Abstract
The occurrence of pollen food syndrome (PFS) is increasing in the pediatric population. Investigators in Europe, Australia, and Mexico have documented the prevalence of PFS as high as 10–24% and related the increase to the rise in seasonal allergic rhinitis worldwide. Tree pollen allergy patients, especially birch pollen allergic patients, are the most likely to develop PFS. Patients experience PFS when eating fresh fruits, raw vegetables, nuts, and spices. Symptoms begin when fresh fruit contacts the oral mucosa producing itching, tingling, and swelling of the mouth, tongue, and throat. The symptoms usually resolve quickly with swallowing. PFS is a class 2 food allergy caused by cross-reacting antibodies that have developed via the respiratory tract in response to pollen allergens, in contrast to class 1 food allergy, where antigen sensitization to milk, egg, soy, wheat, peanut, or tree nuts occurs in the gastrointestinal tract. It is important to distinguish between the clinical symptoms of PFS and food allergy because PFS is limited to the oral mucosa and infrequently causes systemic symptoms leading to anaphylaxis. Standard skin testing with commercial as well as IgE immunoassay testing for foods responsible for PFS is sometimes unhelpful, and clinical history is very important in interpreting results. It is hoped that component-resolved diagnostics (CRD), as an alternative to or in combination with traditional testing, can help identify the significant molecular antigens associated with clinical symptoms and lead to improved diagnosis and effective immunotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- OAS
- PFS
- PR proteins
- Class 1 food allergy
- Class 2 food allergy
- Pan-allergens
- Cross-reacting antibodies
- Profilins
- Lipid transfer proteins
Introduction
Pollen allergy has increased in the last 20 years both in the United States and worldwide leading to an associated rise in reports of pollen food syndrome (PFS) [1]. The initial descriptive term for PFS was oral allergy syndrome (OAS) [2, 3]. The term OAS is ambiguous because it has been used indiscriminately in the literature without regard to the antigens or mechanisms causing the oral symptoms. The diagnosis and treatment of PFS require an understanding of the antigens implicated, path of antigen sensitization, the associated plant foods and the potential clinical syndromes involved. Many authors are now advocating the use of the term “pollen food syndrome” (PFS) or “pollen food allergy syndrome” (PFAS) to describe specifically the cross-reactivity to shared cross-reactive antibodies among the pollens, fresh fruits, raw vegetables, nuts, and spices. This is because the term OAS is used to describe the oropharyngeal symptoms resulting from eating any food, not just plant food [4,5,6,7]. PFS is used precisely to mean class 2 food allergy resulting from plant food allergens cross-reacting with pollen allergens and causing oral symptoms in pollen allergic patients [8, 9].
Definition
PFS is the most common food allergy in adolescents and adults and the incidence is rising in young children [6, 10]. PFS is an allergic reaction to fresh fruits, raw vegetables, nuts, or spices which can cause swelling and pruritus of the lips, oral mucosa, tongue, and throat. The symptoms occur within seconds to minutes as the food contacts the oral mucosa. It is usually isolated to the oral mucosa and infrequently associated with systemic signs of anaphylaxis [6, 11].
Epidemiology of PFS
Tuft and Blumstein first described the phenomena of OAS in 1942 [2]. They observed a reaction to foods, particularly raw fruits and vegetables, among their patients. Patients described localized itching of the inner cheeks, roof of the mouth, with itching often extending to the throat, plus swelling of the lips with occasionally an urticarial rash around the mouth. Patients noted, however, that cooked or canned produce did not cause the same reaction and their reactions were worse during pollen season. The authors demonstrated that the antigens causing the reaction could be detected by skin testing with the suspected fresh fruit. In the 1940s, the idea of using fresh foods to skin test was novel, but the description of the oral allergy syndrome and its clinical significance were not fully appreciated until the late 1980s.
Amlot used the term OAS in 1987 when evaluating 80 highly atopic patients who either had atopic eczema or were skin test positive for food allergy [3]. He used this term because patients described the immediate onset of symptoms of oral irritation and throat tightness upon the ingestion of their food allergen. It reflected a precise time frame in the patient’s experience of food allergies – the oral symptoms occurred first with small amounts of food, with progression to a systemic reaction with larger quantities of food. He also noted that in a subgroup of patients, urticaria, asthma, or anaphylaxis developed following the oral symptoms.
