Abstract
Traditional Chinese medicines (TCM) have been used in China for thousands of years. Although TCM has been generally perceived to be safe, adverse reactions to Chinese materia medica (CMM) have been reported. Most of the adverse reactions are allergic in nature, but other mechanisms may play a role. This review focuses on the mechanism and clinical presentation of these allergic reactions. Allergic reactions can occur as a result of the active and inactive ingredients of CMM. Impurities and chemicals generated during the production process can also lead to allergic or adverse reactions. Environmental factors such as temperature, humidity, and light can cause changes in the allergenicity of drugs. Human error in formulating CMM drugs also contributes to adverse drug reactions. The management of allergic reactions to CMM includes taking a good history, avoidance of medications in the same class as those which caused prior reactions, the proper training of staff, adherence to manufacturer guidelines and expiration dates, evaluation of benefit and risk balance, and the formulation of a risk management strategy for the use of CMM. A small test dose of a considered drug before using, improvements in drug purification technology, and proper storage and clinical administration help reduce allergic reactions due to CMM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional Chinese medicine (TCM) has been widely used in China, Japan, and Korea to treat various diseases for thousands of years. The 2015 Nobel Prize in Physiology or Medicine was awarded to Tu Youyou for the discovery of artemisinin and its antimalarial properties, which is believed to have saved millions of lives in third world countries where Western pharmaceuticals are inaccessible. But this level of recognition of TCM is a rarity. Acceptance of TCM by the Western scientific community has been slow, primarily because of the lack of scientific evidence and scant knowledge of the mechanisms of action of TCM methods. Despite this, the general public of the Western world has been much more apt to try these methodologies, despite a lack of coverage by insurance companies. In other words, patients are often willing to pay out of pocket for Chinese herbal medications and other forms of therapy, such as massage therapy and acupuncture, ironically even while they simultaneously refuse to seek mainstream Western medicine treatments due to the high cost.
In general, given its reputed effectiveness, low cost, and favorable safety profile, TCM is attracting great interest in Western societies as a source of therapy for an array of illnesses, including allergies and asthma [1]. TCM distinguishes itself from Western medicine by its principles, which involve treating the whole person and alleviating symptoms using an individualized, holistic approach. TCM encompasses a variety of methods and medications including acupuncture, medicinal herbs, tuina (Chinese medical massage), taichi, qigong (a form of exercise), moxibustion and cupping, as well as advice on dietary and various lifestyle interventions [2].
CMM
The History of CMM
The Chinese materia medica (CMM) is based on TCM theory and reflects centuries of the natural resources, history, and culture of China [3]. The CMM is used for the prevention and treatment of diseases and the preservation of functional health.
The TCM classic “Shennong’s Classic of Materia Medica” [4], written in the first century BC, listed 365 types of CMM and divided them into upper, middle, and lower grade drugs. In the Ming Dynasty, Li Shizhen spent 30 years writing the “Compendium of MateriaMedica” [5], which was divided into 52 volumes, recording 1892 types of CMM and describing the characteristics, methods of collection, features, preparation, and administration of specific drugs. Currently, the Pharmacopoeia of the People’s Republic of China includes 618 types of drug materials and prepared drugs, 47 types of botanical oils and extracts, and 1493 types of prescription preparations and single active ingredient preparations. There are many forms of CMM, ranging from botanical-based drugs and animal-based drugs to mineral-based drugs that are derived from the flowers, roots, stems, and leaves of plants, such as Chrysanthemum and Xanthium sibiricum, or from insects, birds, fish, iron ore, and minerals (Figs. 1 and 2). Among these, botanical-based drugs are the most common. Therefore, CMM often refers to Chinese herbal medicine.
Chinese Medicine Preparation
Decoction is the earliest and most widely used drug form in China. Decoctions are rapid in absorption and also potent. A decoction is the medicinal liquid made by solubilizing the medicinal substance with water or other medicinal liquids, such as alcohol or vinegar. It is the medicinal form used most widely in clinical practice, and the correct method for decoction is vital to ensure the quality of decoction and to acquire the expected curative effects. Earthenware pots and jars are mostly used to prepare decoctions. Medicinals are immersed in water for a specified time, allowing active ingredients to dissolve. The decocting time can be adjusted to avoid loss of active ingredients. Maximum heat is initially applied; then, the decoction is allowed to simmer to avoid overflowing or excessive drying out. To develop topical or aromatic medications, high heat is used to bring the decoction to boil; then, simmering is limited to 5 to 10 min. For minerals, bones, horns, shells, crust, and tonics whose active ingredients are difficult to dissolve out, low heat is applied for 30 to 40 min. After the decocting, the material is squeezed to extract all the decoction liquid. The process is repeated 2 to 3 times. Each time the herbs are boiled down until about 250 to 300 ml of liquid remains. Some ingredients in the same formula must be separately treated during the decocting process as their property, potency, and clinical use are different.
There are also pills and powder made from CMM. A Chinese medicine pill is a round solid preparation made by grinding Chinese medicine into fine powder and mixing it with water, honey, or other excipients. Powders are manufactured by crushing CMMs and sieving to obtain a fine powder. Various powders can be compounded together to create a multicomponent drug [6].
Traditionally, CMMs were not administered by injections. Modern technology has allowed for the development of CMMs administered parentally. CMM injections can be sterilized solutions or emulsions; suspensions extracted from medicinal materials. These extracts can be injected into the human body, including muscles; acupuncture points; intravenous injections and intravenous drip infusions; or preparations made from sterile powders or concentrates [7]. Compared with other Chinese medicinal administration routes, injections have the characteristics of high bioavailability, high curative effect, and rapid action.
Mechanism of Allergic Reactions
The World Health Organization (WHO) defines “drug allergy” as an immune-related hypersensitivity reaction mediated by drugs. The mechanisms of action underlying drug allergies can be IgE mediated or non-IgE mediated [8]. Since CMM is derived from nature and has a long history, it is extensively used worldwide, especially in China and other parts of Asia [9–12]. Most Chinese doctors still consider CMM to have few side effects because there are few reports of allergic reactions caused by CMM. However, the reality is that CMM can cause drug allergy and can even induce serious allergic reactions resulting in death [13]. Adverse effects of CMM have been reported not only in Asia but also in America and Europe [14, 15]. According to the Gell and Coombs classification since 1963, allergic and immunologic reactions have been categorized based on four separate mechanisms [16].
Type I Hypersensitivity
Many conventional Chinese medicines, including tree peony root bark (Cortex Moutan), Belamcanda chinensis, Pueraria lobata root, and Houttuynia cordata, as well as many CMM injections, can produce type I hypersensitivity reactions [17–20]. For example, Shuanghuanglian Injection (SHLI) has caused many serious anaphylactic reactions, to the point that SHLI was forced to amend its instruction with a warning of allergic shock by the Chinese National Medical Products Administration (NMPA, www.nmpa.gov.cn) in 2018. Using a rat model, Li et al. [18] studied SHLI-induced anaphylaxis. Total and SHLI-specific IgE levels were significantly increased after SHLI sensitization. Systemic symptoms, local skin reactions, elevated histamine levels, and decreased blood pressure were observed after challenge. Histological examination revealed that slight pathological changes occurred in the lungs, while no obvious tissue alteration was found in the gastrointestinal tract. IL-4, but not IFN-γ, levels were significantly increased in spleen cells from SHLI-sensitized rats, indicating that SHLI-induced anaphylaxis may be Th2 mediated. This rat model represents a useful tool for studying the mechanisms underlying SHLI-induced anaphylaxis, and it can draw on the experience of type I hypersensitivity caused by other CMM.
Type II Hypersensitivity
Clinical manifestations of type II hypersensitivity reactions may include hemolytic anemia, thrombocytopenia, or vasculitis. An example of a Chinese Materia Medica drug that causes a type II reaction is puerarin. Puerarin is a common Chinese herbal drug that is administered as an injection to treat hypertension and cervical osteoarthritis. Extensive research has indicated that puerarin injections can cause acute intravascular hemolysis [21]. Puerarin becomes immunogenic by combining with macromolecular proteins when it enters the body and then stimulating the immune system to produce specific antibodies, leading to a type II hypersensitivity [22].
Type III Hypersensitivity
Serum sickness and serum sickness-like reactions are a form of type III hypersensitivity. There are a few reports on the induction of this type of hypersensitivity by CMM, and most of them involve allergic cutaneous vasculitis [23]. For example, Mao has reported Houttuynia injection—a Chinese herbal injection to treat infectious diseases owing to its anti-inflammatory effect—causing two cases of allergic purpura. Both patients developed hemorrhagic papules on their distal limbs, and one also had pain in her knee joints [24].
