJ, Berestka JS, Sommer M, Hayes J, Cope FO. Diminishment of respiratory sinus arrhythmia foreshadows doxorubicin-induced cardiomyopathy. Circulation. 1991;84:697–707.
Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61.
Hoitsma E, Reulen JP, de Baets M, Drent M, Spaans F, Faber CG. Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci. 2004;227:119–30.
Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–53.
Rahman MH, Jha MK, Suk K. Evolving insights into the pathophysiology of diabetic neuropathy: implications of malfunctioning glia and discovery of novel therapeutic targets. Curr Pharm Des. 2016;22:738–57.
Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. International journal of molecular sciences. 2019;20:1451 This paper is a fantastic summary of the mechanisms of chemotherapy-induced peripheral neuropathy. Given the similarities between autonomic fibers and peripheral sensory nerves, many of the same processes may be contributing to chemotherapy-induced cardiovascular AD.
Wani TH, Chakrabarty A, Shibata N, Yamazaki H, Guengerich FP, Chowdhury G. The dihydroxy metabolite of the teratogen thalidomide causes oxidative DNA damage. Chem Res Toxicol. 2017;30:1622–8.
Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–14.
LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–9.
Ganz PA, Dougherty PM. Painful hands and feet after cancer treatment: inflammation affecting the mind-body connection. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:649–52.
Hirvonen HE, Salmi TT, Heinonen E, Antila KJ, Valimaki IA. Vincristine treatment of acute lymphoblastic leukemia induces transient autonomic cardioneuropathy. Cancer. 1989;64:801–5.
Goodman BP, Schrader SL. Radiation-induced cranial neuropathies manifesting as baroreflex failure and progressive bulbar impairment. Neurologist. 2009;15:102–4.
Sharabi Y, Dendi R, Holmes C, Goldstein DS. Baroreflex failure as a late sequela of neck irradiation. Hypertension. 2003;42:110–6.
Kong L, Lu JJ, Liss AL, Hu C, Guo X, et al. Radiation-induced cranial nerve palsy: a cross-sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1421–7.
de Waard DEP, Verhorst PMJ, Visser CA. Exercise-induced syncope as late consequence of radiotherapy. International Journal of Cardiology. 1996;57:289–91.
Kamath MV, Halton J, Harvey A, Turner-Gomes S, McArthur A, Barr RD. Cardiac autonomic dysfunction in survivors of acute lymphoblastic leukemia in childhood. Int J Oncol. 1998;12:635–40.
Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991;18:464–72.
Ishise H, Asanoi H, Ishizaka S, Joho S, Kameyama T, et al. 1998. Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure. J Appl Physiol. 1985;84:1234–41.
Eaton GM, Cody RJ, Nunziata E, Binkley PF. Early left ventricular dysfunction elicits activation of sympathetic drive and attenuation of parasympathetic tone in the paced canine model of congestive heart failure. Circulation. 1995;92:555–61.
Malik B, Stillman M. Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2008;8:56–65.
Adams SC, Schondorf R, Benoit J, Kilgour RD. Impact of cancer and chemotherapy on autonomic nervous system function and cardiovascular reactivity in young adults with cancer: a case-controlled feasibility study. BMC Cancer. 2015;15:414.
Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53:94–104.
Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, et al. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart. 1999;81:419–23.
Lipshultz SE, Sallan SE. Cardiovascular abnormalities in long-term survivors of childhood malignancy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1993;11:1199–203.
Kaplan BM, Miller AJ, Bharati S, Lev M, Martin GI. Complete AV block following mediastinal radiation therapy: electrocardiographic and pathologic correlation and review of the world literature. Journal of Interventional Cardiac Electrophysiology. 1997;1:175–88.
van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Peterson EJ, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–17.
Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;2005:214–23.
Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am JHeart J. 2005;150:977–82.
Cuomo JR, Sharma GK, Conger PD, Weintraub NL. Novel concepts in radiation-induced cardiovascular disease. World J Cardiol. 2016;8:504–19.
Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302.
Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–25.
Bennett GJ. A neuroimmune interaction in painful peripheral neuropathy. Clin J Pain. 2000;16:S139–43.
Dobrek L, Baranowska A, Thor PJ. The influence of oxazaphosphorines alkylating agents on autonomic nervous system activity in rat experimental cystitis model. Acta Pol Pharm. 2013;70:1097–105.
Orzan F, Brusca A, Gaita F, Giustetto C, Figliomeni MC, Libero L. Associated cardiac lesions in patients with radiation-induced complete heart block. International Journal of Cardiology. 1993;39:151–6.
Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73–7.
Cohen SI, Bharati S, Glass J. Radiotherapy as a cause of complete atrioventricular block in Hodgkin’s disease. Arch Intern Med. 1981;141:676–9.
Slama MS, Le Guludec D, Sebag C, Leenhardt AR, Davy J, et al. Complete atrioventricular block following mediastinal irradiation: a report of six cases. PACE. 1991;14:1112–8.
Palma G. Paraneoplastic syndromes of the nervous system. West J Med. 1985;142:787–96.
Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146:3–7.
Koike H, Tanaka F, Sobue G. Paraneoplastic neuropathy: wide-ranging clinicopathological manifestations. Curr Opin Neurol. 2011;24:504–10.
Low PA. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7:451–8.
Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes CY, et al. Norepinephrine pre-cursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–8.
Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, et al. Pyridosgitmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–8.
Provitera V, Nolano M, Pagano A. Acetylcholinesterase inhibition and orthostatic hypotension. Clin Auton Res Off J Clin Auton Res Soc. 2006;16:136.
Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol Epub ahead of print. 2017.
Gu Y, Menzies AM, Long GV, Fernando S, Herkes G. Immune mediated neuropathy following checkpoint immunotherapy. Journal of Clinical Neuroscience. 2017;45:14–7.
Ekholm E, Rantanen V, Syvanen K, Jalonen J, Antila K, Salminen E. Docetaxel does not impair cardiac autonomic function in breast cancer patients previously treated with anthracyclines. Anticancer Drugs. 2002;13:425–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Teng, A.E., Noor, B., Ajijola, O.A. et al. Chemotherapy and Radiation-Associated Cardiac Autonomic Dysfunction. Curr Oncol Rep 23, 14 (2021). https://doi.org/10.1007/s11912-020-01013-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-020-01013-7
Keywords
- Cardiology
- Cardio-oncology
- Oncology
- Autonomic dysfunction
- Dysautonomia cancer survivorship
- Peripheral neuropathy
- Chemotherapy
- Radiation therapy
- Chemoradiation
- Autonomic testing
- Postural orthostatic tachycardia
- Orthostatic hypotension
- Tachycardia
- Syncope
- Presyncope
- Sympathetic
- Parasympathetic
- Baroreflex
- Heart rate variability
- Valsalva
- Tilt test