In 1988, Ortolani et al. studied 262 patients and used the term OAS to apply specifically to subjects with hay fever who developed oral allergy syndrome after fruit and vegetable ingestion. The patients had local reactions, and a few had systemic reactions [11]. There was a close connection between age of onset of hay fever and the occurrence of their oral allergy symptoms. In addition, the authors described an association between allergy to some pollens and certain fruits. For example, apple, carrot, pear, and cherry correlated with birch pollen allergy, and while tomato, melon, and watermelon correlated with grass pollen allergy. The presence of pollen and plant cross-reacting antibodies had not been demonstrated; therefore, the term OAS was connected with the spectrum of reactions associated with food allergy based on the presence of oral symptoms but not the type or source of the antigen.
In 1999, Kazemi-Shirazi et al. demonstrated convincingly that as distinguished from OAS, PFS is the result of cross-reacting antibodies between pollen and plant food [12]. Pre-incubation of sera from patients with PFS in the presence of natural pollen allergens led to an almost complete inhibition of IgE binding to plant food allergens in Western blots as well as in RAST inhibition experiments. When incubating the patients’ serum with the plant food, there was poor inhibition of IgE binding. The key antigens were the pollen antigens causing the reactions. Sensitization was occurring through the respiratory tract because of pollen antigens, which shared epitopes with plant-based food. Patients were the bystanders in the reactions and were responding to the plant food as if they were eating the pollen directly due to the cross-reactive antibodies.
Researchers proposed that food allergy reactions could be classified according to the type of antigen and the path to sensitization; and categorized the food reactions into class 1 food allergy or class 2 food allergy [9]. In class 1 food allergy, IgE antibodies develop against food proteins, which were “complete,” meaning not affected by proteolytic digestion or affected by heat, and were the result of direct sensitization occurring primarily in the gut. Class 1 food allergies cause the allergic reactions most common in early childhood to milk, egg, soy, fish, shellfish, peanut, and tree nuts [9, 13]. Alternatively, class 2 food allergy sensitization takes place through the respiratory tract when pollen antigens share epitopes with plant-based food. The allergens are often heat labile and known as “incomplete allergens” [8].
Cross-Reactive Pan-Allergens
Pollen and food are not botanically related but do contain homologous proteins [8]. The shared epitopes between the pollen and the plant food involve both primary and tertiary structures [4, 8]. These shared proteins are highly conserved across the plant kingdom and widely distributed and are known as pan-allergens. Depending on the pan-allergen inducing the reaction, symptoms can range from PFS to anaphylaxis [6, 14].
Pathogenesis-Related Proteins (PRPs)
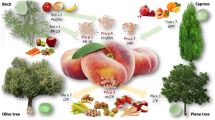
The most common pan-allergens are pathogenesis-related proteins (PRPs), including lipid transfer proteins (LTPS) and profilins. PR proteins are defense proteins that plants express to protect themselves in response to fungi, bacterial or viral infections, and injury or exposure to chemicals which mimic both infections and stress [9]. PR proteins were first discovered as proteins present in tobacco plants infected with tobacco mosaic virus [15]. They did not occur in non-infected plants but were evident after viral infection. The PRs are composed of 17 families with very similar biochemical functions and sequences [16]. The PR protein families constitute a repertoire of protective responses for the plant kingdom. In particular, the PR-10 protein family, represented by the Bet v 1 homologous proteins, is linked to an ancient primordial gene and is responsible for the majority of cross-reacting epitopes causing PFS [17]. The Bet v 1 antigen is the major birch pollen allergen. The homologous proteins in birch pollen-related fruits are apple (Mal d 1), cherry (Pru av. 1), apricot (Pru ar 1), and pear (Pyr c 1). The vegetables that are cross-reactive are carrot (Dau c 1), celery (Api g 1), parsley (pcPR), and potato (pSTH). Hazelnut allergy is related to another: Bet v 1 homolog and Cor a 1. Other important PRs allergens include PR-2, 3, 4, 5, and 14. The PR-2 family is known as B-1, 3 glucanases and can degrade fungal cell walls of actively growing hyphae. Most are extracellular but the basic glucanases are located intracellularly in vacuoles. The most important is Hevea brasiliensis (Hev b 2) from the latex of the tropical rubber tree and one the epitopes that causes latex allergy. It provokes an antibody responsible for the latex-fruit syndrome, causing hypersensitivity to avocado, banana, chestnut, fig, and kiwi [9, 18, 19].
The chitinases, PR-3, are found in seed-producing plants and digest the chitin that is in the skeleton of most insects and fungal cell walls. The latex prohevein, Hevein (Hev b 6.02), belongs to the chitin-binding proteins and also contributes to the epitopes present in the fruit associated with the latex-fruit syndrome [9, 19, 20] (Table 5.1).
PR-4 represents another family of chitinases occurring in potatoes in response to trauma. The PR-5 family constitutes thaumatin-like proteins with diverse antifungal functions. The foods that contain these proteins are cherry, apple (Mal d 2), paprika, and bell pepper (P23), and these cross-react with the pollen of mountain cedar (Jun a 3) [9].