Type IV Hypersensitivity
In 2013, we reported and confirmed a case of allergic contact dermatitis from a topical Chinese herbal medicine (white mustard seed) used for the treatment of tracheitis [29] (Figs. 3 and 4). There was also a case report of Stevens–Johnson syndrome in a 50-year-old female patient who used Pulsatilla chinensis (Bunge) Regel for 5 days [25]. Li et al. studied consecutive adult patients with strong clinical evidence of allergic contact dermatitis caused by traditional Chinese herbal medicines [26]. Patch testing was performed with the standard series developed by the Beijing Medical University in Beijing, China. The suspected herbs, including mast, myrrh, safflower, and cassia, were also patch tested. Herbs were ground into their powdered form and dispersed in petroleum. A 10% concentration of the herbs was used for patch testing. The names, ingredients, composition, and suspected allergens in 12 other traditional Chinese herbal medicines are shown in Table 1.
Allergic contact dermatitis caused by the Chinese herbal medicine, white mustard seed. a Clinical picture of allergic contact dermatitis caused by white mustard seed. b Positive (+ + +) patch-test reaction to white mustard seed (patient). c Negative patch test reaction to white mustard seed (control subjects). d Skin lesions after treatment
The clinical characteristics of patients with allergic contact dermatitis (ACD) due to traditional Chinese herbal medicines and the results of patch testing are shown in Table 2. The rates of positive responses to common contact allergens are shown in Table 3. The rates of positive results on patch testing to herbal allergens are shown in Table 4. As expected from their main ingredients, the positivity rate to either fragrance mix or colophonium or both on patch testing in this study was 91.3%, which was much higher than that in controls. These results support the hypothesis that fragrance and colophonium are the main contact allergens in traditional Chinese herbal medicines. These results also indicate that patch testing with preparations of the herbs is useful for the detection of traditional Chinese herbal medicine induced ACD.
Nonallergic Hypersensitivity Reactions and Nonallergic Anaphylaxis
Nonallergic Hypersensitivity Reactions
In addition to true allergic reactions, adverse reactions to traditional Chinese medicine injections include nonallergic hypersensitivity reactions (NHRs) and pseudoallergic reactions (PARs). Nonallergic hypersensitivity (pseudoallergy or idiosyncratic) is a nonimmune hypersensitivity reaction that mimics allergic reactions [27]. According to revised terminology proposed in 2003, the European Academy of Allergy and Clinical Immunology has recommended that each condition be categorized as allergic or nonallergic and is no longer referred to as idiosyncratic (now hypersensitivity), pseudoallergic (now nonallergic hypersensitivity), and anaphylactoid (now nonallergic anaphylaxis) reactions.
Nonallergic hypersensitivity reactions (NHRs) are generally recognized as occurring after the first exposure to antigen and not mediated by pre-existing IgE antibodies and account for more than 77% of all immune-mediated immediate hypersensitivity reactions. As in drug-induced allergic reactions, NHRs can present with urticaria, angioedema, or bronchospasm. NHRs comprise 15% of all adverse drug reactions [28]. Animal experiments have indicated that 5 pathways are involved in the mechanism of NHR, including direct stimulation, complement, coagulation, kallikrein-kinin, and IgE integrated in an anaphylatoxin-triggered pathway (Figs. 3 and 4) [27].
NHRs can occur the first time a drug is used. The drug may directly bind to immune receptors, such as the HLA molecule or to the TCR and stimulate T cells directly without haptenization and processing of a hapten-modified protein. This direct binding capacity is an inherent pharmacologic feature of most of the low molecular weight drugs designed to fit into pockets of enzymes and block their function. This kind of immune stimulation via pharmacological interaction with immune receptors (p-i concept) bypasses the classical control mechanisms of our immune system and can result in severe forms of hypersensitivity [29].
In animal experiments, NHRs were induced by CMM injections with different injection rates and doses. The serum histamine level was significantly increased in CMM injection models. CMM injection induces the release of inflammatory factors via a non-IgE-mediated immune pathway, stimulating mast cells to release histamine and β-hexosaminidase [30, 31]. The most common reported triggers were injections of Qingkailing and Houttuynia [32]. Hypersensitivity reactions resulting from the first instance of drug use account for 80 to 90% of hypersensitivity reactions due to CMM injection, indicating that NHRs play an important role in adverse reactions to CMM injections [33].
Nonallergic Anaphylaxis
Anaphylaxis is a severe, life-threatening, generalized, or systemic hypersensitivity reaction, as defined by the World Allergy Organization (WAO) [8]. Yin et al. analyzed a total of 1952 episodes of anaphylaxis in 907 outpatients and found that 36% of drug-induced anaphylaxis cases could be attributed to CMM [32]. A total of 13 herbs were found to induce anaphylaxis in that study. The most common triggers for CMM-induced anaphylaxis were injections of Qingkailing, Shuanghuanglian, Houttuynia, and Xuesaitong [32, 34].
Anaphylaxis can be classified into four types based on the pathophysiologic mechanism. The classical IgE-dependent immunologic mechanism depends on both interleukin-4 and IL-4 receptors and is characterized by the allergen-mediated cross-linking of FcεRI receptors (high-affinity IgE receptors) on mast cells and basophils. This induces the release and fulminant propagation of inflammatory mediators and cytokines causing the smooth muscle constriction and increased vascular permeability associated with clinical anaphylaxis [35, 36]. The second mechanism is an IgE-independent immunologic mechanism mediated by IgG, FcγRIII receptors, and either macrophages or basophils (depending on the experimental system). This mechanism requires proportionately more antigen and antibody than the IgE-dependent pathway. The third mechanism is nonimmunologic, in which mediators are triggered by factors such as drugs to be directly released from mast cells and basophils. Anaphylaxis due to a nonimmunologic reaction is called “nonallergic anaphylaxis.” The fourth mechanism is idiopathic [38]. Nonallergic anaphylaxis is a syndrome with varied triggers and clinical presentations that is mediated predominantly by mast cells and basophils. Some agents can cause degranulation of mast cells and basophils without help from immunoglobulins, which can lead to nonimmunologic anaphylaxis.
Xuesaitong injection, a traditional Chinese medicine with total saponins of Sanqi ginseng as active ingredients, has been used for more than 500 years to treat coronary artery disease in China [37]. Anaphylactoid reactions induced by Xuesaitong injections are the main adverse reactions that have commonly been reported in recent years [34]. RBL-2H3 is a basophilic leukemia cell line with high affinity IgE receptors from Wistar rat basophilic cells that are maintained as tumors. They can be activated to secrete histamine and other mediators by aggregation of these receptors or with calcium ionophores. They have been used extensively to study FcεRI and the biochemical pathways for secretion in mast cells. Hong [38] found the quantitation of histamine release from RBL-2H3 cells using ELISA to be a suitable in vitro method for assaying the anaphylactoid reactions of Xuesaitong injection to confirm mast cell activation. Their investigation into anaphylactoid components in Xuesaitong injections indicated that proteins greater than 10 kDa in molecular weight, but not ginsenosides, are the main constituents inducing the release of anaphylactoid mediators from RBL-2H3 cells. A high-performance liquid chromatography (HPLC) method for determining the proteins in Xuesaitong injection was subsequently established. The level of proteins with molecular weights over 10 kDa in the injections showed an obviously positive correlation with injection-induced histamine release. This experiment in vitro confirmed that Xuesaitong injections can cause anaphylaxis by inducing the degranulation of mast cells and RBL-2H3 cells.
To analyze the mechanism behind nonimmunologic anaphylaxis, we successfully established animal models for testing nonimmunologic anaphylaxis due to Chinese herbal medicine injections (e.g., mouse and rat models for Qingkailing, Shuanghuanglian, and Houttuynia [39, 40, 41] and Beagle dog models for Houttuynia [42]). Specifically, beagle dog models were used to study Houttuynia. A method for quantitative determination of histamine GC–MS in canine plasma was established. Twelve beagle dogs were randomly divided into 4 groups. The drug-administered group was injected with high-, medium-, and low-concentration Tween-80 in the treatment of Houttuynia injection and saline. After 30 min, the dog plasma was taken and derivatized by adding ethyl chloroformate/trichloromethane. The experimental method is simple, is accurate, and has a good recovery rate. It can quickly detect the release of histamine into the medium caused by traditional Chinese medicine injection [42].
