Abstract
Purpose of Review
Plant-derived foods are one of the most common causative sources of food allergy in China, with a significant relationship to pollinosis. This review aims to provide a comprehensive overview of this food-pollen allergy syndrome and its molecular allergen diagnosis to better understand the cross-reactive basis.
Recent Findings
Food-pollen cross-reactivity has been mainly reported in Northern China, Artemisia pollen is the major related inhalant source, followed by tree pollen (Betula), while grass pollen plays a minor role. Pollen allergy is relatively low in Southern China, with allergies to grass pollen being more important than weed and tree pollens. Rosaceae fruits and legume seeds stand out as major related allergenic foods. Non-specific lipid transfer protein (nsLTP) has been found to be the most clinically relevant cross-reacting allergenic component, able to induce severe reactions. PR-10, profilin, defensin, chitinase, and gibberellin-regulated proteins are other important cross-reactive allergen molecules.
Summary
Artemisia pollen can induce allergenic cross-reactions with a wide range of plant-derived foods in China, and spring tree pollens (Betula) are also important. nsLTP found in both pollen and plant-derived food is considered the most significant allergen in food pollen cross-reactivity. Component-resolved diagnosis with potential allergenic proteins is recommended to improve diagnostic accuracy and predict the potential risk of causing allergic symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food allergy is an increasing public health problem worldwide. Up to 60% of food allergies in older children, adolescents, and adults are related to inhalant allergy due to cross-reactivity [1]. China accounts for over 20% of the world’s population, and allergy is now a serious health problem, affecting more than 400 million people [2, 3]. The overall prevalence of food allergy in China is similar to Western countries (5–8%) [4], with geographic variation of 3.1–11.9% in Northern and 3.5–21.1% in Southern China [4]. The observed incidence in children is significantly higher than in adults [5]. Plant-derived foods, particularly fruits, are among the most common causative sources of food allergy in China [6,7,8], which is significantly related to pollinosis. Pollen allergy can also induce allergenic cross-reactivity with vegetables, nuts, cereals, legumes and herbs used in Chinese medicine [9,10,11,12,13,14,15,16,17,18]. Recent studies have shown that the clinical symptoms of pollen-food allergy vary from mild to moderate and in some cases may even be life-threatening [19].
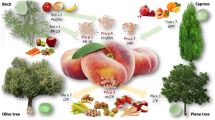
China is a vast territory, with significant differences in environment, climate, and dietary habits between the Northern and Southern areas, resulting in different allergy patterns (Fig. 1). Plant-food allergy is often associated with pollen allergy patterns which are dependent on the geographic distribution of the major allergenic pollen sources. Northern and Western China have much higher prevalence of weed, grass and tree pollens, with the most dominant species for each group being Artemisia spp. (50%), Cynodon dactylon (31%), and Ulmus spp. (41%), respectively. Other allergenic pollen sources include Humulus spp., Broussonetia papyrifera., Chenopodium spp., Betula spp., Juniperus spp., Platanus spp., Alnus spp. and Cryptomeria spp. Pollen allergy in Southern China is relatively low, with allergies to grass pollen being more important than weed and tree pollens [20, 24]. Artemisia pollen allergy is the major seasonal allergic respiratory disease of late summer and autumn in a very large area north of the Yangzi River and South-Western China [25, 26]. The prevalence of food allergy is very low in patients with grass pollen allergy [27], but much higher among Artemisia pollen allergic patients [9, 28], resulting in the higher prevalence of plant-derived food allergy in Northern compared to Southern China.
This study provides a comprehensive overview of food-pollen allergy syndromes and molecular allergen diagnosis including component resolved diagnosis (CRD), to better understand allergenic cross-reactivity in China, aiming to improve allergenic risk assessment and allergy prevention.
Aspects of Allergenic Food-Pollen Cross-Reactivity in China
Cross-Reactivity
Allergy caused by cross-reactivity refers to when a specific antibody (sIgE) can respond to two different allergens which have a similar secondary and/or tertiary structure [29, 30]. It occurs in a patient reacting to highly identical iso-allergens from the same species or to similar allergens from different sources through a single specific IgE antibody type. A single bi-valent allergenic protein (carrying two specific IgE-epitopes) can bridge two such IgE antibodies on the surface of mast cells and basophils, leading to the release of inflammatory mediators, such as histamine. A single protein with only one (monovalent) IgE-epitope may acquire allergenicity after di-/multimerization (bi-/multivalent). A protein from any source with a certain degree of similarity to a primary sensitizing allergen can also act as an allergen, without having sensitizing ability itself, if it has exposed amino acid residues that match the conformational IgE-epitope/s of the sensitizing allergen. This makes such proteins allergenically cross-reactive. The cross-sensitivity and cross-reactivity phenomenon is highly relevant in the allergology field because it moves the focus from individual allergenic sources, e.g. Artemisia pollen and peach fruit, to common protein molecules with similar biochemical and immunological characteristics. IgE antibodies induced by the primary sensitizing source will generally bind with the highest affinity to the original sensitizing allergens [31]. Here we extracted diagnostic IgE data to show the correlation of sIgE values to pollen and fruit allergen components in China (Fig. 2). The induced IgE positivity and values are patient-specific related to epitope recognition, which have consequences for the degree of IgE binding affinity to a cross-reactive allergen, and for the intensity of the cross-reactive allergic response in an individual patient.
The correlation of sIgE values to pollen and fruit allergen components. Figure 2A correlates the specific serum IgE responses of individual patients against the allergens Mal d 1 (from apple, Malus domestica), Pru p 1 (peach, Prunus persica) and Bet v 1 (from birch, Betula pendula ( Betula verrucosa); Fig. 2B, to the fruit allergens Pru p 3 (from peach, Prunus persica) and Art v 3 (from Artemisia vulgaris); and Fig. 2C, to the allergens Man i 4 (from mango, Mangifera indica) and Art an 4 (from Artemisia annua). The data is from our previous studies and published references: A [32, 33]; B [11]; C [34]; **P < 0.01, ***P < 0.001
Allergen molecules with similar sequential or conformational structures are the basis of cross-reactivity. Higher sequence homology increases the likelihood of cross-reactivity. An attempt to set a firm identity threshold is challenging, but generally, having more than 50% sequence identity to the primary protein sequence is considered a reliable guideline. With the increasing importance of novel foods, predicting cross-reactivity based on sequence similarity with known allergens using bio-informatics has become a common tool [35].