Lipid Transfer Proteins (nsLTP)
Another significant group is the non-specific lipid transfer proteins (PR-14) that transfer phospholipids from liposomes to mitochondria, which are located in the outer cell layer of the plant and form part of the plant defense system against fungus and bacteria. They are highly resistant to heat and changes in pH. They are the most important allergens in the Rosaceae family, which contains three significant subfamilies: Prunoideae (peach, apricot, plum, almond cherry), the Pomoideae subfamily (apple, pear); and the Rosoideae subfamily (blackberry, strawberry) [21]. The oral reactions that these proteins cause are different from the other defense proteins because pollen allergy is not a prerequisite. Sensitization can occur via class 2 sensitization and is limited to the oral mucosa; however, if sensitization occurs via class 1 sensitization the reactions can be systemic [22].
Profilins
Profilins are monomeric, actin-binding proteins that regulate the actin filaments to form the cytoskeleton and are present in trees, grasses, and weeds. It is estimated that 20% of pollen allergic subjects are reactive to profilins which are shared by a wide variety of inhalant and food allergens [23]. Bet v 2, also another birch pollen allergen, is an example of a profilin that will cause birch pollen allergic patients to react to apple, celery, carrot, and pear. It is a complete antigen and often is not degraded by heating. It is associated with anaphylaxis in the celery-mugwort syndrome. (Table 5.1).
Pollen Food Syndrome Worldwide
The increase in PFS is related to multiple factors. Environmental changes worldwide and the agricultural practices of developing more resilient plants seem to have increased the expression of homologous proteins possibly leading to plant food becoming more allergenic [4]. Other factors include specific geography, climate, local diet, and food preparation [1, 9, 24].
Investigators in England, Italy, Australia, and Mexico recently examined their pediatric patients with allergic rhinitis for signs of PFS and found some surprising results. Studies from these countries have demonstrated a PFS prevalence of 10–24%. The effects of predominant plant foods varied among countries, possibly in relation to geography, climate, local dietary habits, and pollen exposures [7, 22, 25, 26].
The Australian investigators studied atopic children in southwest Sydney to assess the occurrence of PFS in that pediatric population. They considered OAS to include PFS, food allergy, and latex-fruit syndrome. They found that the prevalence of PFS alone in patients with allergic rhinitis and pollen sensitization was 12.1%. The fruits causing PFS symptoms were all tropical fruits, and watermelon was the most common. In the broader definition of OAS, where reactions begin with oral symptoms but progress to systemic symptoms, OAS was compatible with typical reactions characteristic of food allergy, class 1, most frequently caused by peanut (13.6%) [26].
In Mexico, researchers evaluated children 6–14 years seen for the first time in their allergy clinic. They were given questionnaires to assess for PFS and skin testing for pollens and foods. In 267 patients PFS occurred in 10–12% of patients with allergic rhinitis to pollen. Pineapple was the most common food cited, related to the pollen of the Quercus species [25].
In Italy, Mastororilli et al. tested for pan-allergens to estimate the prevalence of PFS and then to identify endotypes of PFS in children with seasonal allergic rhinoconjunctivitis (SAR). They examined 1271 children from 4–18 years of age. They skin tested with both commercial pollen extracts and the pan-allergens. Foods eliciting symptoms were determined by questionnaire. The pan-allergens Phl p 12 (profilin), Bet v 1(PR-10), and Pru p 3 (nsLTP) were tested by immunoCAP FEIA. They found PFS in 24% of patients. They identified five PFS endotypes associated with pan-allergen IgE sensitization. There was a multi-pan-allergen group (sensitization equal to two or more pan-allergens) who had more severe allergic disease comorbidities and multiple foods causing symptoms; mono pan-allergen group (only reacting to one of the three pan-allergens tested) or no pan-allergen sensitization. The sensitization patterns were informative. The group who were sensitized to two or more pan-allergens (PR-10, profilin, nsLTP) lived in Northern Italy (84%). This region has a continental climate with more birch and alder pollen than in Southern Italy. They had more asthma, atopic dermatitis, urticaria, and anaphylaxis, as well as higher total IgE levels and more foods that triggered symptoms. The mono-sensitized patients who were reactive to profilin were more frequently from Central Italy, had a high IgE level and a defined group of foods that caused symptoms from the Cucurbitaceae family (watermelon, melon, and cucumber) as well as peach, banana, and kiwifruit. This pattern is characteristic of sensitization to grass, plantain, plane, and olive trees seen in Central Italy. The LTP endotype was more common in Southern Italy where birch is rare but Rosaceae fruits (apple, peach, and pear), bananas, and nuts cause symptoms. Both class 1 and class 2 food allergy sensitizations can be present in this LTP endotype and if the sensitization is via class 1 “complete antigens” the reactions are more likely to be systemic. PR-10 endotype was more common in Central Italy. Interestingly, it was not related to the birch pollen but to other Fagales (Quercus spp.) or beech tree pollen. The related plant foods which caused symptoms were apple, peach, and kiwifruit. Finally, children with no pan-allergen sensitivity detected usually had mild allergic disease and comorbidities. Forty percent of these children reacted to kiwifruit. Further prospective studies are needed to assess the value of the endotype classification and how it might provide strategies for prevention and therapy [22].
