Abstract
Background
The vast majority of IgE-mediated food allergies in adults are based on sensitization to pollen, followed by reactions to structurally related, often unstable allergens, in particular in fruit (including edible nuts), vegetables, and spices.
Materials and methods
This article provides an up-to-date overview of selected scientific works on pollen-related food allergy and has been drawn-up on the basis of PubMed research, the German Study on Adult Health (Studie zur Gesundheit Erwachsener in Deutschland, DEGS) conducted by the Robert Koch Institute, as well as the national and international guideline registries.
Results
Birch pollen-related symptoms are generally the commonest form of pollen-related allergy observed in Northern Europe. The types of fruit that most frequently cause symptoms belong to the Rosaceae (e. g., apple, cherry) and Fagales families (hazelnut). Reactions to legumes (e. g., peanut, soy) and vegetables, including celery, carrot, tomato, and bell pepper, are also worthy of note. In addition to oropharyngeal contact urticaria, the clinical symptoms of pollen-related food allergy can range from the involvement of other organ systems to anaphylactic shock. The main plant food allergens belong to a handful of protein families: Bet v 1 homologs, profilins, lipid transfer proteins, storage proteins, and thaumatin-like proteins.
Conclusion
The diagnosis of pollen-related food allergy has seen significant advances in recent years in the wake of component-resolved/molecular allergology, thereby, enabling reliable identification. Treatment comprises dietary counseling and the prescription of emergency medication. In addition, allergen-specific immunotherapy for cross-reactive pollen allergens appears to positively affect concomitant food allergies in some patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
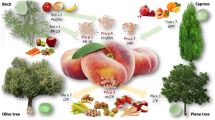
According to current data from the Study on Adult Health in Germany (Studie zur Gesundheit Erwachsener in Deutschland, DEGS), almost 5% of adults have a food allergy [1]. The majority of IgE-mediated food allergies in adults are based on sensitization to aeroallergens (in particular pollen), followed by reactions (cross reactions) to structurally related, often unstable allergens [2], especially in (plant) foods such as fruit, vegetables, and spices. This type of food allergy is referred to as a secondary food allergy, as distinct from the primary form, which is presumed to involve sensitization via the gastrointestinal tract [2,3,4]. The types of fruit most commonly involved in pollen-related food allergy belong to the Rosaceae and Fagales families; celery and carrot from the Apiaceae family in terms of vegetables, as well as tomato and bell pepper, are worthy of note ([5]; Table 1).
A number of typical associations have been described, such as birch-fruit syndrome, celery-birch-mugwort-spice syndrome, and mugwort-mustard syndrome, among others (Table 2). The geographical distribution of pollen and regional dietary habits affect the incidence and development of various forms of food allergy [4]. For example, hazelnut allergy in Northern Europe is usually attributed to sensitization to the birch pollen-related allergen Cor a 1, whereas sensitizations to the lipid transfer protein (LTP) Cor a 8 are more common in the Mediterranean region. The latter is likely a case of combined sensitization resulting from peach consumption (as a cross reaction with Pru p 3) and pollen [6]. Due to the multitude of possible pollen and food sensitizations with, to an extent, unknown allergens [7,8,9], it is not always possible to reliably distinguish a primary from a secondary food allergy.
As a whole, molecular (component-resolved) allergy diagnostics have contributed much to our understanding of pollen-related food allergy in recent years [5, 10, 11].
This paper is intended to provide an overview of the most important aspects of pollen-related food allergy; having said that, it has been necessary to make a selection due to the breadth of increases in our knowledge.
Clinical presentation of pollen-related food allergy
The clinical reactions associated with secondary food allergy (FA) can occur as early as upon first intake of a food, since sensitization takes place via pollen allergens as opposed to contact with the food. As such, patients are often caught completely unaware [2, 12, 13]. Symptoms typically manifest within a few minutes to up to 2 h following intake of the food. Reactions may be seen in one or more target organs, including the oral mucosa, skin, gastrointestinal tract, respiratory tract, and cardiovascular system [2,3,4].