Food- Pollen Allergy Syndrome Reported in China
There is no accurate measure of food allergy prevalence in China, but plant-derived foods have been recognized as the major cause. Among patients from Northern China with anaphylaxis induced by foods, 77% are allergic to plant-derived foods, the most common food allergens being wheat (37%) and fruits/vegetables (20%), with peach the dominant fruit allergen and mugwort as the most frequent allergenic pollen [36]. Fruit and vegetable allergy has increased dramatically over the last 20 years, and is currently estimated at 3–5% in Taiwan [37]. In Chinese patients, peach and mango have been shown to be the major allergenic fruits [6,7,8, 38, 39]: both are associated with Artemisia and birch pollen allergy [11, 14, 34].
Studies on food-pollen allergy in China have been mainly in Northern regions, where the exposure to weed and tree pollen is high, while in Southern China, the major inhalant allergen originated from house dust mites, the positivity and clinical relevance of pollen allergy are low [20, 40, 41]. About 30.0–49.4% of pollinosis patients have reported plant-food allergies in Beijing, Northern China, and over 20% experienced food-induced anaphylaxis, especially associated with weed and tree pollen allergies [27, 42]. In pollen-allergic children with anaphylaxis from Northern China, 22.3% report fruit/vegetable allergy, with Artemisia pollen being the major sensitizing pollen (93.5%), followed by ragweed (65.8%), and birch (40.7%). Peach, mango, and dragon fruit are the number one triggers for anaphylaxis in children with combined Artemisia and Betula pollinosis [19, 36].
The most frequent clinical symptoms of food-pollen allergy fit in with the oral allergy syndrome (OAS), urticarial, respiratory and gastrointestinal symptoms [19, 43,44,45]. Some patients also suffer hypotension and anaphylaxis [14, 19]. The symptoms are associated with the allergenic pollen sources: in China, the Artemisia pollen-related food allergy tends to cause more severe and systemic reactions [14].
Season-Related Plant-Food Allergies
Based on the pollen season, pollinosis patients can be divided into three groups: spring-tree pollinosis, autumn-weed pollinosis, and combined spring and autumn groups (especially in Northern China) [42]. Cross-reactive food allergy occurs most often in single-season pollen allergy [42]. Grass pollinosis is less frequent or not significant compared to tree and weed pollinosis. Table 1 lists the food-pollen allergy syndrome reported in China, where Artemisia pollen-related food allergies (to peach, apple, mango, peanuts, plum, apricot, etc.) are the most dominant [9, 11, 14, 36, 44].
Artemisia produces the most prevalent allergenic autumn pollen. This genus is widely spread in China, with more than 180 species [46]. In China, 26.7% of patients with allergic respiratory diseases are allergic to Artemisia [20]. Ten groups of allergen components from various Artemisia spp. have been characterized, and groups 1 (Defensin-like protein), 3 (non-specific lipid transfer protein, nsLTP), and 7 (putative galactose oxidase) identified as the major allergen molecules in China [46,47,48]. Due to the presence of pan-allergens (nsLTP and profilin), there is a high degree of cross-reactivity between Artemisia spp. and various plant-derived foods, as shown in Table 1, especially between Artemisia and Rosaceae fruits. Seventy-two percent of mugwort-allergic patients report food allergy, with the major allergenic foods being peach (68%), apple (24%), mango (20%), and peanut (16%); 48% of the patients experience anaphylaxis [14]. This differs from the nsLTP syndrome in the Mediterranean region where food nsLTP allergen, especially peach allergen Pru p 3, is the primary sensitizer [49], Artemisia pollen has been identified as the primary cross-reactive sensitizer in Northern China. However, a recent study found that food, notably peach [50], can also be the primary sensitizer, but this needs further confirmation to distinguish between co-sensitization and cross-reactivity. nsLTP has been shown to be the major cross-reacting allergen, tending to induce systemic reactions due to its high molecular stability [11, 14, 42, 45]. Profilin can induce cross-reactivity between Artemisia and tropical fruits (e.g. mango, litchi, pineapple) [51]. Other Artemisia allergens can also induce cross-reactivity: Art v 1, Art v 2, and Art an 7 have been identified as the possible allergen components inducing cross-reactivity between mugwort and kidney beans [52]. Artemisia-allergic patients are also potentially allergic to Chinese herbal medicine ingredients, such as pollens of Chrysanthemum, Artemisia apiaceae, Artemisia argyi, Lonicera japonica, and Carthamus tinctorius [53, 54].
The prevalence of birch pollen sensitization is between 7 and 25% in China, mainly in Northern and Central regions [55, 56], with Bet v 1 (82.4%) being the major cross-reactive allergen, 75.9% of birch pollen allergic patients report food allergy in Northern China, of which 72.7% are allergic to apple [18]. In addition, of the Bet v 1-positive patients, 78.8% are also sensitized to the soybean allergen Gly m 4 [33]. All patients from Northern China with spring pollen allergy and experiencing food allergy are allergic to birch pollen and Bet v 1, and a strong correlation of sIgE levels between Bet v 1 and Mal d 1, Pru p 1 was observed (Fig. 2A). Patients mono-sensitized to birch pollen do not suffer from food-induced anaphylaxis [42]. The profilin in birch pollen (Bet v 2) is also able to induce wide cross-reactivity with plant foods, such as litchi [57]. Besides birch, related tree-plant species in Southwestern China also need further investigation, such as Alnus nepalensis which is abundant in the Yunnan province and induces pollen allergy [58, 59]. Other tree and weed pollens can also lead to cross-reactivity with plant-foods, such as peanut allergy caused by pollen of Platanus and Juglans [60].
Genuine grass pollen allergy in China has been found to be relatively low [61]. Most grass pollen-sensitized patients experience other weed or tree pollen allergies, with profilin and cross-reactive carbohydrate determinants (CCD) identified as significant contributors [27]. This is a good indication that cross-sensitization between grass pollen and plant-foods in China is probably caused by these pan-allergens. CRD should be used to distinguish between genuine and spurious grass pollen allergy.