In London, Ludman et al. set out to discern the patterns of PFS and their relationship to three age groups: 0–5, 6–10, and 11–15 years of age. Overall, PFS was present in 48% of all the children recruited from their specialty allergy clinic. Starting from youngest to the oldest group the occurrence was 17%, 50%, and 78% of PFS, respectively. From microarray data, pan-allergen sensitization was demonstrated at 2.8 years of age and symptoms started at 4.5 years much earlier than expected [7].
PFS and Associated Atopic Conditions
Atopic Dermatitis
The study of birch pollen allergy (Bet v1) and its cross-reactive allergens in plant food has provided insights into the immunologic connections between pollen sensitization via upper respiratory allergy and other atopic manifestations. Reekers et al. demonstrated that birch pollen-related foods trigger atopic dermatitis in a subgroup of patients that were highly allergic to birch pollen. They evaluated 37 patients without immediate reactions to birch pollen-related food. After an elimination diet avoiding the cross-reactive foods for 4 weeks, the patients underwent an oral challenge of carrots, celery, hazelnuts, and apple mashed together and masked with carob and an orange flavor. Seventeen out of 37 patients responded with worsening of their eczema within 48 hours. The blood lymphocytes of the food responsive patients with atopic dermatitis expressed CLA+, a homing antigen that facilitated the appearance of these lymphocytes in patients’ lesional skin from the punch biopsies when these patients were exposed to the birch pollen. None of them realized that they were sensitive to birch pollen-related foods and were unaware of its relationship to worsening of their eczema [27].
Bohle et al. extended the observations of Reekers in another study looking at patients with birch pollen allergy and eczema. Allergists usually counsel their patients to cook the foods cross-reacting with birch pollen because heat will denature the tertiary structure. In many cases, this allows the patient to consume the food without any oral allergy symptoms. The authors, however, demonstrated that heating does not destroy the expression of birch pollen (Bet v 1) T cell epitopes and can cause an increase in the patient’s eczema. Eating birch pollen-related foods supports the pollen-specific TH2 inflammation and ongoing synthesis of IL-4. The continuing stimulation, even with small concentrations of Ig E binding allergens, through mucosal surfaces might foster perennial IgE synthesis in B cells [28].
Eosinophilic Esophagitis
The insight that aeroallergens could contribute to eosinophilic esophagitis (EoE) was articulated first by Mishra et al. [29]. In their mouse model, after respiratory exposure to Aspergillus fumigatus they noted esophageal eosinophilia. To explain this finding they postulated that aeroallergens could be topically spread to the esophageal mucosa and contribute to ongoing inflammation in EoE. This theory was supported by Fogg et al. who reported a case study involving a 21-year-old woman who recounted worse symptoms, proven by esophageal biopsy of her EoE in pollen season with no change in diet or medication [30]. In another study in children with EoE, Ram et al. demonstrated that seasonal allergic rhinitis is associated with seasonal flares of esophageal eosinophilia. This was seen in 14% of patients with EoE; 84% were male and all had allergic rhinitis. They hypothesize that the allergic rhinitis may contribute to exacerbations of EoE in pollen season and by intensifying anti-inflammatory therapy during pollen season, the disease could be better controlled [31]. The impact of pollen allergy was also seen in another study by van Rhijn, whose patients with eosinophilic esophagitis (EoE) had a higher prevalence of sensitization to pan-allergens including profilins and PR-10. Thirty-nine percent of their patients with EoE were sensitized to birch pollen (rBet v 1) and corresponding food allergen components supporting a link with PFS [32]. Mahdavinia et al. surveyed a group of adults with EoE and found that greater than 50% of patients with EoE had PFS with pollen sensitization. They postulated that uncontrolled nasal inflammation due to pollen exposure along with the ingestion of pan-allergens in fruits and vegetables, prior to denaturation by stomach enzymes, could contribute to esophageal eosinophilia and subsequently the esophageal inflammation [33].