Clinical Features of Allergic Reactions to CMM
Traditional CMM can be administered in a variety of ways, including extract injection, oral CMM decoctions, Chinese patent medicines, and external application, all of which cause different allergic reactions (Table 5) [43–63]. Typical allergic reactions occur in the skin, digestive tract, and respiratory and cardiovascular systems. Among these, the skin is the most commonly involved site. Chen and his group used patch testing to investigate the incidence of contact sensitivity to the components of CMM in patients with clinical contact dermatitis due to CMM [64]. Their research showed that of the main ingredients in CMM, the positive reaction rate to fragrance mix, colophonium, or both fragrance mix and colophonium on patch testing was much higher in patients with contact sensitivity than in controls, suggesting that fragrance mix and colophonium are the main contact allergens in CMM. Similar experiments have also been completed in other areas of China. CMM allergens in 11 Hong Kong topical medications were studied for their ability to cause skin reactions under occlusive conditions. The medications included White Flower Oil, Hung Far Oil, Kwan Loong Medicated Oil, Tiger Oil, Jaminton Oil, Bee Brand Oil, Tiger Balm, Au KahChuen Skin Lotions, Mopiko Ointment, Oronine H Ointment, and Mentholatum. The former 7 are traditional Chinese herbal medicaments, the last one is made in the USA, and the remaining 2 are Japanese products. Twenty patients were patch tested with the 11 medicaments. Hung Far Oil caused mild to moderately severe irritation in 8 patients; White Flower Oil, Jaminton Oil, and Tiger Balm caused mild irritation in 1 to 3 patients; and the rest showed no positive reactions at all. The authors concluded that with the exception of Hung Far Oil, all the other medicaments should be safe when applied topically. For Hung Far Oil, additional warnings as to its irritant properties and the proper method for its application should be provided for the protection of its users [65, 66].
The skin is commonly the only site of an allergic reaction. However, skin reactions are often accompanied by symptoms or signs in other organ systems. CMM-induced allergic reactions in the skin, including pimples, angioedema, exudation, blisters, erythema multiforme, Stevens–Johnson syndrome, and toxic epidermal necrolysis which can range from mild to severe [67, 68, 69]. These can also occur as a result of acupoint sticking therapy. The treatment uses CMM powders to attach to specific acupoints to stimulate acupuncture channels and collaterals.
Reactions to CMM can be limited to one organ system or may present with multisystem involvement. Allergic reactions in the respiratory system, manifested by coughing, asthma, and laryngeal edema, are also common [70, 71]. Allergic reactions in the digestive system, such as abdominal pain, diarrhea, and vomiting, are usually caused by the ingestion of specific foods or drugs [23]. In cases of severe allergy, anaphylactic shock and death may occur [13, 72, 73]. However, tryptase was not tested in most Chinese reports because the test is not yet approved by NMPA.
Allergens
Drug Impurities
In the past 20 years, there have been many reports in China of allergic disorders caused by CMM injections (Table 6), [19, 74–84]. Some researchers believe the majority of allergic reactions induced by CMM injections result from the presence of soluble substances in the injected material when the purification process does not meet quality standards [85]. The hypersensitivity reactions are similar type I reactions [20, 86]. Allergic symptoms induced by CMM include skin flushing, headache, edema, hypotension, and shock [86]. Anaphylaxis, which has been reported often, is the most severe adverse reaction induced by CMM injections [20, 87, 88]. In the case of decoctions, alcohol may be present but has not often been reported to cause allergic reactions. A past history of an allergic reaction to alcohol helps make a proper diagnosis of alcohol allergy.
Unique Allergen
Heterologous immunogenic proteins are an important cause of allergic reactions induced by CMM. The immune system remains in a highly reactive state after recognizing heterologous pathogens and molecules. When the human body encounters a heterologous protein or peptide, an MHC-antigenic peptide complex is formed. This initial interaction can occur at any of the host–environment interfaces in the body, including the gastrointestinal tract (oral medications), skin (topical preparations), or intramuscular or subcutaneous tissue (injections). The complex specifically interacts with T cell antigen receptors on the surface of nonsensitizing T lymphocytes to produce specific antigen recognition signals that induce allergic reactions [84].
The sensitizing heterologous proteins in CMM are derived from two main sources. One source includes drugs prepared from animals such as scorpions, leeches, earthworms, and cicada slough, all of which have heterologous proteins as important components [89]. The other source is herbal plants, such as Radix trichosanthis, an herb used in diabetes, hepatitis, and respiratory disease [90]. Trichosanthin, which is extracted from Radix trichosanthis, has been associated with acute allergic reactions [91, 92]. All of these preparations contain large amounts of heterologous proteins, many of which are not removed during the preparation process. In some cases, the heterologous proteins are active ingredients of CMM [93].
For oral drugs, if proteins that are capable of inducing allergic reactions are not rendered nonimmunogenic in the digestive system, they will induce a Th2 reaction, which occurs through polarization of T cells and isotype switching of B cells with production of antigen-specific IgE, resulting in a hypersensitivity reaction [94]. The same reaction can occur through any of the other administration routes, with slight variations in mechanism due to local differences in immune cells and mediators in various tissues. In addition, due to limitations in the purification process, some CMM injections may contain residual heterologous proteins unrelated to the therapy that can induce an allergic reaction. This is one of the major mechanisms underlying CMM injection-induced hypersensitivity responses [95].
Mineral medicine containing heavy metals is one of the forms of traditional Chinese medicine and is also an indispensable part of TCM. Examples include Cinnabar, a common sedative and tranquilizing drug, and realgar, an important drug for treating leukemia. The 2015 edition of the Chinese Pharmacopoeia contains 1493 traditional Chinese medicine preparations [7, 88]. About 7% of them contain one or more heavy metals. Due to the potential toxic effects of heavy metals, they are used less frequently now. Cinnabar is still widely used orally or topically [96]. A patient taking Cinnabar experienced itchy skin, diffuse erythema on the trunk and limbs in a symmetrical distribution, and a red millet-sized rash after taking Cizhu pills; the rash subsided after drug withdrawal and hormone therapy [97]. A 1% Cizhu pill (composed of magnet, cinnabar, and medicated leaven) reagent was used for the spot test, and a positive reaction was observed at 24 h [97]. A patient with repeated exposure to cinnabar developed skin papules, blisters, and itching, which improved after treatment with anti-allergic drugs [98].
Complex CMM Ingredients
CMM itself contains many potential antigens, including proteins, flavonoids, polypeptides, and polyglycosides [93]. In fact, any single CMM decoction is usually a complex consisting of many compounds. The resulting soup is what is used for treatment [99]. In addition to the original components, additional products may be generated during the preparation process. Occasionally, patients are not allergic to an individual CMM but experience allergic reactions to CMM compounds after decoction. Studies have begun to focus on identifying the allergenic components of CMM decoctions. However, the task of identifying antigens in compounded drugs is still far from complete [100, 101].
CMM Mercury Preparations
The heavy metal components of mineral based medications used in CMM can often cause allergic reactions. Mercury is present in some CMM, such as cinnabar and calomel, and is the most common component causing harmful reactions. Mercury-containing preparations are used to treat a variety of skin diseases [102, 103]. Allergic reactions caused by these preparations are not rare and have been reported in China and the Western world [96, 104]. Mercury can become a superantigen after entering the blood, inducing lymphocyte proliferation and stimulating lymphocytes to anchor to the peptide-binding groove of MHC class II molecules. Hypersensitivity occurs when another region of this molecule engages with T cells [105]. If the patient has a hypersensitivity to mercury, drugs containing mercury should be avoided.
Mercury can also be toxic to humans. Standardizing dosing methods, controlling medication dosage and duration of use, and reducing the level of soluble mercury and free mercury in cinnabar through careful processing can reduce the incidence of toxic reactions [96].
Cross-reactivity
Cross-reactivity refers to the condition that occurs when a specific antibody or T cell receptor can respond to two different allergens of similar secondary and/or tertiary structure. This phenomenon occurs quite often in clinical practice, and there are specific disorders that occur as a result of cross-reactivity, such as oral allergy syndrome [106].
Cross-reactivity can also occur in CMM. Common cross-reactivity in CMM usually involves similar species. For example, patients who are allergic to Artemisia pollen often display high sensitivity to medicinal materials containing pollens such as Chrysanthemum, Artemisia apiaceae, Artemisia argyi, Lonicera japonica, and Carthamus tinctorius [107, 108]. However, different products from the same species can also show cross-reactivity. To clarify the allergenicity of the nonpollen containing components of Artemisia annua, Leng collected and extracted Artemisia annua leaves and stems before the pollination period; their results showed that the pollen-free plant extracts had in vivo allergenic activities [109]. Cocklebur is a common CMM drug for treating sinusitis and allergic rhinitis, and patients who are clinically allergic to cocklebur may also be allergic to cocklebur seed present in CMM [110].