Oropharyngeal contact urticaria, also formerly referred to as oral allergy syndrome (OAS), describes manifestations limited to the oral cavity and pharynx. Typical subjective symptoms include oral paresthesia or pruritus of the lips, tongue, gums, ears, and/or larynx, a feeling of swelling and/or difficulties swallowing. Objective symptoms may comprise swelling of the lips or tongue, hoarseness, and/or laryngeal edema. Some patients exhibit red patches or transient, small blisters on the oral mucosa (Fig. 1). Symptoms generally resolve within 10–30 min; however, patients can also go on to develop systemic reactions [2,3,4, 13, 14].
Where this is the case, the skin is most frequently affected [2,3,4], particularly in the form of acute generalized urticaria; angioedema and flushing are observed more rarely. Respiratory symptoms (e. g., respiratory distress, drop in peak flow, asthma attacks) can also be seen as a result of pollen-related food allergy. Gastrointestinal and cardiovascular symptoms are rarer and are generally not seen as sole manifestations [2,3,4].
Why some patients develop systemic reactions in addition to local symptoms has not been conclusively explained. The bioavailability of the allergen, the patient’s degree of sensitization, as well as other possible co- or augmentation factors all play a role here ([15, 16]; Table 3). Concomitant food allergies may be more pronounced in pollen-allergic individuals during the relevant pollen season [2,3,4].
In addition to immediate-type reactions, eosinophilic esophagitis [17] has also been associated with pollen-related food allergy, and a transient exacerbation of eczema is sometimes seen in a subgroup of atopic dermatitis patients in the context of pollen sensitization following oral provocation with the cross reactive food [2,3,4].
The diagnosis of a food allergy generally has a considerable impact on patients: not only does it result in restrictions in everyday life due to the necessary dietary measures, it is also associated in many instances with living under the constant threat of a sudden allergic reaction, including life-threatening anaphylaxis. A study of patients with birch pollen-related food allergy [18] showed a clear reduction in quality of life, which was more pronounced in women than in men, and which worsened with the number of foodstuffs not tolerated, age, and the severity of previous symptoms. The main issues were associated with the patients’ general concern about their health and their anxiety about experiencing a sudden and unexpected allergic reaction [18].
Plant food allergens
The allergen content in plant foods can vary according to growth and storage conditions. Moreover, varying degrees of allergenicity, along with evidence of high- and low-allergen types, could be shown for a number of apple cultivars (see also the section Therapeutic consequences: dietary measures [19]). The main plant food allergens belong to a handful of protein families, the best known among these being Bet v 1 homologs (Table 1), LTPs, storage proteins, and thaumatin-like proteins (TLP) [2,3,4,5, 10, 11]. In addition, profilins and cross-reactive carbohydrate determinants (CCD) play a role as panallergens capable of yielding positive allergy tests of generally dubious clinical relevance. Polcalcins (calcium-binding proteins) are found only in pollen and therefore play no role in pollen-related food allergy [2,3,4,5, 10, 11].
Using a group of children in Italy as an example, an attempt was recently made based on molecular sensitization patterns to the allergens Phl p 12 (profilin), Bet v 1, and Pru p 3 (LTP) to classify five different types of pollen-related food allergy [20]. To what extent this classification may be helpful in the areas of diagnosis and treatment remains to be elucidated.
Bet v 1 homologs
The main allergen component in birch pollen, Bet v 1, belongs to the plant proteins in the pathogenesis-related protein family (PR-) 10. Bet v 1 homologs are widespread in the plant world, as well as in fruit and vegetables (Table 1). Hazelnut, apple, celery, carrot, and cherry belong to the food allergens most commonly associated with birch pollen allergy [2,3,4]. Although symptoms are generally mild and take the form of oropharyngeal contact urticaria, they can extend to allergic shock, particularly in the case of ingestion of protein-rich soy products [12,13,14]. A Danish study on a group of patients with Cor a 1 sensitization (hazelnut) also revealed that 49% experienced objective symptoms [21]. Therefore, even in monosensitization, the risk of anaphylaxis should always be taken into consideration. Since heat typically destroys the thermolabile PR-10 allergens, cooked, baked, or heavily processed foods are usually well tolerated. However, even roasted hazelnuts [22, 23] or cooked celery [24] can cause symptoms in some strongly sensitized patients.