Seasonal Intestinal Inflammation and Irritable Bowel Syndrome
Seasonal intestinal inflammation also correlates with aeroallergen sensitization in patients. Magnusson et al. evaluated nine patients with documented birch pollen allergy and PFS [34]. The patients had two duodenal biopsies: one at the end of birch pollen season and one 6 months later (out of season). They found during birch pollen season there was an increase in activated eosinophils (MBP+) and IgE+ mast cells present in the mucosa, villi, and basal lamina propria compared with off-season biopsies. They noted that five of nine patients satisfied the criteria for irritable bowel syndrome (IBS) during the pollen season. Another study of patients with irritable bowel syndrome (IBS) by Tobin et al. found that in patients identified with diarrhea-predominant IBS, 80% had seasonal allergic rhinitis (SAR) and 51% reported atopic eczema (AE). The patients were specifically asked about symptoms of itching or swelling of the mouth, tongue, and throat, and fruits were cited most often as the cause [35]. It is thought provoking that respiratory sensitization to pollen allergens might be related and contributes to seasonal immunologic inflammatory changes in the small intestine as well as in the esophagus and skin.
Pollen Food Syndromes (Table 5.1)
Birch fruit syndrome is the most common of all the pollen food syndromes. It is rarely associated with anaphylaxis. There is a risk of reaction to at least one food of 55% [1].
Ragweed-melon-banana syndrome in North America is related to weed pollen and usually associated with at least one other food approximately 90% of the time, i.e., avocado, banana, kiwi, and peach [1]. In Spain, melon allergy is associated with several pollens, especially grass. In Australia, watermelon is seen in patients reporting grass, tree, and weed pollen allergies [26].
Celery-mugwort-spice syndrome is seen with severe reactions especially in patients who are allergic to both the birch and mugwort pollen [8]. Spice allergy is usually related to shared epitopes of profilins and Bet v 1.
Mugwort-peach syndrome is related to sensitization to extensive cross-reactivity toward the LTPs, Bet v1, and profilins. In Spain, the allergen is the LTP, Pru p 3, the cause of the peach allergy [36]. If there is no pollen sensitization, systemic reactions are more common. Cross-reactivity with the Rosaceae fruits is 55% [1].
Mugwort-mustard syndrome in patients with mustard allergy 97% were sensitized to mugwort. Ten percent reported anaphylaxis and 40% reported reactions to cauliflower, cabbage, and broccoli [37].
Grass pollen syndrome is associated with reactions to kiwi, melon, tomato, and watermelon [38].
Latex-fruit syndrome can cause patients to experience reactions to plant foods. Almost 50% of patients allergic to natural rubber latex (NRL) will respond to avocado, banana, kiwi, chestnut, peach, tomato, white potato, and bell pepper [1, 20].
Quality of Life
In the study of children in the United Kingdom, a quality of life questionnaire was administered to families with children having PFS. The questions encompassed family and social activities, such as school and camp, social activities involving food, vacation, restaurant meals, leaving children in the care of others, and children being near others who are eating. The questionnaire discussed the time needed for meal preparation and diet precautions observed when leaving the home. Parents’ concerns about nutrition and feeling empowered to manage a reaction were addressed. In addition, the questionnaire assessed emotional issues including anxiety about reactions, frustration in dealing with others, and worries about the lack of a “normal childhood” [39]. All of the parameters measured showed moderate disruption with the most notable being the caregiver’s anxiety regarding the need to read labels and spend extra time to preparing foods [7]. Ludman’s results were in line with a similar caregiver survey by Springston et al. [40].
Clinical History
In atopic patients, seasonal allergic rhinitis develops first and then PFS. The more symptomatic the patient is with itchy eyes and nose as well as rhinorrhea the more likely they are to develop PFS, which can occur in the first 5 years of life [7]. Documenting the months when the patient has allergic rhinitis symptoms will help to isolate potential foods if unrecognized by the patient. Fresh fruits, nuts, and raw vegetables are most frequently implicated. Inquiring whether cooked food causes symptoms is important. It is essential to inquire about the associated symptoms that the food causes. The questions should focus on the presence of mucosal itching, tingling, burning, or swelling of the checks, tongue, lip swelling, change in voice or problems swallowing, gastrointestinal symptoms, urticarial lesions or anaphylaxis. The time course is informative because symptoms are apparent almost as soon as or within minutes of the food being in the mouth. It is usually relieved by swallowing or drinking water. Occasionally antihistamines are needed. Some patients will complain of gastrointestinal symptoms. Less than 8% suffer from systemic symptoms such as urticaria, angioedema, profuse diarrhea, coughing, wheezing, and hypotension [6].