Risk Factors for Allergy
Patients’ Physical Characteristics
Adverse drug reactions due to allergies are considered to be either Gell or Coombs type I or IV hypersensitivity reaction. Both type I and type IV hypersensitivity reactions are not only based on the pharmaceutical properties of the drug but also determined by host factors (type B drug reactions). Susceptible populations should be highly alert to the possibility of allergic reactions to CMM. It has been generally found that a risk factor for drug allergy is a previous history of allergy to other drugs. The susceptible population may also include those with atopy and may depend on the age of the patient. Atopy is associated with a high risk of allergic reactions [111].
Age may present a varying risk factor for allergic reactions to CMM. Research based on the Beijing Pharmacovigilance Database (BPD) showed that the age range of 135 patients who suffered anaphylaxis induced by TCM injections was 4 to 90 years, with children under 18 years comprising 5%; adults between 18 and 59 years, 58.6%; and adults over 60 years, 36.4% [112]. The rate of many forms of atopic diseases, including food allergy and environmental allergies, has also been increasing in the developed world, which includes the affluent cities in China. Since many forms of TCM utilize components that are derived from insects and plants, there is a chance that these may cross-react with common allergens such as dust mite and pollen and this raises the possibility of developing an allergic reaction to TCM based on previous sensitization to common environmental allergens. Many manufacturers of TCM are aware of this possibility and include a warning in their packaging.
Contraindications
The use of CMM has very strict indications. The use of CMM generally follows guidelines defined by syndrome differentiation and the application of established treatment strategies based on TCM theory. CMM is described as having four properties and five tastes. The four properties are cold, hot, warm, and cool; the five flavors are sour, bitter, sweet, spicy, and salty. Different diseases call for the use of symptomatic drugs based on the differentiation of the patient’s patterns. As an empirical medical system independent of conventional Western medicine (CWM), over thousands of years, traditional Chinese medicine (TCM) has established its own unique method of diagnosis and treatment based on the different symptoms. In TCM clinical practice, the relationship between symptoms and patterns can be seen as a form of latent group analysis in which patterns of symptoms may present various syndromes. The usage of CMM is based on the above-mentioned theory [113, 114]. The use of the wrong medication can also result in allergic reactions [115].
Poor Storage
Improper storage methods can also lead to CMM sensitization. Long-term storage under improper conditions of temperature and humidity may change the pH of CMM or catalyze the formation of metabolites, potentially leading to the production of insoluble microparticles and new antigens [116]. CMM and its processed products are exposed to the air during the preservation process. Factors such as temperature, light, and humidity can have an effect on allergenicity. On the other hand, dryness can impact the effectiveness of herbal medications. For example, ginseng is a relatively rare herb which is expensive and reserved for severe illness in TCM. When stored for long periods in dry conditions, it will gradually lose its effectiveness [117]. High temperatures can cause decomposition of starch, protein, sugar, and other active components, and extremely high temperatures can denature some proteins. Elevated humidity can cause drug metamorphosis, and medications can become contaminated by molds, which can lead to allergy reactions [118, 119].
Insoluble microparticles may mediate the production of immune complexes during allergic reactions. These immune complexes may eventually cause vascular obstruction and organ failure in a type III hypersensitivity reaction [120]. During the preparation process, stabilizers or antioxidants are often added to CMM injection formulations to extend the shelf life of the product. The wrong use of solvents may change the pH of the solution, resulting in the formation of insoluble substances that can cause allergic reactions [116, 121, 122]. For example, Xiangdan injection is an injection used in the CMM treatment of coronary heart disease. It has been reported that erroneous use of low molecular dextran rather than saline solution during reconstitution has resulted in allergic reactions, including anaphylactic shock [123].
Drug Incompatibility
The use of inappropriate drug combinations can result in severe allergic reactions. The combined use of Chinese and Western medicine may induce allergic reactions. Some doctors have reported that combinations of CMM and antibiotics, such as the combined use of methacycline and Phellodendron amurense capsules, can induce anaphylactic shock. The possible mechanism underlying these effects includes the generation of new compounds and the resulting cascade reactions. The mechanism may involve the production of new antigens [124]. Houttuynia cordata is commonly used to treat pulmonary infections in China. It has been reported that its use in conjunction with Ciprofloxacin has been associated with anaphylaxis [125, 126].
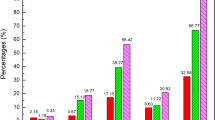
According to the annual report of national adverse drug reaction monitoring from 2009 to 2015, CMM injections account for half of CMM Adverse Drug Reactions (ADRs). Based on these reports from the National Center for Adverse Drug Reaction Monitoring (CNCADRM) [127] in China, we found that CMM injections account for 50.1–52.5% of adverse reactions (Fig. 5). Combined use of various CMM and other products is the primary cause of allergy resulting from CMM injections (CMMI). For example [128], in Shanghai Jiading District Central Hospital, there were 65 cases of CMMIADR between 2013 and 2015, with 59 of those cases (90.77%) involving combined use of CMMIs. One favorable observation was that the increase in the rate of reactions from CMM injection is lower than that for ADRs in general (Fig. 6). The proper training of medical workers in drug errors and patient safety has been deemed a national priority in China.
Prevention of Allergy
Allergy History
Before administration of CMM, doctors should inquire about the patient’s history of allergies. Patients who have a history of allergy caused by CMM should be more cautious about using other forms of CMM [129]. A detailed allergy history includes the names of drugs that cause allergic reaction, dosage, dosage form, symptoms, and treatment that was used during allergic reactions. The history can help determine if the reaction was allergic or nonallergic in nature. The patient’s past history of allergy can not only indicate whether the patient is atopic but also assist in determining whether cross-allergic reactions are likely to be triggered by the same group of drugs based on the types of drugs that have been previously shown to cause allergy in the patient. For example, Shuanghuanglian should not be used if a patient is allergic to Artemisia, because it originates from Artemisia apiaceae. The use of the same class of drugs that previously caused allergic reactions should be avoided as much as possible.
Minimizing Combined Use with Other Drugs
Lin [130] has studied the risk factors of allergic reactions caused by traditional Chinese medicines. The results suggest that the chi-square test of severe allergic reactions caused by combination drugs and traditional Chinese medicine injections is significantly different (P = 0.001), and the relative risk (OR) is 1.479. High-risk combination drugs are penicillin, vitamin C, dexamethasone, and cephalosporin antibiotics. Combined use with other drugs should be avoided to reduce the generation of compounds that cause sensitization, especially in the case of antibiotics and chemotherapeutic drugs, which readily induce sensitization. If it is necessary to combine CMM preparations with other preparations, they should be used at different time points with at least a 30-min interval between administrations. When combining Chinese and Western medicines in clinical practice, the metabolic mechanism should be understood and actively applied in clinical practice. The effects of the combined use on drug activity and dosage should be fully considered to ensure the safety and effectiveness of the medication. If necessary, drug monitoring can be carried out. Improvements in the education and the safety awareness of doctors and pharmacists on the combined use of Chinese and Western medicine are necessary. Doctors and patients should be encouraged to actively report adverse reactions of combined use of Chinese and Western medicine. At the same time, with the rapid development of modern computer technology, computers can be used to establish pharmacovigilance-related databases for the combined application of Chinese and Western medicines, and new methods such as data mining and a spontaneous reporting system for adverse reactions can be used to monitor and identify the combined use of Chinese and Western medicines. An emergency treatment plan helps to minimize the risk of combined use of Chinese and Western medicines [131].
Storage
Drugs should be stored in the proper environment and used within their shelf life [122]. Many CMM injections require storage at ambient temperatures in a dark, clean, and dry environment. It should be ensured that CMM preparations are appropriately stored according to the specific requirements.
Elderly Patients and Patients with Liver or Kidney Dysfunction Should Use CMM with Caution
Elderly patients (> 60 years old) and patients with liver or kidney dysfunction should be especially cautious about using CMM preparations because variability in drug clearance in these patients may extend the time required for the metabolism of drugs. Because drugs may have decreased clearance and a longer half-life in such patients, drug concentrations in serum and tissue may remain persistently high over a more extended period, and these patients may be at higher risk for sensitization [132]. In addition, severe allergic reactions may affect liver and kidney function, thereby potentially aggravating pre-existing disease in these organs. Patients should inform their physician regarding the presence of such conditions.