Clinical Diagnosis with Allergen Molecules
The majority of cross-reacting plant allergens are involved in the plant defense system [62], such as the pathogenesis-related (PR) proteins PR-10 (e.g. Bet v 1) and PR-14 (nsLTP). Currently, clinical diagnosis still relies on crude allergen extracts, which have low levels of allergenic molecules, and are challenging in terms of standardization. Moreover, cross-sensitization and genuine allergies cannot be distinguished using these extracts, resulting in limited diagnostic efficiency. The increasing number of available allergen molecules creates novel diagnostic opportunities. Highly purified native or recombinant allergens are increasingly used for in vitro diagnosis, known as component-resolved diagnosis (CRD) [63]. CRD overcomes the disadvantages of traditional extract-based diagnosis, and has become an important tool for personalized and accurate diagnosis. Below we describe relevant molecular characteristics of the major cross-reactive plant allergens.
PR-10 Related Food Allergies
PR-10 is an acidic protein with a molecular weight of 16–18 kDa. It has low thermal stability and proteolytic resistance [64], and thermal treatment can easily decrease allergenicity. Patients with PR-10 allergies usually have OAS [65]. The major birch pollen allergen Bet v 1 was the first PR-10 allergen identified, with 56–59% similarity to homologous allergens from several Rosaceae fruits, causing relatively high cross-reactivity. In China, some birch pollen allergic patients subsequently developed Rosaceae fruit allergy, with the sIgE levels of Bet v 1 in general higher than Mal d 1 or Pru p 1 (Fig. 2A), indicating primary sensitization from Bet v 1. Peach Pru p 1 and apple Mal d 1 cross-react with the Bet v 1 homologue from local birch pollen allergen in Northern China, resulting in mild OAS, similar to the situation in Central and Northern Europe [33, 44, 45, 48, 66]. Since there are a limited number of birch trees planted in populous urban regions, Pru p 1 and Mal d 1 positivity is very low in Northern China. Bet v 1-related allergies are also rare in Southern China, where birch trees are virtually absent. In addition to Mal d 1 and Pru p 1, cross-reactivity occurs between Bet v 1 and other plant-foods, such as soybean Gly m 4. Bet v 1 also appears to be the potential sensitizer causing cross-reactivity with the mango allergen Man i 2 [34].
nsLTP Related Food Allergies
nsLTP is a class of small molecular proteins that can be divided into nsLTP1 (9–10 kDa) and nsLTP2 (6–7 kDa). Most allergens belong to nsLTP1, with an isoelectric point close to nine [67]. The protein contains four α-helices connected by disulfide bonds that fold into a stable compact structure [68]. LTP is usually accumulated in the outer epidermal cell layers of plants: it has high stability and resistance to thermal processing and pH changes. It can cause severe allergic reactions through respiratory or gastrointestinal contact [69]. Unlike the highly conserved PR-10 allergen, nsLTP allergens from different sources have significant sequence variations with similarity ranging from 25 to 67% [70]. LTP is a pan-allergen widely present in numerous plant tissues and is the major allergen involved in pollen-food cross-reactivity in China [11, 14]. Pru p 3 and Art v 3 (Fig. 2B) are the most representative nsLTP allergens. LTP from Artemisia pollen is considered the biomarker for severe food-pollen allergies in China [11, 14, 44, 71]. In Northern China, over 90% of peach allergic patients are sensitized to Pru p 3 due to cross-reactivity to Art v 3 homologous pollen allergens from different Artemisia spp. In these patients, Art v 3 sIgE titers are higher than those against Pru p 3 (Fig. 2B), as is also demonstrated by homologous and heterologous ImmunoCAP inhibition experiments [11]. These results support the hypothesis of Artemisia pollen nsLTP being the primary sensitizer for Pru p 3-allergy in Northern China. Some Pru p 3 sensitized patients from Southern China reported more severe symptoms when they consumed peach varieties of high Pru p 3 content after traveling to Northern China during the peak Artemisia pollen season. This may be explained by Artemisia nsLTPs enhancing the specific IgE production, as exposures to Art v 3, for example, have been shown to induce subsequent sensitization to peach Pru p 3 in mice [72].
Artemisia pollen nsLTPs are the dominant primary sensitizers for peach and other food (fruit) allergens in China. With the amino acid composition and structural knowledge available for Artemisia nsLTPs, sequence identity searches can provide further insight into the mechanism of cross-sensitization to peach. Alignment of different nsLTP sequences showed two highly conserved regions around two lipid-binding motifs (Fig. 3). These two regions have been identified as major T-cell epitopes for both Art v 3 and Pru p 3 [73, 74]. IgE binding epitopes identified for Pru p 3 and Art v 3 are located mainly in two regions (33-47aa, 72-82aa) and a C-terminal lysine residue (K). These regions overlap with the most conserved amino acids of both nsLTPs, suggesting a role as cross-reactive sites [75,76,77]. Following on from the first epitope region, the alanine residue 48A is unique in Art an 3 from A. annua species in China, which is identical in Pru p 3 and the majority of other plant food nsLTPs. We hypothesize this alanine plays a pivotal role in primary sensitization, and may explain why cross-sensitization to plant food nsLTPs occurs so frequently in Northern China, Japan, Korea and Mediterranean countries, where there is widespread distribution of A. annua [78,79,80,81]. Art an 3 is worth investigating as its IgE binding epitope 42–54 is identical to Pru p 3 and other food nsLTPs (Fig. 3). This may contribute to the high prevalence of cross-reactive peach allergy related to Artemisia-nsLTP sensitization in Northern China. Peach fruit and Artemisia pollen allergy is also common in Mediterranean countries, but peach has been considered as the primary sensitizer [65, 81,82,83]. However, A. annua is the second key pollen source in Southern Europe, after A. vulgaris [78], its impact on airway and nsLTP-related food allergy should be reconsidered.