Certain foods like those in the Rosaceae family can cause anaphylaxis without pollen allergy. They are considered class 1 food allergy and not PFS. Peach is the most common of these fruits to cause systemic symptoms due to LTP. There is a higher rate of clinical cross-reactivity in this family of foods and so questioning regarding reactions to related foods is important (Table 5.1). Also any history of allergic reactions to peanut or tree nut allergy should be treated as a class 1 allergy. Both peanut and hazelnut can cause PFS but it is difficult to distinguish at times from class 1 food allergy. If anaphylaxis is a concern, an epinephrine auto injector should be prescribed. If other than mild symptoms are present, an allergy consultation should be obtained.
Testing
An allergist will do testing for inhalants and suspected foods for children with allergic rhinitis and/or asthma. The season when the symptoms occur, the plant food involved and the history of the reaction will usually suggest an accurate diagnosis. Skin testing with inhalant allergens will confirm sensitization to pollen. For foods, skin testing using fresh or frozen fruit and raw vegetables usually gives better results via the prick method than skin testing with commercial extracts [26]. The commercial extracts contain more stable allergens and may not contain sufficient cross-reacting antigens to elicit a positive skin test [41, 42]. If the patient had anaphylaxis, dermographism, severe dermatitis, or cannot stop antihistamines, the initial step is to draw blood for specific IgE immunoassays. They can be informative especially if the cross-reactive antibody is heat stable. If there are questions regarding safe ingestion of a particular plant food and the testing is equivocal or negative then an oral food challenge should be done with the appropriate part of the fruit, peel, and or pulp. This is especially true if the patient had a systemic reaction to peach because there is a high clinical cross-reactivity with other food in the Rosaceae family, and the patient may want to include it in their diet. Many believe that future testing will involve utilizing component-resolved diagnostics (CRD) with a microarray of specific antigens, like the recombinant PRs, LTPs, and profilins, which will greatly enhance our diagnostic abilities. Using CRD that are available for peanut or hazelnut is helpful. In testing for peanut, the Ara h 2 is elevated in patients with more severe reactions. Not all the molecular components, like Bet v 1, PR-10, or Bet v 2, are routinely available but hopefully will provide a better picture of the natural history and severity of the PFS.
Therapy
There is no cure for food allergy, class 1 or class 2. Avoidance is currently advised for management of these food allergies. As noted, earlier allergists have advised patients to cook the cross-reacting foods to destroy the tertiary structure and render the food safe for consumption. In the presence of associated atopic conditions, when the T cell epitopes are conserved even when the food is cooked, ingesting the food might promote ongoing inflammation as seen in AD and perhaps EoE or even IBS. In the last 20 years, investigators have suggested that pollen immunotherapy could modify the symptoms of PFS. The success rate of remission with subcutaneous immunotherapy with birch pollen has reportedly ranged from 84% success to concerns that birch immunotherapy might have triggered the onset of PFS [43, 44]. There has been no success with sublingual therapy with birch pollen with regard to tolerance for apple [45]. The studies cited are not robust. The hope of future immunotherapy lies in the ability to define and administer immunotherapy with the significant clinical epitopes. Molecularly directed immunotherapy will lead to significant reduction in the oral symptoms induced and is a potential cure for both pollen and related plant food allergy.
Summary
Pollen food syndrome is increasing in the pediatric population. Studies from abroad suggest that it may occur in as high as 24% of the pediatric population and that it will increase in late childhood and adolescence depending on the severity and number of pollen allergens causing the rhinitis [10]. The foods most frequently identified as allergens are related to birch pollen and most often do not involve systemic reactions but only local reactions of the oral mucosa. A good clinical history will elicit the extent of the symptoms and whether further allergy consultation and testing is necessary. If there is any question of a systemic reaction, an epinephrine auto injector should be provided and allergy consultation should be obtained. Depending on associated atopic diseases, cooking the fruits and vegetables may be an acceptable alternative to allow the food to be ingested. Current research with recombinant allergens is hoped to bring about more accurate diagnosis and potential immunotherapy.
Case Studies
Case Study 5.1
A 5-year-old boy comes to the allergy clinic with itchy eyes, runny nose, and sneezing which started during the spring. He has had atopic eczema since he was 6 months old. His eczema is also worse. His mom notes complaints that his favorite fruit, apples, make his mouth and throat feel funny. It starts as soon as the apple is in his mouth and goes away quickly. His skin test shows a significant reaction to birch pollen, grass, and ragweed. The skin test with commercial apple extract is negative. A skin-prick test with the apple, touching the applicator through the peel, and applying pulp to the patient’s forearm is positive.