Lipid transfer proteins
Sensitization to LTP is primarily seen in the Mediterranean region and probably occurs largely via the gastrointestinal tract following the ingestion of peaches [5]. The peach LTP, Pru p 3, exhibits structural similarities to LTP in other fruit and vegetable cultivars. Other clinically important LTP include: Cor a 8 (hazelnut), Ara h 9 (peanut), and Jug r 3 (walnut) [5]. An association between sensitization to LTP in foods and sensitization to inhalant allergens (LTP) in mugwort, cypress, olive, and plane tree is discussed [25, 26]. LTP are resistant to heat and digestion, and sensitization to these proteins is associated with systemic and severe reactions [5, 26].
Profilins
Due to their ubiquitous occurrence in pollen and plant foods, profilins are considered to be panallergens [27]. There is marked cross reactivity between profilins from different sources (e. g., Bet v 2/birch, Phl p 12/grass, Cor a 2/hazelnut, Pru p 4/peach, Mal d 4/apple, Ara h 5/peanut), which can result in nonspecific coreactions in specific immunoglobulin E (sIgE) diagnostics [5, 10, 11]. Molecular allergy diagnostics with the determination of specific IgE to Bet v 2 can be a helpful investigation method, particularly of polyvalent sensitizations in skin prick testing [28,29,30]. Although profilins are rarely associated with clinical symptoms, they can cause local and also severe reactions in some patients [29, 30]. Clinical relevance has been demonstrated for allergic reactions to melon (Cuc m 2), watermelon, kiwi, tomato, banana, pineapple, apricot, cucumber, and orange [27, 30].
Cross-reactive carbohydrate determinants
Approximately 20% of patients with pollen allergy are believed to have antibodies against proteins >30 kDa, whereby these are mostly carbohydrate determinants. There is much discussion on the clinical relevance of these CCD antibodies [5]. CCD have been identified in birch, timothy, and ragweed pollen, as well as in numerous foods such as celery (Api g 5), bell pepper, pepper, and mango [31].
Thaumatin-like proteins
Thaumatin-like proteins (TLP) are found in plane pollen, as well as in cypress (Cup s 3), birch, mugwort, and olive pollen. They have been identified, for instance, as important allergens in peach allergy. At a molecular weight of between 20 and 30 kDa, TLP have an extremely (heat) stable, three-dimensional structure and are also referred to as PR-5 allergens [5]. Their role in pollen-related food allergy has recently been demonstrated, whereby observations are currently restricted to Spanish regions [32]. Examples of TLP in foods include Act d 2 (kiwi), Mal d 2 (apple), Mus a 4 (banana), Pru p 2 (peach), and Pru av 2 (cherry); they have also been identified in cabbage, lettuce, and chestnuts [5].
Storage proteins
The term “storage proteins” encompasses a large number of structurally related, mostly stable, and thus often clinically relevant food allergens. In all likelihood, sensitization takes place largely via the gastrointestinal tract and not via inhalant allergens. Sensitization to storage proteins is associated with a high risk for systemic symptoms. The allergens are found in many plant allergen sources, e. g., nuts, seeds, legumes, including peanut, soy, lupines, and cereal. Numerous cross reactions are known [5, 10, 11].
Specific aspects of selected plant food allergens
Fruit
In the DEGS study, IgE sensitizations were most commonly seen to peach (Pru p 1/Bet v 1 homolog: 12.4%), cherry (10.1%), apple (9.2%), kiwi (7.5%), and strawberry (5.5%) [1]. Sensitizations to the Bet v 1 homologs Pru p 1 (peach) and Mal d 1 (apple) are detected particularly frequently in birch pollen-allergic individuals in Northern Europe, whereas Pru p 3 and Mal d 3 sensitizations (LTP) are more common in Southern Europe. Other apple allergens include Mal d 2 (TLP) and Mal d 4 (profilin) [5]. Secondary pollen-related food allergy to kiwi is seen in birch (allergens Act d 8/Bet v 1 homolog or Act d 9/profilin), grass (Act d 9), and latex sensitization [5].