Amino acid sequence comparison of different food and pollen nsLTP allergens.Sourced from the WHO/IUIS database (www.allergen.org), only Chinese bayberry (Morella rubra) LTPs were derived from genome sequences (https://www.ncbi.nlm.nih.gov/Traces/wgs/RXIC02). The most conserved regions in different nsLTPs are boxed in red; LTP sequence variants from different Artemisia spp. pollens are in green. Blue lines indicate T cell epitope regions [73, 74] and
 as Pru p 3 IgE binding sites [75, 76],
as Pru p 3 IgE binding sites [75, 76],
 as Art v 3 IgE binding epitopes [77]. Purple bars indicate conserved lipid binding motif position. Minor modification of Fig. 2 at page 225 from Gao ZS, Molecular approaches to peach and Artemisia pollen allergies in China, PhD thesis, University of Amsterdam, 15-Feb. 2022. https://dare.uva.nl/search?identifier=cf8ec9ea-5899-43d7-b4b8-370a83ab3c49
as Art v 3 IgE binding epitopes [77]. Purple bars indicate conserved lipid binding motif position. Minor modification of Fig. 2 at page 225 from Gao ZS, Molecular approaches to peach and Artemisia pollen allergies in China, PhD thesis, University of Amsterdam, 15-Feb. 2022. https://dare.uva.nl/search?identifier=cf8ec9ea-5899-43d7-b4b8-370a83ab3c49
Apart from fruits, cross-reactivity between Art v 3 and peanut allergen Ara h 9 is also significant in Northern China: of the peanut-allergic patients, 66.7% had a specific sensitivity to only Ara h 9, excluding all other peanut allergens. All of them are also sensitized to Art v 3, and a significant correlation of IgE values is observed [84], suggesting a strong cross-reaction.
The greater sequence diversity and higher exposure to Artemisia pollen nsLTP isoforms in China may further increase the possibility and wide range of cross-reactivity to plant foods such as peach, apricot, plum, cherry, apple, pear, chestnut, gouji, Chinese bayberry, peanut, beans, sunflower seed maize and blueberry. The utilization of purified natural or recombinant “China-specific” iso-allergen components and variants of Artemisia nsLTPs will facilitate further investigation of the clinical relevance for Artemisia pollen-associated food allergies and to develop a hypoallergenic vaccine.
Profilin Related Food Allergies
Profilin is a small molecular protein (12–16 kDa) with poor thermal stability and proteolytic resistance, expressed in almost all plants. This protein is highly conserved between different plants and responsible for the extremely common cross-reactions between pollen and plant-derived foods. Pollen-source profilin is the primary sensitizer [85]. Although profilin is known as a minor allergen in most plants and with low abundance, its clinical relevance has been debated [86], and recent studies have shown that profilin can act as a major allergen in certain plants causing clinical symptoms, such as Cit s 2 in citrus [87], Cuc m 2 in melon [88], and Pla a 4 in Platanus acerifolia pollen [89]. Cross-reaction between Artemisia pollen and various fruits (such as peach, mango, litchi, pineapple, etc.) induced by profilin has previously been demonstrated (Fig. 2C) [34, 51]. In birch, the profilin Bet v 2 is also an important pan-allergen which leads to wide cross-reactions with foods [57]. Because the amount of food ingested is much higher than the inhaled pollen, so the potential risk of food profilin is also higher. Besides foods, profilin can also cause the cross-reaction between different pollens, for example between mugwort and ragweed, Bermuda and timothy grasses [90, 91].
Defensin-Related Food Allergies
Plant defensins are classified within the PR-12 protein family, mainly expressed in peripheral cell layers [92]. Artemisia pollen defensin (Art v 1, Art an 1, etc.) is the most representative defensin allergen and is the major Artemisia allergen in China [48, 93]. Although there is no direct evidence from scientific research for this group of allergens being involved in pollen-food allergy in China, research from other countries has indicated the possible association with severe reactions [94]. IgE inhibition assays indicate cross-reactivity between Art v 1 and the celery defensin allergen Api g 7 [95], mango allergen [96] and sunflower seeds [97], with Art v 1 being the potential primary sensitizer. Defensin has also been identified as a food allergen in peanut (Ara h 12 -13) and soybean (Gly m 2) [98]. Considering the wide cross-reactivity between Artemisia pollen and plant foods, and the high prevalence of Art v 1 in Chinese patients, its effect in China needs further investigation.
Gibberellin-Regulated Protein(GRP)-Related Food Allergies
GRP is a small (7–8 kDa), cysteine-rich, highly conserved, heat-stable, and digestion-resistant protein, which can induce severe symptoms [99]. It has been identified as a food allergen in peach (Pru p 7), cherry (Pru av 7), apricot (Pru m 7), chili (Cap a 7), and as a pollen allergen in cypress (Cup s 7) and Japanese cedar (Cry j 7). Food GRP allergens didn’t show any cross-reactivity with Artemisia allergens. Peach GRP-sensitization is probably due to the Pru p 7 allergen itself [99], although primary sensitization to cypress pollen has been presumed in France [100] and Japan [101]. The local junipers (Juniperus chinensis) and cedar (Cryptomeria spp.) pollens are the most common causes of spring pollen allergies in China, also related to clinical symptoms of food allergy in China. GRP is a serious candidate requiring further confirmation.
Other Allergens Related to Food Allergies
For Chinese patients, chitinase has been identified as the major mango allergen (Man i 1) [34], and it is also an important allergen in latex and Japanese cedar pollen. Chitinase is also found in A. annua, sharing 57% sequence identity with Man i 1 in mango, however, their potential cross-reactivity to pollen needs further study. We noticed the instability nature of fruit chitinase which affected the diagnosis.
Cross-reactivity between the ragweed pollen enolase (Amb a 12) and peach, apple, kiwi has been demonstrated [102], and recently, new enolase allergens from A. sieversiana and plane tree pollen [103] have been identified in China, sharing 87% and 86% sequence identity, respectively, with Amb a 12. This suggests that enolases might be pan-allergens in pollen and plant-foods, requiring further investigation to determine the cross-reaction with foods in China.
CCDs are clinically irrelevant cross-reactive carbohydrate determinants, causing spurious cross-sensitization in pollen and food. Carbohydrate determinants used as inhibitor could prevent false positive results in pollen and food allergy tests and improve the diagnostic accuracy [104, 105].
Diagnostic Procedures and Methods in Food-Pollen Cross-Reactive Allergies
Diagnostics
Food-pollen allergies are clinically under-diagnosed as most food-allergic patients tend to avoid the corresponding foods, with most information obtained from self-reporting and questionnaires, whereas only some severe food allergy cases are diagnosed by clinicians. Skin-prick test (SPT) and in vitro sIgE measurement with fresh food or crude extract are the most common diagnostic tools in China. However, they do not establish the existence and origins of cross-reactivity, overall resulting in poor accuracy with regard to the present data. Currently, only a few common allergen components have been used in clinical diagnosis, and single-plex platform (Phadia, etc.) is the major method of in vitro sIgE measurement.