Case 5.1 illustrates a typical birch pollen-related reaction to apple. The reaction is usually worse in the pollen season and may be ameliorated with cooking of the fruit. What is interesting is the cooking of the fruit denatures the tertiary structure so the mediator release from mast cells is inhibited but does not alter the primary structure which has cross-reacting T cell epitopes that can cause worsening of eczema within 24 hours of ingestion. A small percentage, less than 8%, of patients with throat symptoms may progress to a systemic reaction. A prescription for an epinephrine auto injector would be indicated if this is a concern as well as an allergy consultation. In addition, the child is allergic to three pollens which is a risk factor of PFS. As he gets older, he might experience PFS with an increasing number of foods related to each of the pollens.
Case Study 5.2
A 12-year-old girl has a history of seasonal allergic rhinitis, atopic eczema, and moderate persistent asthma. She noted that after she ate peanut, her throat felt like it was closing after just a few bites and she developed urticaria. She has a similar reaction with cantaloupe and tomato.
Case 5.2 illustrates pan-allergen sensitization which is seen in older children with more allergic comorbidities. She may have reactions to Bet v 1, PR-14, and profilin. Her eczema might worsen within 24 hours of ingestion due to the T cell epitopes for birch pollen. Due to her symptoms and severity of atopic comorbidities, strict avoidance of the foods is advised and a prescription for an epinephrine auto injector is indicated. Consultation with an allergist is advised. If her asthma was stable she could be skin tested for the inhalants to confirm pollen sensitivity. Because of her reaction to the foods, specific IgE immunoassay to each food would be ordered. If the results were negative or very low titer, then skin testing would be done. In addition, CRD for peanut would be helpful. A high titer against Ara h 2 is consistent with more serious reactions, whereas Ara h 8 is related to the birch pollen allergens and not likely to be proceed to anaphylaxis.
References
Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108(6):881–90.
Tuft L, Blumstein G. Studies in food allergy-sensitization to fresh fruits: clinical and experimental observations. J Allergy. 1942;13:574–8.
Amlot PL, Kemeny DM, Zachary C, Parkes P, Lessof MH. Oral allergy syndrome (OAS): symptoms of IgE-mediated hypersensitivity to foods. Clin Allergy. 1987;17(1):33.
Hofmann A, Burks A. Pollen food syndrome: update on the allergens. Curr Allergy Asthma Rep. 2008;8(5):413–7.
Kelso JM. Pollen food allergy syndrome. Clin Exp Allergy. 2000;30:905–7.
Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003;112(4):784–8.
Ludman S, Jafari-Mamaghani M, Ebling R, Fox AT, Lack G, Du Toit G. Pollen food syndrome amongst children with seasonal allergic rhinitis attending allergy clinic. Pediatr Allergy Immunol. 2016;27(2):134–40.
Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006;61(4):461–76.
Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106(1):27–36.
Dondi A, Tripodi S, Panetta V, Asero R, Businco ADR, Bianchi A, et al. Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013;24(8):742–51.
Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988;61(6 Pt 2):47–52.
Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, Hoffmann-Sommergruber K, Breiteneder H, et al. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J Allergy Clin Immunol. 2000;105(1):116–25.
Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106(2):228–38.
Asero R, Pravettoni V. Anaphylaxis to plant-foods and pollen allergens in patients with lipid transfer protein syndrome. Curr Opin Allergy Clin Immunol. 2013;13(4):379–85.
Kauffmann S, Legrand M, Fritig B. Isolation and characterization of six pathogenesis-related (PR) proteins of Samsun NN tobacco. Plant Mol Biol. 1990;14:381–90.
Tellis M, Mathur M, Gurjar G, Kadoo N, Gupta V. Identification and functionality prediction of pathogenesis-related protein 1 from legume family. Proteins. 2017;85(11):2066–80.
Radauer C, Lackner P, Breiteneder H. The Bet v 1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol Biol. 2008;8(1):286.
Barre A, Culerrier R, Granier C, Selman L, Peumans WJ, Van Damme EJ, et al. Mapping of IgE-binding epitopes on the major latex allergen Hev b 2and the cross-reacting 1, 3 Beta-glucanase fruit allergens as a molecular for the latex-fruit syndrome. Mol Immunol. 2009;46(8–9):1595–604.
Midoro-Horiuti T, Brooks EG, Goldblum RM. Pathogenesis-related proteins of plants as allergens. Ann Allergy Asthma Immunol. 2001;87(4):261–71.
Blanco C, Diaz-Perales A, Collada C, Sánchez-Monge R, Aragoncillo C, Castillo R, et al. Class I chitinases as potential panallergens involved in the latex-fruit syndrome. J Allergy Clin Immunol. 1999;103(3):507–13.