Tree nuts and legumes
In the DEGS study, 15.7% of adults were sensitized to hazelnut, 4.0% to almond (edible nuts), 8.0% to peanut, 10.3% to Gly m 4 (soy), 3.9% to lupines, and 3.7% to soybeans (legumes) [1]. Important food allergens for hazelnut include the following: Cor a 1 (Bet v 1 homolog), Cor a 8 (LTP), Cor a 9, and Cor a 14 (storage proteins); for peanut, Ara h 1, Ara h 2, Ara h 3 (storage proteins), Ara h 5 (profilin), Ara h 8 (Bet v 1 homolog), and Ara h 9 (LTP); and for soy, Gly m 3 (profilin), Gly m 4 (Bet v 1 homolog), Gly m 5, and Gly m 5 (storage proteins) [5].
Vegetables
The DEGS study measured IgE sensitization rates of 8.7% to carrot, 8.6% to celery, and 6.1% to tomato [1]. Celery and carrot belong to the Apiaceae family. Celery allergens worthy of note include Api g 1 (Bet v 1 homolog), Api g 2 (LTP), Api g 4 (profilin), and Api g 6 (LTP). It was also recently reported that 42% of celery-allergic individuals are believed to be sensitized to the high-molecular-weight allergen Api g 5 [33]. Homologies to high-molecular-weight allergens from grass and fennel are believed to exist [34]. Allergens from carrot include Dau c 1 (Bet v 1 homolog), Dau c 4 (profilin), and Dau c 3 (LTP). Patients with mugwort pollen allergy can develop a cross-allergy to celery and carrot through allergens as yet unidentified [5]. Important allergens in tomato include Sola l 1 (profilin), Sola l 3 (LTP), and Sola l 4 (Bet v 1 homolog), and in bell pepper, Cap a 1 (TLP) and Cap a 2 (profilin) [5, 35, 36].
Specific aspects of relevant pollen allergens
Birch family (Betulacea)
The Betulacea family, which includes hazel and alder trees alongside birch, belongs to the order Fagales. The major allergens in Betulacea pollen include molecules in the PR-10 group, the most important being Aln g 1 from alder, Bet v 1 from birch, and Cor a 1 from hazelnut [5]. Birch pollen-related food allergy is of major relevance in Northern and Central Europe [2, 3]. Approximately 17% of adults in Germany are sensitized to birch pollen [1], whereby 95% of those affected are believed to have IgE antibodies to the major allergen Bet v 1 [4]. Bet v 1 exhibits cross-reactivity with a number of Bet v 1-homologous allergens in foods (Table 1). The minor allergens from the molecule groups of profilins (e. g., Bet v 2) and TLPs can also be responsible for cross reactions with foods [5, 27].
Mugwort (Artemisia vulgaris)
Mugwort belongs to the daisy family (Asteracea). Important allergens include Art v 1 (defensin), Art v 3 (LTP), Art v 4 (profilin), Art v 5 (polcalcin), and Art v 6 (Amb a 1 homolog). More than 95% of mugwort-sensitized patients are believed to have antibodies to the major allergen, Art v 1 [31]. Although cross-reactive food allergies appear to be less common than in tree pollen-allergic individuals, a number of clinical cross-reactions have been described (Table 2). A link between inhalant sensitization to the LTP Art v 3 and LTP-related food allergy has already been suspected on more than one occasion. For example, Art v 3 was described as a sensitizing allergen in peach-allergic individuals [37] and associated with immediate-type reactions to broccoli as a possible cross-reaction with the LTP Bra o 3 [38, 39].
Grasses
One can assume extensive cross-reactivity between related grasses. It is well known that the profilin Phl p 12 (timothy grass allergen/Phleum pratense) can cause cross-reactivity with food allergens. Likewise, CCD from grass pollen can produce positive test results [5]. It is questionable whether grass pollen-related food sensitization is of any clinical relevance [4].
Ragweed
Typically indigenous to North America, ragweed is increasingly found in Europe [40]. Amb a 1 is considered the major allergen for inhalation symptoms; other allergens worthy of note include Amb a 4 (defensin), Amb a 6 (LTP), and Amb a 8 (profilin). Although a number of foods have been linked to ragweed sensitization, it remains unclear whether and which ragweed allergen could be responsible here. The allergens Amb a 6 and Amb a 8 are suspected in ragweed-melon-banana syndrome [5]. Since polysensitization is common in ragweed-allergic individuals [41], one can also assume that an associated food allergy in ragweed-allergic individuals is more likely attributable in many cases to a concomitant sensitization to another pollen allergen.