The progress in biotechnology provides the possibility of producing substantial quantities of pure recombinant allergens as a tool for precise diagnosis in vitro or in vivo and for better treatment and prevention of allergenic disorders [106]. Advancements have been made in specific IgE tests using multiplex platforms (such as solid microarray chip), which enable simultaneous testing of IgE reactivity to a large number of allergens from various selected sources [107]. One of the benefits of this technology is that only very small amounts of allergens are required [108]. This method is useful for those patients with multiple and complicated allergen sensitizing patterns. However, the multiplex platform system has been more often used in research settings than in clinical practice, but it is more suitable for the identification of cross-reactivity [107].
CRD has been used in peach [45], mango [34], peanut [84], Artemisia [48], birch [2], walnut [2], and timothy grass [61] in China, and some promising results have been obtained. However, more CRD data is needed to get a comprehensive spectrum for the development of more effective CRD diagnostic panels for Chinese patients.
The basophil activation test (BAT) is also a powerful tool. In Artemisia-related peach-allergic patients, BAT with Pru p 3 using CD63 as a biomarker can discriminate between systemic reaction and Pru p 1-caused OAS [109]. It must be emphasized that all the measurements need to be combined with a detailed clinical history for a final interpretation.
Prevention
Based on CRD results, the risk of symptoms in patients can be predicted, which enables timely implementation of corresponding preventive and treatment measures: patients with nsLTP allergy are at a higher risk of severe allergic reactions. This makes it necessary to strictly avoid the corresponding allergens, and receive appropriate medication and immunotherapy treatments (as shown Fig. 4). A. annua sublingual immunotherapy has been developed in China and has proved to be an effective and safe treatment [110,111,112], its effect on mugwort-related food allergy needs further investigation.
Nowadays, the majority of CRD reagents used in China are from Europe. While these reagents are generally suitable for Chinese patients, their IgE binding capacity may be lower than that of the homologous allergen components from local sources [46]. However, for certain specific allergic sources, locally sourced regents are necessary. To address the specific food-pollen allergy pattern in China, more relevant allergen components, especially for the Artemisia-related molecules, should be identified and produced to construct a high-throughput CRD platform which is suitable for Chinese patients.
Future Perspectives
Food-pollen allergies are under-diagnosed in China due to the lack of effective diagnostic tools and insufficient identification of the relevant allergens. Further efforts should be made to establish a more comprehensive panel and develop efficient diagnostic and treatment approaches. In China, many universal and local plant foodstuffs have been reported as allergenic sources. Some established allergens from Europe can be employed as starting references to confirm their allergenicity and to characterize the molecular and immunological properties of newly identified allergens in China, including their cross-reactivity with pollens. Other novel allergens and special iso-allergens will follow if associated allergies become a public health concern. Some companies are attempting to develop allergen molecular diagnostic platforms and protocols to fill the gap between research and clinical needs.
This review summarizes the food-pollen allergy syndrome and its molecular basis in China, providing effective information for clinical diagnosis and treatment. With the increase in CRD information obtained in China, further analyses can facilitate the development of algorithms and models to better predict the risk of severe allergic reactions. This risk may eventually be quantified using predictive formulas to help clinicians manage allergic diseases more effectively.
Data Availability
No datasets were generated or analysed during the current study.
References
Werfel T, Asero R, Ballmer-Weber BK, et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70(9):1079–90.
Xu LN, Luo WT, Lu Yh, et al. A comprehensive analysis of the components of common weed pollen and related allergens in patients with allergic diseases in southern China. Mol Immunol. 2022;147:180–6.
D’Souza N, Weber M, Sarzsinszky E, et al. The molecular allergen recognition profile in China as basis for allergen specific immunotherapy. Front Immunol. 2021;12:719573.
Tang R, Wang ZX, Ji CM, et al. Regional differences in food allergies. Clin Rev Allergy Immunol. 2019;57(1):98–110.
Zhang XW, Liu SY, Li X, et al. Environmental influences on food allergy. Chinese J Preventive Medicine. 2023;57(12):1921–8.
Hao G, Lai X, Song Z, et al. Self-reported questionnaire survey on the prevalence and symptoms of adverse food reactions in patients with chronic inhalant diseases in Tangshan city. China Allergy Asthma Clin Immunol. 2018;14:3.
Feng H, Liu Y, Xiong XJ, et al. Epidemiological survey of self-reported food allergy among university students in China. Medicine. 2022;101(31): e29606.
Sha L, Shao MJ, Chuanhe L, et al. A cross-sectional study of the prevalence of food allergies among children younger than ages 14 years in a Beijing urban region. Allergy Asthma Proc. 2019;40(1):e1–7.
Wen Z, Ye S. A report of 50 patients with Artemisia pollinosis and plant food allergy. Nati Med J China. 2002;82:626–9.
Zhou XP, Li H. One case of anaphylaxis of chestnut and Artemisia pollinosis. Chinese J Allergy and Clin. 2011;5:148–50. https://doi.org/10.1111/all.16073.
Gao ZS, Yang ZW, Wu SD, et al. Peach allergy in China: a dominant role for mugwort pollen lipid transfer protein as a primary sensitizer. J Allergy Clin Immunol. 2013;131(1):224–6.
Deng S, Yin J. Allergy to cumin: mugwort pollen-related food allergy. Allergy. 2015;70(S101):608.
Wang XY. Food Allergy: Clinical management and typical case analysis. Beijing Science and Technology Press. 2016.
Deng S, Yin J. Mugwort Pollen-Related Food Allergy: Lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese population. Allergy Asthma Immunol Res. 2019;11(1):116–28.
Jiang NN, Guan K, Xaing L. Clinical characteristics of self-reported food allergy in children with pollinosis. Chinese J Allergy Clin Immunol. 2020;14(06):552–9.
Tang R, Wang LL, Yin J, et al. History of hay fever in China (in Chinese). Sci Sin Vitae. 2021;51:901–7.
Shi Y, Tang R, Luo FM, et al. The diagnosis and management of allergic reactions caused by Chinese materia medica. Clin Rev Allergy Immunol. 2022;62(1):103–22.