Patient Allergen Screening
Many patients with allergic diseases receive only symptomatic treatment. The underlying causes of the allergy are often not identified, so counseling the patient on avoidance is challenging. Targeted prevention and treatment of the underlying cause or trigger solely based on the history often cannot be accomplished, leading to recurrent attacks of allergic reactions that may vary in severity. It is suggested that patients who frequently experience allergic reactions undergo allergen screening. The possibility of cross-reacting allergens should also be considered. For example, patients who are allergic to pollens, especially Artemisia pollen, have a high probability of experiencing allergic reactions after using Reduning injection, Ciwujia injection, or other CMM that contain similar species, such as Artemisia apiaceae or Chrysanthemum [133].
The main allergens identified in most tree nuts such as Chinese chestnut [134], almonds, ginkgo seeds, and peanut, which are commonly used in traditional Chinese medicines, include a 2S Albumin and 7S, 11/12 S globulins, which have been reported to cause allergic reactions [135]. Tree nuts may also be contaminated by peanut, which is the most common cause of fatal anaphylaxis among foods. Pericarpium zanthoxyli is a form of CMM used in gastroenteritis (oral route) and arthritis (topical use). Li analyzed 15 cases of with Pericarpium zanthoxyli allergy and studied its allergenic components. The patients suffered from multiple food allergies to cashew, pistachio, orange, kumquat, sesame, almond, hazelnut, pine nut, and mango, suggesting that possible allergenic cross-reactivity was present in these cases [136]. Pericarpium zanthoxyli cap can be obtained from Thermo Fisher Scientific Co., Ltd for research purposes.
Xiao et al. [137] have developed a novel fast allergy skin test detector for TCM injections, combining high-amplitude pulses with a permeation device for drug solution with different shapes that is compatible with skin test electrodes, nano-sponge patch adsorption, and flexible liposome coverage. It functions as a form of rapid patch test utilizing ultrasonic technology. A patent was submitted to the China National Intellectual Property Administration (CN201968714U). This device used 0.9% normal saline and 10% glucose as negative controls. Advantages include a simple design structure, convenient operation, low-drug dose for a skin test, human safety, no skin irritation, and a low-false positive rate. The accuracy of this device has not been validated. Early trials in five patients suggest that there may be a role for this device in evaluating allergy to injected CMM drugs. This device is currently in clinical trials and is not currently commercially available for use in patients.
Identification of Allergenic Components and Improvement of Drug Purification Technology
Drug impurity plays an important role in allergic reactions. Determining how to identify active ingredients, inactive ingredients, and allergenic components and improve drug purity is a significant undertaking. For example, Shuanghuanglian injection (SHLI) is derived from an extract of Scutellaria baicalensis root, Honeysuckle, and Fructus forsythiae and is a widely used form of CMMI to treat pneumonia, bronchitis, and COPD in China [138]. However, it has been reported to cause serious allergic reactions [139]. In vitro and in vivo models have been used in the identification of allergic components. Yi and his group [140] screened the main ingredients in SHLI extract (extract of Scutellaria baicalensis, japonica, and Forsythia suspensa) and found that forsythoside A and arctiin, which are present in the extract and have high chemical polarity, cause pseudoallergic reactions. The removal of forsythoside A from SHLI with HPLC–MS technology reduced the incidence of pseudoallergic reactions and significantly improved safety.
RBL-2H3 is a basophilic leukemia cell line from Wistar rat basophilic cells that are maintained as tumors. These cells have high affinity IgE receptors. They can be activated to secrete histamine and other mediators by aggregation of these receptors or with calcium ionophores. They have been used extensively to study FcεRI and the biochemical pathways of secretion of mediators in mast cells. The effects of baicalin, forsythin, phillyrin, forsythiaside A, chlorogenic acid, and cryptochlorogenic acid on RBL-2H3 cells have been studied. Chlorogenic and cryptochlorogenic acid were associated with the degranulation of RBL-2H3 cells and enhanced production of 5-HT [138, 141]. With the use of RBL2H3 cells in this degranulation model, a novel in vitro detection method for anaphylactoid reaction was established based on fluorescent labeling and high content screen (HCS) system. Thirty samples of Danhong injection (DHI) with clinical allergy symptoms were used to confirm the reliability of this HCS method. The HCS results showed high consistency with the clinical data [142].
Ultrafiltration is an effective way to decrease the incidence of CMMI-induced allergy. For instance, Reduning injection is derived from an extract consisting of Artemisia apiaceae, Honeysuckle, and Cape jasmine. It is a TCM given by injection that has multiple TCM effects, such as clearing heat, dispelling wind, and detoxification. There have been multiple reports of allergic reactions to Reduning injection. A review of the literature on adverse drug reactions caused by Reduning injection showed that the longest time to onset of an ADR following drug administration was 7 days, whereas the shortest interval was 2 min. A breakdown of the data showed that 70.16% occurred after 30 min, 16.57% occurred between 30 and 60 min, and 13.27% occurred after 1 h. The main clinical manifestations were urticaria, diarrhea, abdominal pain, anaphylactic shock, lethargy, convulsions, confusion, difficulty breathing, laryngeal edema, and facial edema (Table 7)[143]. Most patients experiencing an ADR fully recover. The authors also noted that honeysuckle contains chlorogenic acid, which is a hapten, is sensitizing and usually combines with proteins to form a complex that causes allergies.
Ultrafiltration may also significantly reduce sensitization to Reduning injection, which is likely due to the decrease of Tween-80 [144]. Tween-80 is a nonionic detergent and is mostly used in Chinese medicine injections to assist in solubilization. However, Tween-80 can induce hypersensitivity reactions, including anaphylaxis [144-148]. Refining methods of Reduning injection by ultrafiltration is effective and Jiangsu Kanion Pharmaceutical Co., Ltd, has succeeded in using ultrafiltration to develop higher purity in Reduning injections [149]. Ultrafiltration has also been used by many Chinese pharmaceutical companies to produce products of higher purity (e.g., Qiingkailing, Shuanghuanglian, Danshen, Qiingkailing, Chuanshentong) [150].
The advances in biomedical, chemical, and computational technology have provided an opportunity to take advantage of multidisciplinary approaches for investigating evidence-based aspects of TCM practice [151]. The ultrafiltration used in decreasing the incidence of Reduning-induced allergy above is a good example. Research involving quality control in the manufacture of TCM products, relatively new systems in biology, and experience-based TCM principles is vital to integrating the holistic approach of TCM with mainstream medicine. Networking, cross-cultural research collaboration, and an open-minded approach are required to support scientific innovations and investigations to ensure that TCM treatments provide benefits in maintaining good health and treatment of diseases and are free of dangerous side effects. Continued efforts to develop an advanced scientific methodology (functional genomics, proteomics and metabolomics) and a commitment to research and development are the key to providing new knowledge for training the new generation of physicians in integrative medicine and TCM in particular.
Summary and Outlook
As medicine continues to develop, the application of CMM will become more extensive. Ongoing research to elucidate the mechanisms of CMM is increasing year by year. As these products are more extensively used, the occurrence of allergic reactions caused by CMM will present a more common scenario for both doctors and patients. It is particularly worrisome that a review of the literature revealed reports of fatal reactions to CMM. In these cases, a risk benefit analysis would almost certainly curtail some of the use of these medications, since the benefits would be outweighed by potentially rare but serious outcomes. As CMM use becomes more mainstream in various cultures, it is imperative that we understand the mechanism of action of these pharmaceutical products, as well as the associated side effects, so that judicious use by patients and physicians can be achieved. Unfortunately, current data is still insufficient and much of the literature is not peer reviewed.
