Abstract
Purpose of Review
Stress urinary incontinence (SUI) remains a strikingly common condition faced by women with substantial economic and quality of life impact. Embarking upon treatment for incontinence often culminates with invasive surgical procedures with recognized complication profiles. Innovative directions for SUI therapeutics are on the horizon, including the utilization of adult autologous muscle-derived cells for urinary sphincter regeneration (AMDC-USR).
Recent Findings
Current published literature presents safety and efficacy data regarding AMDC-USR injection in 80 patients at 12-month follow-up. No adverse events attributed to the cellular product were reported. Compared to lower dose groups, the higher dose groups demonstrated enhanced percentages of patients with at least 50% reduction in stress leaks and pad weight at 12-month follow-up. All dose groups had statistically significant improvement in patient-reported incontinence-specific quality of life scores at 12-month follow-up. Conclusions from the pooled analyses indicate that injection of AMDC-USR across a range of dosages appears safe. Efficacy data suggests a dose response with more patients responsive to the higher doses of AMDC-USR.
Summary
Promising technologies for utilization of autologous cellular therapies for treatment of SUI, and conceivably multiple additional indications, are approaching fruition. Multiple phase III randomized, placebo-controlled studies for AMDC-USR are ongoing to bring this regenerative option forth for the millions of patients who may ultimately benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI), defined by the International Continence Society (ICS) as the involuntary loss of urine on effort or exertion, remains an astonishingly common urologic condition associated with striking clinical and economic sequelae. Despite increasing public recognition, as well as appreciation by the medical community of the impact of SUI, the projected prevalence between 26 and 44% of adult women is likely substantially underestimated secondary to social factors such as embarrassment and fear that preclude open discussion of incontinence symptoms [1•]. Although a diverse suite of pathophysiologic processes contributes to the manifestation of the symptoms of SUI, loss of pelvic floor anatomic support combined with dysfunction of the external urethral sphincter due to both structural and neuromuscular compromise often represents primary etiologies [2, 3].
Strategies for treatment of stress urinary incontinence are currently experiencing a judicious re-evaluation. Our very concepts of the pathologies resultant in SUI symptoms, in addition to the intricacies patients have experienced with regard to treatments, have propagated massive efforts to both revisit the past and define novel technologies to drive future innovations.
Historical Management of SUI
With regard to contemporary management of SUI, a variety of non-surgical interventions including bulking agents are available which generally provide modest improvement and frequently mandate repeat procedures [4••, 5]. Due to the relatively short operative time, generally limited morbidity, rapid convalescence, and long-term efficacy, the synthetic mid-urethral sling (MUS) remains the most widely employed procedure for surgical correction of female SUI [6]. Joint position statements from the Society of Urodynamics, the Female Pelvic Medicine and Urogenital Reconstruction (SUFU), and the American Urogynecologic Society (AUGS) strongly support standard of care use of polypropylene mesh MUS for the treatment of female SUI [7]. However, as previously alluded, surgical management of SUI is undergoing a critical metamorphosis. The current climate of mesh litigation drives both initial patient presentation and clinician management of MUS complications [8]. Although, over the past decade, MUS has overwhelmingly emerged at the primary surgical treatment option for SUI, the autologous fascia pubovaginal sling has experienced resurgence due to the complex environment encompassing MUS complications [9]. Indeed, several countries around the globe are currently prohibiting the use of any transvaginal mesh product, including MUS, until further clarity regarding the long-term risk/benefit profile is established. What is not in question is the mandate that an innovative alternative will be essential to provide durable and safe treatment options for patients with SUI.
Regenerative Technologies for SUI
In the era of regenerative medicine, a novel paradigm shift is underway to substantially modify treatment strategies for SUI. As opposed to our current therapies designed to mask the symptoms of a dysfunctional urethral sphincter closure mechanism, regenerative medicine offers the promise of reversal of the primary pathophysiology of intrinsic urinary sphincter deficiency (ISD) [10, 3]. Indeed, regenerative technologies hold immense promise to create functional tissue to repair or replace damaged organs of many body systems, including myriad of aspects of the genitourinary system [11]. By virtue of their ability to proliferate, self-renew, and regenerate tissues, stem cells encompass ideal attributes for urethral sphincter regeneration. Although continued debate exists regarding pluripotent embryonic stem cells capable of differentiation into an array of cell types, most current therapies utilize somatic multipotent stem cells derived from adult tissues. Such adult stem cells are present in a variety of tissue types and are necessary for local tissue renewal while unable to transdifferentiate into cell types outside their native environment. With regard to SUI, studies have employed adult stem cells which have been commonly isolated from adipose tissue, skeletal muscle, total nucleated cells and platelets, and bone marrow mesenchymal stromal cells [12, 11].