Wang XY, Chen LJ, Ding JQ, et al. Profiles of birch allergen component sensitization and its association with pollen food allergy syndrome in Northern China. J Asthma Allergy. 2023;16:1241–50.
Jiang NN, Xu W, Huang HJ, et al. Anaphylaxis in Chinese children with pollen sensitization: Triggers, clinical presentation, and acute management. J Asthma Allergy. 2022;15:633–43.
Luo WT. National multi-center study on allergen sensitization patterns and risk factors of allergic diseases based on the biobank resource sharing platform. 2023. PhD thesis, Guangdong Medical University.
Qiao BS. Color Atlas of air-borne pollens and plants in China. Beijing: Peking Union Medical College Press; 2005.
Gao ZS. Molecular approaches to peach and Artemisia pollen allergies in China. 2021. PhD thesis, University of Amsterdam.
Jiang ZH, Xiao H, Zhang HT, et al. Broussonetia papyrifera (paper mulberry) pollen is an important cause of allergic rhinitis in Southwest China. Clin Exp Allergy. 2022;52:1448–51.
Hou XQ, Hou WT, Wu LQ, et al. Associations of Four sensitization patterns revealed by Latent class analysis with clinical symptoms: A multicenter study of China. eClinicalMedicine. 2022;46:101349.
Wang XY, Zhang Y, Chen YL, et al. Prevalence of adult eczema, hay fever, and asthma, and associated risk factors: a population-based study in the northern Grassland of China. Allergy Asthma Clin Immunol. 2021;17(1):27.
Sun AZ, Sun XL, Li XY, et al. Sensitization characteristics in allergic rhinitis and transport pathway for Artemisia pollen in northern Beijing. China Sci Total Environ. 2023;884: 163795.
Li JD, Gu JQ, Xu YY, et al. Serum IgE profiles in Chinese pollinosis patients with grass pollen sensitization. World Allergy Organ J. 2022;15(1):100624.
Zhang W, Zhao Y, Wang CS, et al. Food allergen sensitization in patients with allergic rhinitis. J Capital Medical University. 2011;32(1):8–12.
Aalberse RC, Akkerdaas J, van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56(6):478–90.
Guryanova SV, Finkina EI, Melnikova DN, et al. How do pollen allergens sensitize? Front Mol Biosci. 2022;9: 900533.
Gilissen L, Gao ZS, Chen Z. Allergen protein families and cross-reactivity, in “Multidisciplinary Approaches to Allergies” edited by Gao ZS, Shen HH, Zheng M, Frewer L and Gilissen L, Zhejiang University Press and Springer, 2012, Hangzhou China. 81–90.
Gao ZS, Zhou X, Yang ZW, et al. IgE-binding potencies of three peach Pru p 1 isoforms. Mol Nutr Food Res. 2016;60:2457–66.
Hao GD, Zheng YW, Wang ZX, et al. High correlation of specific IgE sensitization between birch pollen, soy and apple allergens indicates pollen-food allergy syndrome among birch pollen allergic patients in northern China. J Zhejiang Univ-Sci B (Biomed & Biotechnol). 2016;17(5):399–404.
Zhao L, Xie HB, Wang XF, et al. Molecular characterization of allergens and component-resolved diagnosis of IgE-mediated mango fruit allergy. Allergy. 2023;78(6):1699–703.
van Ree R, Ballerda BD, Berin MC, et al. The COMPARE database: A public resource for allergen identification, adapted for continuous improvement. Front Allergy. 2021;2: 700533.
Jiang NN, Yin J, Wen LP, et al. Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: A retrospective study of 1,952 episodes. Allergy Asthma Immunol Res. 2016;8(4):353–61.
Li SK, Liu ZY, Huang CK, et al. Prevalence, clinical presentation, and associated atopic diseases of pediatric fruit and vegetable allergy: A population-based study. Pediatr Neonatol. 2022;63(5):520–6.
Feng H, Zhou JD, Lu YA, et al. Prevalence of self-reported food allergy among adults in Jiangxi, China. World Allergy Organ J. 2023;16(5): 100773.
Feng H, Luo N, Xiong XJ, et al. Prevalence of food allergy in the Chinese population: A systematic review and meta-analysis of population-based studies. Allergy Asthma Proc. 2023;44(5):315–25.
Huang Z, Feng W, Wei W, et al. Prevalence of food-allergen and aeroallergen sensitization among people in Sichuan, western China: An 8-year observational study. J Vlin Lab Anal. 2019;33(3): e22723.
Hu H, Huang H, Liao C, et al. A study of allergen detection panel in Guangzhou, southern China based on real-world data from the past 7 years. Sci Rep. 2023;13(1):14855.
Li JD, Du ZR, Liu J, et al. Characteristics of pollen-related food allergy based on individual pollen allergy profiles in the Chinese population. World Allergy Organ J. 2020;13(5): 100120.
Sun Y, Yu XL. Clinic analysis of plant food allergies in 22 pollinosis patients. Inner Mongolia Med J. 2007;39(9):1121–2.
Ma SK, Yin J, Jiang NN, et al. Component-resolved diagnosis of peach allergy and its relationship with prevalent allergenic pollens in China. J Allergy Clin Immunol. 2013;132(3):764–7.
Guan K, Hao CL, Liu ZF, et al. Expert consensus on the diagnosis and management of pollen-food allergy syndrome. Chin J Prev Med. 2024;58(6):1–16.
Zhao L, Fu WY, Gao BY, et al. Variation in IgE binding potencies of seven Artemisia species depending on content of major allergens. Clin Transl Allergy. 2020;10(1):50.
Fu WY, Gao ZS, Gao L, et al. Identification of a 62-kDa major allergen from Artemisia pollen as a putative galactose oxidase. Allergy. 2018;73(5):1041–52.
Gao ZS, Fu WY, Sun YM, et al. Artemisia pollen allergy in China: Component-resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy. 2019;74(2):284–93.
Rial MJ, Sastre L. Food allergies caused by allergenic lipid transfer proteins: What is behind the geographic restriction? Curr Allergy Asthma Rep. 2018;18(11):56.
Di Y, Yu RL, Du H, et al. Observation on pollinosis with plant food allergy. J Clin Otorrhinolaryngol Head Neck Surg (China). 2018;32(23):1779–83.