Granular dissection of the components of CMM will help to identify the culprits of allergic reactions so that they may be removed or mitigated, allowing patients to continue to enjoy the benefits of the nonallergic component of CMM. On the other hand, patients with a history of allergy should be cautious about using CMM preparations. Patients should request that a small dose of a considered drug be tested in a provocation test such as the CMM patch test or the local injection skin test. The management of drug usage and storage, improvements in drug purification technology, and appropriate clinical administration can also reduce the occurrence of allergic reactions due to CMM. More studies should be performed to identify possible allergenic substances in CMM prior to their clinical use.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACD:
-
Allergic contact dermatitis
- ADR:
-
Adverse drug reactions
- BC:
-
Blood circulation
- CMM:
-
Chinese materia medica
- CMMI:
-
Chinese materia medica injection
- HMs:
-
Herbal medicines
- RC:
-
Rheumatic condition
- SHLI:
-
Shuanghuanglian injection
- sIgE:
-
Specific IgE
- TCHM:
-
Topical Chinese herbal medicine
- TCM:
-
Traditional Chinese medicines
- UTI:
-
Urinary tract infection
References
Kern J, Bielory L (2014) Complementary and alternative therapy (CAM) in the treatment of allergic rhinitis. Curr Allergy Asthma Rep 14(12):479
Robinson N, Lorenc A, Ding W, Jia J, Bovey M, Wang XM (2012) Exploring practice characteristics and research priorities of practitioners of traditional acupuncture in China and the EU-A survey. J Ethnopharmacol 140(3):604–613
Gao XM (2005) Chinese traditional medicine, vol. 1, 1st edn. Beijing: China Press of Traditional Chinese Medicine
Sun XY, Sun FJ (2003) Shennong’s classic of materia medica. Scientific and Technical Documentation Press, Beijing
Li SZ. (1590) Compendium of materia medica. Beijing: People's Medical Publishing House
Teng JL (2019) Chinese materia medica. 2nd edn. People’s Publishing House, Beijing
Commission CP (2015) Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing
Johansson SG, Bieber T, Dahl R et al (2004) Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 113(5):832–836
Borrelli F, Capasso R, Izzo AA. Garlic (2007) (Allium sativum L): adverse effects and drug interactions in humans. Mol Nutr Food Res 51(11):1386–1397
Nagai M, Fukamachi T, Tsujimoto M et al (2009) Inhibitory effects of herbal extracts on the activity of human sulfotransferase isoform sulfotransferase 1A3 (SULT1A3). Biol Pharml Bull 32(1):105–109
Coppola M, Mondola R (2012) Potential action of betel alkaloids on positive and negative symptoms of schizophrenia: a review. Nord J Psychiatry 66(2):73–78
Kim BS, Song MY, Kim H (2014) The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J Ethnopharmacol 152(3):532–539
Ji KM, Li M, Chen JJ, Zhan ZK, Liu ZG (2009) Anaphylactic shock and lethal anaphylaxis caused by Houttuynia Cordata injection, a herbal treatment in China. Allergy 64(5):816–817
Sadler C, Vanderjagt L, Vohra S (2007) Complementary, holistic, and integrative medicine: butterbur. Pediatr Rev 28(6):235–238
Calapai G, Miroddi M, Minciullo PL, Caputi AP, Gangemi S, Schmidt RJ (2014) Contact dermatitis as an adverse reaction to some topically used European herbal medicinal products - part 1: Achillea millefolium-Curcuma longa. Contact Derm 71(1):1–12
Coleman JW, Blanca M (1998) Mechanisms of drug allergy. Immunol Today 19(5):196–198
Huang C, Luo X, Li H, Liu J, Mei X, Xu J. Effect of traditional Chinese medicine injections on type I allergy. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica. (2011) 36(6):801–805
Li Z, Gao Y, Wang H, Liu Z (2010) A rat model of Shuang Huang Lian injection-induced anaphylaxis. Asian Pac J Allergy Immunol 28(2–3):185–191
Bian ZX, Tian HY, Gao L et al (2010) Improving reporting of adverse events and adverse drug reactions following injections of Chinese materia medica. J Evid Based Med 3(1):5–10
Guo YJ, Wang DW, Meng L, Wang YQ (2015) Analysis of anaphylactic shock caused by 17 types of traditional Chinese medicine injections used to treat cardiovascular and cerebrovascular diseases. Biomed Res Int (2015):420607
Chen XH (2010) Analysis of 266 cases of ADRs induced by puerarin injection. Chin Pharm Aff 24:203–205
Deng PY, Zhang J, Zhu YZ (2004) Clinical analysis of acute hemolytic anemia caused by puerarin injection. Chin J New Drugs. 13(8):140
Satpute P, Yadav L, Ahmed R, Kashid A, Peter K (2014) Herbal toothpowder induced erythema multiforme. J Clinical Diagnostic Res 8(3):275–276
Mao XC (2003) 2 cases of anaphylactoid purpura caused by Houttuynia injection. Chin J Integr Med 23(7):510
Xing Y. (2011) Three cases of contact dermatitis caused by topical Chinese traditional medicine. Int J Dermatol Vener 37(3):191-192
Li LF, Wang J (2002) Patch testing in allergic contact dermatitis caused by topical Chinese herbal medicine. Contact Derm 47(3):166–168
Xu Y, Guo N, Dou D, Ran X, Ma X, Kuang H (2016) Proteomics study on nonallergic hypersensitivity induced by compound 4880 and ovalbumin. PLoS ONE 11(2):e0148262
Gomes ESR, Marques ML, Regateiro FS (2019) Epidemiology and risk factors for severe delayed drug hypersensitivity reactions. Curr Pharm Des 25(36):3799–3812
Hausmann O. (2017) Chapter 9 - Drug Allergy A2 – O’Hehir, Robyn E. In.: Holgate ST, Sheikh A, (eds). Middleton’s allergy essentials. Elsevier, p 225–247
Huang CG, Mo ZC, Wu SL, Luo CL, Wang M. (2014) Study on anaphylactoid reaction induced by Shuanghuanglian injection. Chin J Info on TCM. 21(9):64-67
Li LM, Jin RM, Fu SG, Yao GT (2014) Studies on anaphylactoid reactions induced by traditional Chinese medicine injections of Qingkailing and Xuesaitong. Chin J Info on TCM. 21(9):53-57
Jiang N, Yin J, Wen L, Li H (2016) Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy, Asthma Immunol Res 8(4):353–361
Liang AH, Yi Y, Zhang YS, et al. (2015) Pseudoallergic reactions of traditional Chinese medicine injections and the approaches for risk prevention and control. Chin Pharm J 50(15)
Xiang Z, Qiao T, Xiao H et al (2013) The anaphylactoid constituents in Xue-Sai-Tong injection. Planta Med 79(12):1043–1050
Finkelman FD. (2007) Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 120(3):506–515; quiz 516–507
Kalesnikoff J, Galli SJ (2010) Anaphylaxis: mechanisms of mast cell activation. Chem Immunol Allergy 95:45–66
Long W, Zhang SC, Wen L, Mu L, Yang F, Chen G (2014) In vivo distribution and pharmacokinetics of multiple active components from Danshen and Sanqi and their combination via inner ear administration. J Ethnopharmacol 156:199–208
Hong Z (2013) Adverse drug reactions induced by Xuesaitong injection: ADR of 44 cases. Lishizhen Med Materia Medica Res 8(24):2
Liang A, Zhao Y, Li C, et al (2012) Methodology for preclinical assay of pseudoallergy of injectable drugs (II) rat model for assay of cutaneous pseudoallergy induced by injections. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chine Materia Medica 37(13):1871–1874.