Autologous Muscle-Derived Cells for Urinary Sphincter Regeneration
Augmentation of urethral sphincter function with adult autologous muscle-derived cells (AMDC) represents the most mature potential therapeutic option with data suggesting tissue integration with neuroregeneration, neovascularization, and ultimately improved sphincter function [13]. Proposed mechanisms of action include direct incorporation into the sphincter complex, secretion of chemokines and growth factors, and additionally paracrine effects from adjacent tissues [10]. As will be outlined in the following section, regeneration of the sphincter complex has been demonstrated in animal models and evidence for clinical efficacy and safety continues to be investigated in ongoing randomized phase III trials.
In selected animal models of SUI, intrasphincteric injection of muscle-derived cells displays multiple qualities which provide advantage for treatment of ISD [14]. Unlike bulking agents, AMDC implantation displays durability when compared to collagen implants [15]. Isolated and expanded populations of AMDCs retain the capacity to fuse and form post-mitotic multinucleated myotubes, a distinct advantage over cellular therapies employing muscle-derived fibroblasts [16,17,18]. AMDCs may additionally undergo neuroregeneration and have revealed capability in animal models to improve urethral sphincter function [19]. Indeed, in a cauterization animal model of SUI, incorporation of AMDCs was verified to incorporate into damaged striated muscle and, more critically, improved leak point pressures on urodynamic analysis [20,21,22].
Initial description of clinical efforts for utilization of autologous myoblasts and fibroblasts for treatment of SUI was originally published in The Lancet in 2007, although regrettably, the manuscript was ultimately retracted due to ethical concerns [23].
Pioneering concurrent clinical studies on AMDC for urinary sphincter repair (AMDC-USR) in North America introduced the first iteration of contemporary technology. Eight courageous patients underwent muscle biopsy and AMDC expansion to the desired cell number, purity, and sterility prior to reinjection into their external urethral sphincter [24]. Although principally designed to evaluate safety, at median 17-month follow-up, five remaining subjects reported improvement with one subject displaying total continence. Due to the presumed mechanisms of action to augment the sphincter, such improvements manifested between 3 and 8 months following cellular injection. Most notably in these early evaluations, no serious adverse events were reported with all events consistent with transient complications expected for biopsy and urethral injection. Overall, this preliminary study was strongly encouraging and suggested intrasphincteric injection of autologous muscle progenitor cells has an acceptable safety profile and reveals clinical efficacy.
A subsequent randomized, blinded feasibility study of 38 female patients ensued with inclusion of a small portion of patients receiving two injections [25•]. Dose ranges were explored in this analysis including use of doses up to six times greater than the primary pilot study. Additionally, patients could elect a second treatment of the same dose after their 3-month follow-up. Quantitative measures included pad tests and incontinence diaries which were combined with quality of life measures to assess outcomes. Of the 33 patients who completed the analysis at 18 months, higher cell dosages were associated with improved incontinence with 88.9% of this group experiencing a 50% or greater reduction in baseline pad weight and 77.8% reporting similar reduction in diary-reported stress leaks. Although chosen by relatively few participants, women who underwent two AMDC treatments also established a statistically significant reduction in both pad weight and stress leak frequency. Concomitant improvement trends were demonstrated in quality of life assessments. In addition to the acceptable safety profile, there was a remarkable dose-dependent profile for AMDC-USR.
Subsequent expanded analyses were conducted at the higher dose of AMDC to include additional patients in Canada and US investigative sites [26].
Pooled data in two phase I/II studies with identical selection criteria and outcome measures were analyzed. Enrolled patients displayed SUI refractory to prior treatment with stable symptoms over the past 6 months. Patients received intrasphincteric injection of 10 (n = 16), 50 (n = 16), 100 (n = 24), or 200 × 106 (n = 24) AMDC-USR, derived from biopsies of each patient’s vastus lateralis. The primary outcome measure was safety, determined by incidence and severity of adverse events. Efficacy was measured by changes in 3-day voiding diaries, 24-h pad tests, and patient-reported quality of life measures including the Urogenital Distress Inventory (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7) scores.
In the pooled analysis, 80 patients underwent AMDC-USR injection, with 72 completing diary and pad test data at 12-month follow-up. No adverse events attributed to the product were reported. Compared to lower dose groups, the 100 and 200 × 106 dose groups demonstrated higher percentages of patients with at least 50% reduction in stress leaks and pad weight at 12-month follow-up. All dose groups had statistically significant improvement in UDI-6 and IIQ-7 scores at 12-month follow-up.
Conclusions from the pooled analyses indicate that injection of AMDC-USR at doses of 10, 50, 100, and 200 × 106 cells appears safe. Efficacy data again suggested a dose response with more patients responsive to the higher doses of 100 and 200 × 106 AMDC-USR.