Zhao L. Identification of allergens in mango and red bayberry fruits and relation to Artemisia pollen allergy. 2022. PhD thesis, Zhejiang University.
Kong R, Yin J. Kidney-bean (Phaseolus Vulgaris) Dependent, exercise-induced anaphylaxis in patients comorbid with mugwort (Artemisia Vulgaris) Pollinosis. Immunol Invest. 2020;50(4):389–98.
Tang R, Sun JL, Yin J, et al. Artemisia allergy research in China. Biomed Res Int. 2015;2015: 179426.
Song L, Wu J, Xi AQ. Clinical analysis of 28 pollenosis cases induced by TCM preparation. China Pharm. 2007;18(12):937–8.
Sun ZB, Zhao YX, An XQ, et al. Effects of airborne pollen on allergic rhinitis and asthma across different age groups in Beijing. China Sci Total Environ. 2024;912: 169215.
Lou H, Ma S, Zhao Y, et al. Sensitization patterns and minimum screening panels for aeroallergens in self-reported allergic rhinitis in China. Sci Rep. 2017;7(1):9286.
Song JJ, Zhang HY, Liu ZG, et al. Cloning of the panallergen profilin from lychee fruit and its cross-reactivity with birch pollen profilin Bet v 2. Food Agric Immunol. 2007;18(2):129–38.
Xie SQ, Gao Y, Gong LZ. Clinical analysis of Alnus nepalensis pollen allergy. Medicine and Pharmacy of Yunnan. 2006;03:276.
Wang J. Research on pollen allergen component and its cross-reaction with fruit and vegetable proteins in Kunming. 2022. PhD thesis, Kunming Medical University.
Luo WT, Yang SW, Huang HM, et al. Analysis of peanut allergen components sensitization and cross reaction with pollen allergen in Chinese Southerners with allergic rhinitis and/or asthma. J Asthma Allergy. 2021;14:1285–93.
Luo WT, Huang HM, Zheng PY, et al. Major grass pollen allergens and components detected in a southern Chinese cohort of patients with allergic rhinitis and/or asthma. Mol Immunol. 2016;78:105–12.
Shahali Y, Dadar M. Plant food allergy: Influence of chemicals on plant allergens. Food Chem Toxicol. 2018;115:365–74.
Heiss S, Mahler V, Steiner R, et al. Component-resolved diagnosis (CRD) of type I allergy with recombinant grass and tree pollen allergens by skin testing. J Invest Dermatol. 1999;113(5):830–7.
Andersen MBS, Hall S, Dragsted LO. Identification of European allergy patterns to the allergen families PR-10, LTP, and profilin from Rosaceae fruits. Clin Rev Allergy Immunol. 2011;41(1):4–19.
Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016;27(S23):1–250.
Burney PG, Potts J, Kummeling I, et al. The prevalence and distribution of food sensitization in European adults. Allergy. 2014;69(3):365–71.
Kader JC. Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Molec Biol. 1996;47:627–54.
Salcedo G, Sanchez-Monge R, Diaz-Perales A, et al. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin Exp Allergy. 2004;34(9):1336–41.
van Ree R. Clinical importance of non-specific lipid transfer proteins as food allergens. Biochem Soc Trans. 2002;30:910–3.
Radauer C, Bublin M, Wagner S, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121(4):847–52.
Jiang NN, Xiang L, Guan H, et al. Blueberry (Vaccinium myrtillus) Induced Anaphylaxis in a Chinese child with lipid transfer protein sensitization. J Asthma Allergy. 2023;16:1253–8.
Wangorsch A, Schülke S, Gadermaier G, et al. LTP cross-reactivity-primary sensitization to mugwort pollen LTP Art v 3, facilitates subsequent sensitisation to peach LTP Pru p 3 in mice. Clin Transl Allergy. 2014;4:014.
Pastorello EA, Monza M, Pravettoni V, et al. Characterization of the T-cell epitopes of the major peach allergen Pru p 3. Int Arch Allergy Immunol. 2010;153(1):1–12.
Deng S, Yin J, Wang RQ. Identification of T-cell epitopes of major peach allergen Pru p 3. Chin J Allergy Clin Immunol. 2018;12(5):498–502.
Garcia-Casado G, Pacios LF, Diaz-Perales A, et al. Identification of IgE-binding epitopes of the major peach allergen Pru p 3. J Allergy Clin Immunol. 2003;112(3):599–605.
Pacios LF, Tordesillas L, Cuesta-Herranz J, et al. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: Peach Pru p 3 allergen as a model. Mol Immunol. 2008;45(8):2269–76.
Muzio MD, Wildner S, Huber S, et al. Hydrogen/deuterium exchange memory NMR reveals structural epitopes involved in IgE cross-reactivity of allergenic lipid transfer proteins. J Biol Chem. 2020;295(51):17398–410.
D’Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62(9):976–90.
Katial RK, Lin FL, Stafford WW, et al. Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann Allergy Asthma Immmunol. 1997;79(4):340–6.
Riggins CW, Seigler DS. The genus Artemisia (Asteraceae: Anthemideae) at a continental crossroads: Molecular insights into migrations, disjunctions, and reticulations among Old and NewWorld species from a Beringian perspective. Mol Phylogenet Evol. 2012;64(3):471–90.
Scheurer S, Van Ree R, Vieths S. The role of lipid transfer proteins as food and pollen allergens outside the Mediterranean area. Curr Allergy Asthma Rep. 2021;21(2):7.
Sánchez-López J, Tordesillas L, Pascal M, et al. Role of Art v 3 in pollinosis of patients allergic to Pru p 3. J Allergy Clin Immunol. 2014;133(4):1018–25.
Ruano-Zaragoza M, Somoza ML, Jimenez-Rodriguez TW, et al. Lipid transfer protein sensitization: risk of anaphylaxis and molecular sensitization profile in Pru p 3-sensitized patients. Int Arch Allergy Immunol. 2020;182(5):425–32.
Ma S, Nie L, Li H, et al. Component-Resolved Diagnosis of peanut allergy and its possible origins of sensitization in China. Int Arch Allergy Immunol. 2016;169(4):241–8.
Breiteneder H, Radauer C. A classification of plant food allergens. J Allergy Clin Immunol. 2004;113(5):821–30.