Liang A, Li C, Yi Y, et al (2012) Methodology for preclinical assay of pseudoallergy of injectable drugs (I)--mouse model for assay of pseudoallergy induced by injections. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica. ) 37(13):1865–1870
Li C, Liang A, Gao S, et al (2011) Development of animal model for anaphylactoid test of rodent. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica 36(4):488–491
Chen Y, Zhang YY, Liang ZL, et al (2012) GC-MS for rapid determination of allergenic media release induced by Houttuynia cordata injection. Chin Tradit Med 34(1):63–66
Cui ZY (2000) A case report of anaphylaxis caused by Patrinia scaniosaefolia China J Chin Materia. Medica 25(5):348
Shi WH, Guo R, Wang YH, Zhang HW (2004) A case report of anaphylaxis caused by Liquidambar formosana Hance. Lishizhen. Med Materia Medica Res 15(6):367
Gou LK (2001) A case report of allergic eruption caused by notoginseng radix rhizome. People’s Military Surgeon 44(8):492
Chen KL (2002) 4 cases of allergic dermatitis caused by aloe. Chin J Drug Abuse Prev Treatment 237(5):45
Huang DW, Wu Y, Liu BJ (2002) A case report of allergic eruption caused by Astragalus membranaceus. Med J Liaoning 16(5):272
Huang LY (2016) A case report of drug eruption caused by Rhizoma smilacis glabrae. J Prac Tradit Chin Med 32(9):934
Li Y (2007) A case report of allergic dermatitis caused by Draconis sanguis. NeiMongol J Tradit Chin Med 26(3):22
Qiu DY (2004) Two cases of urticaria caused by Feculae bombycis. Clinical Misdiagnosis Mistherapy 17(11):828
Wang Y, Guo YP, Wei WP (2002) A allergic case report of Bruceae fructus external used Modern Medicine. J China 4(1):11
Wang LX, Li P (2002) A case report of severe anaphylaxis caused by Carthami flos. Chin J Integr Tradit West Med 22(5):361
Fang XL (2002) A case report of anaphylaxis caused by Scutellaria barbata herba decoction. China J Chin Materia Medica27(8):634
Niu KY (2009) A case report of anaphylaxis caused by herb decoction. Chin J Info Tradit Chin Med 16(6):110
Lin ZL (2004) Two cases of eupatorium anaphylaxis. Zhejiang J Integr Tradit Chinand West Med 14(2):82
Liang HF, Kong XL, Lu GL, Gu YG, Nie ZH (2012) Toxic epidermal necrolysis caused by Huahongpian. J Clinl Dermatol 10:610–611
Wang LX, Li P (2002) A severe anaphylaxis caused by safflower Carthamus. Chin J Integr Tradit West Med (5):361
Luo XN, Wang SM (2001) Two allergic cases caused by traditional Chinese medicine. J New Chin Med 4:67
Cao YH, Lu J (2001) Allergic asthma caused by Thunberg Fritillary Bulb power. Lishizhen Med Materia Medica Res 8:755
Gao WW (2011) A case report of anaphylaxis caused by Daturae. China’s Naturopathy 1:59
Li W, Li SX, Zhu JS (2004) 5 cases of anaphylaxis caused by radix trichosanthis. Hebei Med J 26(1):79
Zhou B, Jiang QF (2012) A case report of children allergic caused by Cordyceps. Chin J Ethnomed Ethnopharm 15:125
Zhang YZ, Peng Y (2012) A case report of anaphylaxis caused by Leonuri herba. Hubei J Tradit Chin Med 34(10):61
Chen HH, Sun CC, Tseng MP, Hsu CJ (2003) A patch test study of 27 crude drugs commonly used in Chinese topical medicaments. Contact Derma 49(1):8–14
Li LF (1995) A clinical and patch test study of contact dermatitis from traditional Chinese medicinal materials. Contact Derma 33(6):392–395
Lee TY, Lam TH (1990) Patch testing of 11 common herbal topical medicaments in Hong Kong. Contact Derma 22(3):137–140
Batra S (2006) Serious cutaneous adverse reactions to traditional Chinese medicines. Singapore Med J 47(7):647
Wu ML, Deng JF (2013) Toxic epidermal necrolysis after extensive dermal use of realgar-containing (arsenic sulfide) herbal ointment. Clin Toxicol (Phila) 51(8):801–803
Nakajima H, Sawaguchi H, Tsuji F, Miyamoto T, Nakajima S, Tohda Y (2008) Case of acute respiratory failure caused by drug-induced lung injury by shiniseihaito. Arerugi = [Allergy] 57(1):59–63
Minov J, Karadzinska-Bislimovska J, Vasilevska K, Risteska-Kuc S, Stoleski S (2007) Occupational asthma in subjects occupationally exposed to herbal and fruit tea dust. Arhiv Za Higijenu Rada I Toksikologiju 58(2):211–221
Suzuki S, Tanaka A, Arai T, Adachi M (2004) Case of interstitial pneumonitis induced by a Chinese herbal medicine, bofu-tsusho-san. Nihon Kokyuki Gakkai zasshi = J Jap Re Soc 42(8):777–781
Chen LJ, Wang Y, He S, Liao GP, Zhang ZY (2011) Study on the toxic reaction induced by single dose of Qingkailing injection. Zhong yao cai = Zhongyaocai = J Chin Med Mat 34(2):254–258
Sun Z, Lian F, Zhang J, Sun J, Guo Y, Zhang Y (2013) A literature analysis on 14 cases of allergic shock caused by safflower injection. Afr J Tradit Complement Altern Med / Afr Netw Ethnomed 10(6):563–567
Chen ZY, Zhang YQ, Gao HQ (2012) Allergic reactions caused by Astragalus. Guide China Med 10 (10):648–649
Gong S (2008) A case report of Acanthopanax injection cause allergic reactions. Prac Pharm Clin Remedies 11(5):284
Li L (2010) Analysis of adverse reactions of tanshinone IIA sodium sulfonate injection. Prac Pharm Clin Remedies 13(1):55–56
Wang HL, Zhang J (2007) Adverse reactions of puerarin. Chin J Misdiag 7(8):1915
Wu L, Wang Y, Zhu Y (2005) Analysis of allergic reaction of Carthamus tinctorius injection. Zhejiang J Integr Tradit Chin West Med 15(7):457–458
Liang B, Zhang J (2009) Bronchospasm caused by Shengmai injection. Internat J Tradit Chin Med 31(5):430
Gao X, Li P (2001) Adverse reactions of puerarin. Shanxi Med J 30(2):135
Wang C (2004) One case of laryngeal edema caused by Houttuynia cordata injection. J Prac Tradit Chin Med 20(2):104
Zhao Z, Zhao Z (2005) Two cases of allergic shock caused by Astragali radix injection. Mod J Integr Tradit Chin West Med 14(1):102–103
Zheng H (2007) 128 cases of allergic reflection caused by Chuanhuning injection. China Pharm 10(10):1033–1034
Tao S (2010) Analysis of 16 anaphylactic reactions caused by Shenmai injection. Zhejiang J Tradit Chin Med 45(5):380
Liang JQ, Zou KP, Wang NS (2009) Literature study on occurrence law and characteristics of adverse reactions of traditional Chinese medicine. Guangzhou Zhong Yi Yao Da Xue Xue Bao 26(6):574
Cheng F, Liu ZP (2009) Safety assessment and research of key technique of Chinese medicine injection. Zhong Guo Zhong Yao Za Zhi 34(8):1052–1054
Meng WJ, Li Y, Zhou ZG (2012) Anaphylactic shock and lethal anaphylaxis caused by compound amino acid solution, a nutritional treatment widely used in China. Amino Acids 42(6):2501–2505
Du XL (2010) One case of anaphylactic shock caused by Qingkailing. Hainan Med J 21(3):122
Xia L, Bai LP, Yi L et al (2007) Authentication of the 31 species of toxic and potent Chinese Materia medica (T/PCMM) by microscopic technique, part 1: three kinds of toxic and potent animal CMM. Microsc Res Tech 70(11):960–968
Li Q, Ye XL, Chen X (2012) Study on the effective part of Trichosanthes kirilowii for its hypoglycemic action. J Changchun Univ Tradit Chin Med 28(1):36
Song XM, Wang JR, Ding ZD (2001) Rescue and nursing of 1 case of acute anaphylactic shock induced by injection of trichosanate powder. Nursing J PLA. 18(4):56
Li W, Li SX, Zhu JS (2004) 5 cases of allergic reaction caused by trichosanthin powder. Hebei J Med 26(1):79
Abdualkader AM, Ghawi AM, Alaama M, Awang M, Merzouk A (2013) Leech therapeutic applications. Indian J Pharm Sci 75(2):127–137
Chin S, Vickery BP (2012) Pathogenesis of food allergy in the pediatric patient. Curr Allergy Asthma Rep 12(6):621–629
Hu CQ, Xu MZ, Ma Y, et al (2008) Determination of the allergic impurities in the parenteral injection of Chinese traditional medicines containing Salvia miltiorrhiza. Yao xue xue bao = Acta Pharm Sin 43(5):518–522
Liang AH, Xu YJ, Shang MF (2005) Analysis of adverse effects of cinnabar. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica30(23):1809–1811
Zhang SY (1982) A case report of drug dermatitis caused by Cizhuwan. Chin J Dermatol 15(2)
Zhi Y (1997) Exposure to cinnabar and other causes of skin mercury allergy. Chin Pat Med 19(6):49
Niu KY (2009) One case of allergic reaction caused by decoction. Chin J Info TCM 16(6):110
Xie YY, Xiao X, Luo JM et al (2014) Integrating qualitative and quantitative characterization of traditional Chinese medicine injection by high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J Sep Sci 37(12):1438–1447