Rodriguez J, Crespo JF, Lopez-Rubio A, de la Cruz-Bertolo J, Ferrando-Vivas P, Vives R, et al. Clinical cross-reactivity among foods of the Rosaceae family. J Allergy Clin Immunol. 2000;106(1):183–9.
Mastrorilli C, Tripodi S, Caffarelli C, Perna S, Di Rienzo-Businco A, Sfika I, et al. Endotypes of pollen-food syndrome in children with seasonal allergic rhinoconjunctivitis: a molecular classification. Allergy. 2016;71(8):1181–91.
Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175(2):377–85.
Yagami T, Sato M, Nakamura A, Komiyama T, Kitagawa K, Akasawa A, et al. Plant defense–related enzymes as latex antigens. J Allergy Clin Immunol. 1998;101(3):379–85.
Bedolla-Barajas M, Kestler-Gramajo A, Alcalá-Padilla G, Morales-Romero J. Prevalence of oral allergy syndrome in children with allergic diseases. Allergol Immunopathol (Madr). 2017;45(2):127–33.
Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014;50(10):795–800.
Reekers R, Busche M, Wittmann M, Kapp A, Werfel T. Birch pollen–related foods trigger atopic dermatitis in patients with specific cutaneous T-cell responses to birch pollen antigens. J Allergy Clin Immunol. 1999;104(2):466–72.
Bohle B, Zwölfer B, Heratizadeh A, Jahn-Schmid B, Antonia YD, Alter M, et al. Cooking birch pollen–related food: divergent consequences for IgE- and T cell–mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. 2006;118(1):242–9.
Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(1):83–90.
Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112(4):796–7.
Ram G, Lee J, Ott M, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115(3):224–228.e1.
van Rhijn BD, van Ree R, Versteeg SA, Vlieg-Boerstra BJ, Sprikkelman AB, Terreehorst I, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy. 2013;68(11):1475–81.
Mahdavinia M, Bishehsari F, Hayat W, Elhassan A, Tobin MC, Ditto AM. Association of eosinophilic esophagitis and food pollen allergy syndrome. Ann Allergy Asthma Immunol. 2016;118(1):116–7.
Magnusson J, Magnusson O, Lin XP, Dahlman-Höglund A, Hanson LÅ, Telemo E, et al. Seasonal intestinal inflammation in patients with birch pollen allergy. J Allergy Clin Immunol. 2003;112(1):45–51.
Tobin MC, Moparty B, Farhadi A, DeMeo MT, Bansal PJ, Keshavarzian A. Atopic irritable bowel syndrome: a novel subgroup of irritable bowel syndrome with allergic manifestations. Ann Allergy Asthma Immunol. 2008;100(1):49–53.
Pascal M, Muñoz-Cano R, Reina Z, Palacín A, Vilella R, Picado C, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012;42(10):1529–39.
Figueroa J, Blanco C, Dumpierrez AG, et al. Mustard allergy confirmed by double-blind placebo-controlled food challenges: clinical features and cross-reactivity with mugwort pollen and plant-derived foods. Allergy. 2005;60(1):48–55.
Ricci G, Righetti F, Menna G, Bellini F, Miniaci A, Masi M. Relationship between Bet v 1 and Bet v 2 specific IgE and food allergy in children with grass pollen respiratory allergy. Mol Immunol. 2005;42(10):1251–7.
Cohen BL, Noone S, Munoz-Furlong A, Sicherer SH. Development of a questionnaire to measure quality of life in families with a child with food allergy. J Allergy Clin Immunol. 2004;114(5):1159–63.
Springston EE, Smith B, Shulruff J, Pongracic J, Holl J, Gupta RS. Variations in quality of life among caregivers of food allergic children. Ann Allergy Asthma Immunol. 2010;105(4):287–94.
Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104(2):101–8.
Ortolani C, Ispano M, et al. Comparison of results of skin prick test (with fresh foods and commercial extracts) and RAST in 100 patients with oral allergy syndrome. J Allergy Clin Immunol. 1989;83(3):683–90.
Asero. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28(11):1368–73.
Modrzyński M, Zawisza E. Possible induction of oral allergy syndrome during specific immunotherapy in patients sensitive to tree pollen. Med Sci Monit. 2005;11(7):CR351–5.
Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119(4):937–43.
Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70(11):1393–405.
Price A, Ramachandran S, Smith GP, Stevenson ML, Pomeranz MK, Cohen DE. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26(2):78–88.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fox, S., Tobin, M.C. (2020). Environmental Allergies and Pollen Food Syndrome (PFS). In: Gupta, R. (eds) Pediatric Food Allergy . Springer, Cham. https://doi.org/10.1007/978-3-030-33292-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-33292-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33291-4
Online ISBN: 978-3-030-33292-1
eBook Packages: MedicineMedicine (R0)