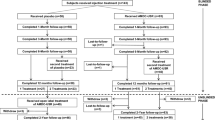
Two subsequent studies were designed to determine AMDC-USR efficacy via double-blind, randomized, placebo-controlled trials (ClinicalTrials.gov Identifiers NCT01382602 and NCT01893138). The first study was conducted at 10 investigative sites within Canada, the UK, and Germany with planned enrollment of 246 subjects randomized 2:1 to receive AMDC-USR or placebo and 1:1 to receive one treatment or two treatments [27]. Although AMDC-USR were again confirmed to be safe and well-tolerated, enrollment was halted due to an unusually high placebo rate using a composite primary outcome. Post hoc analysis employing higher stringency endpoints demonstrated reduction in stress episodes with effective decrease of placebo responder rates [28]. Most interestingly, analysis of a small subpopulation with recurrent or persistent SUI after prior surgical therapy treated with AMDC-USR indicated potential enhanced effect for these women [29]. Overall, this randomized study allowed an exceptional opportunity to enhance our understanding the challenges of progressing from early clinical studies to randomized, controlled trials of a novel treatment paradigm. Multiple lessons from this initiative have allowed subsequent study designs to include more robust inclusion/exclusion criteria and more rigorous efficacy endpoints. Indeed, employing the experiences from this primary study, a second randomized, placebo-controlled phase III study conducted at 29 sites in the USA, Belgium, and Germany just completed enrollment of 311 patients. A confirmatory adaptive, two-stage, double-blind, stratified, randomized, controlled trial with proposed enrollment of 320 patients has recently been accepted by the FDA to complete the data series for final submission for regulatory approval and commercial use of AMDC-USR (ClinicalTrials.gov Identifiers NCT03104517).
Multiple similar initiatives to investigate use of muscle-derived cells have been published, although wide variations in isolation techniques, dosing, and measured efficacy parameters prevent adequate comparison to AMDC-USR [30,31,32,33,34,35].
Conclusions
Accumulating evidence suggests that with the demonstrated safety and efficacy profiles, AMDC-USR represents the next generation of treatments for SUI. Future initiatives for AMDC in the realm of urology include treatment of post-prostatectomy incontinence, fecal incontinence, and detrusor underactivity [36, 37]. With regard to understanding of the mechanism of action, it will be critical to develop strategies to monitor the fate of implanted cells with regard to survival, proliferation, migration, differentiation, and formation of functional muscle. Appreciation of the role of the neuromuscular continence mechanism including the pudendal nerve will undoubtedly prove pivotal [3]. Many questions remain regarding the paracrine role of the implanted AMDC as well as the capacity to augment survival and efficacy with in vitro and in vivo addition of exogenous factors [10]. Additional parameters for continuing consideration for ADMC-USR include durability of treatment response and most prominently, enduring safety.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
• Reynolds WS, Dmochowski RR, Penson DF. Epidemiology of stress urinary incontinence in women. Current Urology Reports. 2011;12(5):370–6. Historically significant manuscript detailing current concepts of SUI etiology.
Petros PE, Woodman PJ. The integral theory of continence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):35–40.
Gill BC, Damaser MS, Vasavada SP, Goldman HB. Stress incontinence in the era of regenerative medicine: reviewing the importance of the pudendal nerve. J Urol. 2013;190(1):22–8.
•• Thompson IM, Kaufman MR. Nonsurgical interventions for incontinence: where is the evidence? Curr Blad Dysfn Rep. 2010;5(3):163–7. Critical guidelines for utilization of surgical therapies for SUI.
Kobashi KC, Albo ME, Dmochowski RR, Ginsberg DA, Goldman HB, Gomelsky A, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J Urol. 2017;198(4):875–83.
Wood LN, Anger JT. Urinary incontinence in women. BMJ. 2014;349:g4531.
SUFU. http://sufuorg.com/docs/news/augs-sufu-mus-position-statement.aspx.
Koski ME, Chamberlain J, Rosoff J, Vaughan T, Kaufman MR, Winters JC, et al. Patient perception of transvaginal mesh and the media. Urology. 2014;84(3):575–82.
Athanasopoulos A, Gyftopoulos K, McGuire EJ. Efficacy and preoperative prognostic factors of autologous fascia rectus sling for treatment of female stress urinary incontinence. Urology. 2011;78(5):1034–8.
Williams JK, Dean A, Badlani G, Andersson KE. Regenerative medicine therapies for stress urinary incontinence. J Urol. 2016;196(6):1619–26.
Hwang K, Lewis S, Lamb DJ. Stem cell research: implications for urology. AUA Update Series. 2010;29(8):77–84.
Vinarov A, Atala A, Yoo J, Slusarenco R, Zhumataev M, Zhito A, et al. Cell therapy for stress urinary incontinence: present-day frontiers. J Tissue Eng Regen Med. 2018;12(2):e1108–e21.