Shaw LH, Chen WM, Tsai TH (2013) Identification of multiple ingredients for a traditional Chinese medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Mol 18(9):11281–11298
Wiwanitkit V (2012) Mercury and vitiligo. J Cutan Med Surg 16(6):387
Lin Y, Dai MK, He XJ, Li P (2013) Effects of Zhuhong ointment on MMPs activities and production by HSF. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica38(11):1795–1799
Cook J, Metcalf J (2009) Images in clinical medicine. Tattoo Allergy. New England J Med 361(1):e1
Cederbrant K, Hultman P (2000) Characterization of mercuric mercury (Hg2+)-induced lymphoblasts from patients with mercury allergy and from healthy subjects. Clin Exp Immunol 121(1):23–30
Gong YX (2009) Molecular mechanisms of homologous cross-reaction. J Biol 26(6):59–61
Tang R, Sun JL, Yin J, Li Z (2015) Artemisia allergy research in China. Biomed Res Int 2015:179426
Song L, Wu J, Xi AQ (2007) Clinical analysis of 28 pollenosis cases induced by TCM preparation. China Pharm 18(12):937–938
Leng X, Ye ST (1987) An investigation on in vivo allergenicity of Artemisia annua leaves and stems. Asian Pac J Allergy Immunol 5(2):125–128
Guo YH, Guo J, Zhai Y (2018) Effects of Canglue Xinyi decoction on elderly patients with allergic rhinitis with bronchial asthma and its influence on serum IgE and inflammatory factors. Chin Archiv Tradit Chin Med 36(2):460–464
Skaaby T, Husemoen LL, Thuesen BH, Hammer-Helmich L, Linneberg A (2014) Atopy and cause-specific mortality. Clin Exper Allergy: J British Soc Allergy Clin Immunol 44(11):1361–1370
Zhao Y, Sun S, Li X et al (2018) Drug-induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database. Intl J Clin Pharma 40(5):1349–1358
Li YZ, Li GZ, Gao JY et al (2015) Syndrome differentiation analysis on Mars500 data of traditional Chinese medicine. Sci World J 2015:125736
Yan E, Song J, Liu C, Hong W (2017) A research on syndrome element differentiation based on phenomenology and mathematical method. Chin Med 12:19
Liu FH, Deng AF (2014) Discussion on adverse reaction and rational drug use of traditional Chinese medicine. Chin Foreign Med Res 22:158–160
Kroetsch AM, Sahin E, Wang HY, Krizman S, Roberts CJ (2012) Relating particle formation to salt- and pH-dependent phase separation of non-native aggregates of alpha-chymotrypsinogen A. J Pharm Sci 101(10):3651–3660
Xie H, Liu LM, Chen LL (2014) Comparison of superoxide dismutase activity of ginseng and American ginseng in different storage. J Prac Chin Med 5:470–471
Xie XY (2018) Analysis on storage and management of traditional Chinese medicine and its processed products. China Health Ind 15(20):87–88
Chen J (2003) Causes and preventive measures of mildew of traditional Chinese medicine. Shi zhen Chin Med 14(7):431–432
Shen Y, Shen Y, Wang SH (2011) Analysis of adverse drug reaction and risk control measures of traditional Chinese medicine injection. China Med Herald 8(29):98–100
Wang H (2008) Anaphylactic shock caused by traditional Chinese medicine injections: analysis of 448 cases in literature. Chin J Drug Appl Monit 5(4):45–47
Li TQ (2010) Avoiding adverse drug reactions to Chinese medicine injections. Chin J Evidence-Based Med 10(2):111–115
Ma CH (2014) Literature analysis of 73 cases of adverse reactions of Xiangdan injection. Chin J Tradit Chin Med Info 6:33–37
Li YL, Ya L, Wang CY et al (2008) Anaphylaxis caused by combined use of metacaycline and Phellodendron amurense capsules in one patient. Chin J New Drugs Clin Remedies 27(6):479–480
Chen Y, Jiang M (2009) Literature analysis, prevention and treatment of anaphylactic shock induced by traditional Chinese medicines. China Med Herald 6(1):114–116
Guo H. (2004) Anaphylactic shock induced by ciprofloxacin combined with herba htuynia. Youjiang Med J. 32(4):401
Li XQ, Qiu XW, Shen J et al (2016) Adverse reactions of traditional Chinese medicine injection in Jiading district central hospital of Shanghai from 2013 to 2015. Mod Med Clinic 31(6):909–913
Zhang JM (2012) Investigation and analysis of allergic history to medications in 8860 hospital records. Chin Pharm Aff 26(7):770–786
Lin MB (2013) Risk factors of allergic reactions induced by Chinese medicines and allergenic ingredients of Chinese patent medicines the study In Hangzhou
Song JR, Xu DS (2016) Research status of traditional Chinese medicine combined with western medicine. China Pharm 25(1):1–5
Rampton D, Folkersen J, Fishbane S et al (2014) Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 99(11):1671–1676
Zhang XW, Song L (2004) Allergic reactions to traditional Chinese medicines in 7 patients with pollen hypersensitivity. Adv Drug Reactions J 6(4):239
Tawde P, Abolhassani M, Seamon V et al (2007) IgE-reactive 60S ribosomal protein P2 in almond (Prunus dulcis) and walnut (Juglans regia), a new class of food allergen cross-reactive with fungal aeroallergens. Clin Immunol 123:S79
Zhi F, Zhang DX, Huang CP et al (2018) Literature analysis of adverse reactions induced by dual-use traditional Chinese medicine. China Pharm 29(17):2411–2415
Li H, Cheng X, Yin J et al (2009) Analysis of components of severe allergic reaction and sensitization of Chinese prickly ash. Chin J Clin Immunol Allergy 3:184–189
Xiao Y, Zhao Y, Xie Y (2017) Design and implementation of fast allergy skin test detector for traditional Chinese medicine injections. Exper Therapeut Med 13(5):1884–1890
Liu T, Wang HD, Di LQ (2015) HPLC specific chromatogram spectrum-effect relationship for Shuanghuanglian on MDCK cell injury induced by influenza A virus (H1N1). China J Chin Materia Medica 40(21):4194–4199
Tang J, Wang GJ (2015) The adverse reactions analysis of TCM injections. Chin J Clin Rational Drug Use 4:38–39
Yi Y, Zhang YS, Li CY (2015) Study of screening pseudoallergenic substances of Shuanghuanglian injection. China J Chin Materia Med 40(14):2727–2731
Wang F, Li CY, Zheng YF (2016) The influence of 8 ingredients of Shuanghuanglian injection on RBL-2H3 cells. Chin Tradit Pa Med 7(38):1615–1617
Sun PP, Zhang N, Liu YX (2019) Bibliometric study on adverse reactions of reduning injection. Pract Drugs Clin Prac 22(2):190–193
Wang H, Li ZH, Wu HH (2016) A high content screening method for detection of anaphylactoid reaction of injection formulations. China J Chin Materia Med 41(10):1903–1909
Yu FP, Dai XM (2013) Research progress on effects of ultrafiltration for safety and effective components of traditional Chinese medicine injection. China Pharm 24(31):2972–2974
Shelley WB, Talanin N, Shelley ED (1995) Polysorbate 80 hypersensitivity. Lancet 345(8960):1312–1313
Palacios Castaño M, Venturini Díaz M, Lobera Labairu T, González Mahave I, Del Pozo GM, Blasco SA (2016) Anaphylaxis due to the excipient polysorbate 80. J Investig Allergol Clin Immunol 26(6):394–396
Coors EA, Seybold H, Merk HF, Mahler V (2005) Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Annals Allergy Asthma Immunol 95(6):593–599
Perino E, Freymond N, Devouassoux G, Nicolas J-F, Berard F (2018) Xolair-induced recurrent anaphylaxis through sensitization to the excipient polysorbate. Ann Allergy Asthma Immunol 120(6):664–666
Xiao W, DaiI XL, Ling Y (2007) Different indexes in evaluating rationality of extraction by different refining methods of reduning injection. Chin J Nat Med 5(1):42–44
Yang Y, Wang WZ, He BH (2010) Application advances of ultrafiltration in injection of traditional Chinese medicine. Chem Bioeng 27(27):5–7
Chan K, Hu X-Y, Razmovski-Naumovski V, Robinson N (2015) Challenges and opportunities of integrating traditional Chinese medicine into mainstream medicine: a review of the current situation. European J Integr Med 7(1):67–75
Funding
This project was sponsored by the grants from National Natural Science Foundation of China (No.81703908, No. 81771725 and No. 81873291) and CAMS Innovation Fund for Medical Sciences (CIFMS:2016-I2M-1003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Y., Tang, R., Luo, F. et al. The Diagnosis and Management of Allergic Reactions Caused by Chinese Materia Medica. Clinic Rev Allerg Immunol 62, 103–122 (2022). https://doi.org/10.1007/s12016-020-08812-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-020-08812-7