Rodriguez del Rio P, Diaz-Perales A, Sanchez-Garcia S. Profilin, a Change in the Paradigm. J Invest Allergol Clin Immunol. 2018;28(1):1–12.
Lopez-Torrejon G, Ibanez MD, Ahrazem O, et al. Isolation, cloning and allergenic reactivity of natural profilin Cit s 2, a major orange allergen. Allergy. 2005;60(11):1424–9.
Lopez-Torrejon G, Crespo JF, Sanchez-Monge R, et al. Allergenic reactivity of the melon profilin Cuc m 2 and its identification as major allergen. Clin Exp Allergy. 2005;35(8):1065–72.
Yang YS, Xu ZQ, Zhu W, et al. Molecular and immunochemical characterization of profilin as major allergen from Platanus acerifolia pollen. Int Immunopharmaco. 2022;106: 108601.
Liao CX, Hou XQ, Wu LT, et al. Major Grass Pollen Allergen components and cross-reactive carbohydrate determinants in mugwort-sensitized child patients with allergic respiratory disease in Western China. Front Pediatr. 2022;10: 816354.
Wu LQ, Hou XQ, Luo WT, et al. Three patterns of sensitization to mugwort, timothy, birch and their major allergen components revealed by Latent class analysis. Mol Immunol. 2022;145:59–66.
Khan RS, Iqbal A, Malak R, et al. Plant defensins: types, mechanism of action and prospects of genetic engineering for enhanced disease resistance in plants. 3 Biotech. 2019;9(5):192.
Zhao L, Chen JY, Wang YQ, et al. Association in molecular profiles of IgE sensitization to mugwort pollen allergens in Chinese parents and their offspring. Pediatr Allergy Immunol. 2023;34(8): e14005.
Cosi V, Gadermaier G. The Role of Defensins as Pollen and Food Allergens. Curr Allergy Asthma Rep. 2023;23(6):277–85.
Ukleja-Sokołowska N, Lis K, Graczyk M, et al. The use of inhibition assay in Api g 7 suspected allergy in a female patient with anaphylaxis: A case report. Int J Immunopathol Pharmacol. 2024;38:03946320231223004.
Ukleja-Sokolowska N, Gawronska-Ukleja E, Lis K, et al. Anaphylactic reaction in patient allergic to mango. Allergy Asthma Clin Immunol. 2018;14:78.
Ukleja-Sokolowska N, Gawronska-Ukleja E, Zbikowska-Gotz M, et al. Sunflower seed allergy. International J Immunopathol Pharmacol. 2016;29(3):498–503.
Petersen A, Kull S, Rennert S, et al. Peanut defensins: novel allergens isolated from lipophilic peanut extract. J Allergy Clin Immunol. 2015;136(5):1295–301.
Tuppo L, Alessandri C, Pomponi D, et al. Peamaclein - a new peach allergenic protein: similarities, differences and misleading features compared to Pru p 3. Clin Exp Allergy. 2013;43(1):128–40.
Klingebiel C, Chantran Y, Arif-Lusson R, et al. Pru p 7 sensitization is a predominant cause of severe, cypress pollen-associated peach allergy. Clin Exp Allergy. 2019;49(4):526–36.
Iizuka T, Takei M, Saito Y, et al. Gibberellin-regulated protein sensitization in Japanese cedar (Cryptomeria japonica) pollen allergic Japanese cohorts. Allergy. 2021;76(7):2297–302.
Grijincu M, Hutu I, Weber M, et al. Physicochemical and immunological characterization of Amb a 12, a novel ragweed (Ambrosia artemisiifolia) pollen allergen. Mol Immunol. 2023;157:18–29.
Jiao YX, Song LB, Xu ZQ, et al. Purification and characterization of enolase as a novel allergen in Platanus acerifolia pollen. Int Immunopharmacol. 2022;113: 109313.
Luo WT, Huang HM, Zheng PY, et al. CCD Inhibition test can improve the accuracy of the detection of pollen and seed food allergen-specific IgE in Southern China. J Asthma Allergy. 2021;14:439–47.
Chen H, Jiang Q, Yang YQ, et al. Cross-reacting carbohydrate determinants inhibitor can improve the diagnostic accuracy in pollen and food allergy. J Asthma Allergy. 2022;15:713–25.
Valenta R, Karaulov A, Niederberger V, et al. Molecular Aspects of Allergens and Allergy. Adv Immunol. 2018;138:195–256.
Chen H, Li J, Cheng L, et al. China consensus document on allergy diagnostics. Asthma Allergy Immunol Res. 2021;13(2): e25.
Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66(1):106–19.
Deng S, Yin J. Clinical utility of basophil activation test in diagnosis and predicting severity of mugwort pollen-related peach allergy. World Allergy Organ J. 2019;12(6): 100043.
Tang LX, Wang PP, Ge WT, et al. Artemisia annua sublingual immunotherapy in children with seasonal allergic rhinitis. Allergy. 2024.
Lou HF, Wang XY, Wei QY, et al. Artemisia Annua sublingual immunotherapy for seasonal allergic rhinitis: A multicenter, randomized trial. World Allergy Organ J. 2020;13(9): 100458.
Shen Z, Zhang PF, Kang W, et al. Clinical efficacy in one-year treatment with Artemisia annua-SLIT drops in monosensitized and polysensitized individuals. Am J Otolaryngol. 2023;44(6): 104002.
Funding
This study was carried out with financial support from the National Natural Science Foundation of China (32302482), Talent Cultivation Project of Beijing Shijitan Hospital, Capital Medical University during the “14th Five Year Plan” Period (Leading Talents) (2023LJRCWXY), and Science and Technology Plan “Open list” project of Ordos City (JBGS-2021–006).
Author information
Authors and Affiliations
Contributions
L.Z. , T.M., X. W. and Z.G. wrote the main manuscript text. L.Z., Y. L. and S.W. prepared figures 1-2, XiaoY. W. and H.W. prepared figure 3., L.Z. , T.M., XiaoY. W., L.F. and Z.G. prepared Table 1. L. G, R. van R. revised the manuscript. X.W.and Z. G. supervised the study. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Ma, T., Wang, X. et al. Food-Pollen Cross-Reactivity and its Molecular Diagnosis in China. Curr Allergy Asthma Rep 24, 497–508 (2024). https://doi.org/10.1007/s11882-024-01162-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-024-01162-w