Wang HJ, Chuang YC, Chancellor MB. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J. 2011;22(9):1075–83.
Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19(3):279–87.
Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165(1):271–6.
Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB. The promise of stem cell therapy to restore urethral sphincter function. Curr Urol Rep. 2007;8(5):373–8.
Kwon D, Kim Y, Pruchnic R, Jankowski R, Usiene I, de Miguel F, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68(2):449–54.
Yiou R, Yoo JJ, Atala A. Restoration of functional motor units in a rat model of sphincter injury by muscle precursor cell autografts. Transplantation. 2003;76(7):1053–60.
Yokoyama T, Pruchnic R, Lee JY, Chuang YC, Jumon H, Yoshimura N, et al. Autologous primary muscle-derived cells transfer into the lower urinary tract. Tissue Eng. 2001;7(4):395–404.
Chermansky CJ, Tarin T, Kwon DD, Jankowski RJ, Cannon TW, de Groat WC, et al. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology. 2004;63(4):780–5.
Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62(5):958–63.
Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2003;14(1):31–37; discussion 7.
Kleinert S, Horton R. Retraction--autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: a [corrected] randomised controlled trial. Lancet. 2008;372(9641):789–90.
Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):881–3.
• Carr LK, Robert M, Kultgen PL, Herschorn S, Birch C, Murphy M, et al. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J Urol. 2013;189(2):595–601. Pooled data from phase I/II studies on safety and efficacy of AMDS-USR.
Peters KM, Dmochowski RR, Carr LK, Robert M, Kaufman MR, Sirls LT, et al. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol. 2014;192(2):469–76.
Carr L, Tu LM, Robert M, Quinlan D, Carlson K, Herschorn S, Dmochowski R, Peters K, Kaufman M, Jankowski R, Chancellor M. Autologous muscle derived cells for urinary sphincter repair for recurrent or persistent stress urinary incontinence after continence surgery. AUA Annual Meeting; 2017; Boston.
Pruchnic R, Jankowski R and Kaufman MR. Lessons learned from a multicenter, randomized, double-blind, placebo controlled study of autologous muscle derived cells for urinary sphincter repair. International Continence Society Annual Meeting. 2017; Florence, Italy.
Quinlan D, Carr L, Tu LM, Robert M, Carlson K, Herschorn S, Dmochowski R, Peters K, Kaufman M, Jankowski R and Chancellor M. Autologous muscle derived cells for urinary sphincter repair for recurrent or persistent stress urinary incontinence after continence surgery. IUGA. 2017; Vancouver, Canada.
Blaganje M, Lukanovic A. Intrasphincteric autologous myoblast injections with electrical stimulation for stress urinary incontinence. Int J Gynaecol Obstet. 2012;117(2):164–7.
Blaganje M, Lukanovic A. Ultrasound-guided autologous myoblast injections into the extrinsic urethral sphincter: tissue engineering for the treatment of stress urinary incontinence. Int Urogynecol J. 2013;24(4):533–5.
Sebe P, Doucet C, Cornu JN, Ciofu C, Costa P, de Medina SG, et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. Int Urogynecol J. 2011;22(2):183–9.
Elmi A, Kajbafzadeh AM, Tourchi A, Talab SS, Esfahani SA. Safety, efficacy and health related quality of life of autologous myoblast transplantation for treatment of urinary incontinence in children with bladder exstrophy-epispadias complex. J Urol. 2011;186(5):2021–6.
Stangel-Wojcikiewicz K, Jarocha D, Piwowar M, Jach R, Uhl T, Basta A, et al. Autologous muscle-derived cells for the treatment of female stress urinary incontinence: a 2-year follow-up of a Polish investigation. Neurourol Urodyn. 2014;33(3):324–30.
Gras S, Klarskov N, Lose G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J Urol. 2014;192(3):850–5.
Gerullis H, Eimer C, Georgas E, Homburger M, El-Baz AG, Wishahi M, et al. Muscle-derived cells for treatment of iatrogenic sphincter damage and urinary incontinence in men. ScientificWorldJournal. 2012;2012:898535.
Levanovich PE, Diokno A, Hasenau DL, Lajiness M, Pruchnic R, Chancellor MB. Intradetrusor injection of adult muscle-derived cells for the treatment of underactive bladder: pilot study. Int Urol Nephrol. 2015;47(3):465–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Kaufman serves as the Global Principal Investigator for Cook Myosite.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stress Incontinence and Prolapse
Rights and permissions
About this article
Cite this article
Kaufman, M.R. Autologous Muscle-Derived Cells for Urinary Sphincter Regeneration: Where are we now?. Curr Bladder Dysfunct Rep 13, 252–256 (2018). https://doi.org/10.1007/s11884-018-0486-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-018-0486-z