Plane tree
Sensitization to plane pollen occurs primarily in Southern Europe. The major allergens Pla a 1 and Pla a 2 are believed to be responsible for allergic airway diseases in spring. Of a group of 61 plane-sensitized patients, more than 50% reported a food allergy (in most cases to peach), and of these, a third experienced systemic symptoms. Cross-reactivity with a TLP or LTP (Pla a 3) was deemed responsible for these plane-related food allergies [42, 43].
Olive
Olive pollens play a role as inhalation allergens in Southern Europe. They are only rarely linked with an associated sensitization to food allergens, in which case they can likely be attributed to profilin sensitization [44].
Table 2 lists other food allergens described in association with plant pollen sensitization [45,46,47,48].
Diagnostic work-up
The diagnostic work-up for pollen-related food allergy should be tailored according to the clinical reactions described in the patient history (Table 4).
Skin testing
Sensitization to pollen allergens is classically investigated using the skin prick test (SPT). When diagnosing concomitant food allergy, the prick-to-prick test with fresh material is generally more sensitive for some foods compared with SPT with commercially available food extracts. These are not biologically standardized, have low sensitivity, and often yield false negative findings. The reasons for this include low occurrence and/or the poor stability of many allergens compared with endogenous, enzymatic processes in plant food extracts [4]. Moreover, ever fewer allergen extracts are commercially available and, when they are, they tend to be extremely cost-intensive. On the down side, any evaluation should take into account the lack of standardization in terms of allergen content, which can vary according to growing conditions, maturity, and storage, and a possible irritant aspect associated with some fresh foods [2, 3, 49]. Therefore, the possibility of false-positive results in prick-to-prick testing with native foods should be taken into consideration in the evaluation. Cross-reactivity to panallergens, such as profilins and polcalcins (the latter found only in pollen), can also cause false-positive skin prick tests [5]. Studies have used palm allergen extracts to investigate nonspecific cross-reactions in sensitizations to these panallergens in SPT. These extracts are not yet commercially available in Germany [50, 51]. The use of molecular extracts in component-resolved diagnostics on the skin has hitherto only been possible in studies [51, 52].
In vitro tests
The possibilities offered by in vitro IgE diagnostics, including component-resolved diagnosis (CRD), have broadened significantly in recent years [7, 8]. Untargeted screening (including serological tests), e. g., of numerous fruit and vegetable types or the available single allergens in birch pollen-associated cross-sensitization is not recommended [4, 5]. In vitro testing is indicated in suspected food allergy in the case of the following:
-
Unclear patient history
-
Negative skin tests
-
Foods that are not suited to skin testing
-
Severe anaphylactic reactions (here prior to skin testing)
-
Impracticality of skin testing (e. g., due to active disease in the test area, use medication/antihistamines that may affect results)
-
Very young children [4].
The interpretation of results requires knowledge of the most relevant triggering allergen families in plant foods (see above). Since PR-10 proteins are underrepresented in many food extracts in commercially available assays, the determination of antibodies to the major birch allergen Bet v 1 is generally indicated for the diagnosis of a birch pollen-related food allergy. A further determination of recombinant PR-10 proteins is not additionally required in most cases. By “spiking” extracts with recombinant allergens (as already performed, for instance, with Cor a 1 in hazelnut), it is possible to increase the sensitivity of commercial test systems [5, 10, 11].
The determination of other marker allergens for plant allergens can reveal the extent of possible cross-reactivity and, to a certain degree, predict the severity of clinical symptoms [10, 11]. For example, in the case of proven IgE sensitization to peanut, sIgE antibodies to the Bet v 1 homolog Ara h 8 (PR-10 protein) may suggest a probably mild, localized reaction in the future, whereas a systemic reaction is far more likely in the case of sensitization to the storage proteins Ara h 1, 2, and 3 [53, 54].
Tolkki et al. [55] were able to show that CRD using single (ImmunoCAP) or multiplex (ImmunoCAP ISAC) assays were unable to increase diagnostic accuracy in patients with grass and birch pollen-related food allergy. This confirmed earlier investigations that had found no benefit conferred by multiplex diagnosis in birch pollen-related apple allergy [56].
If in vitro allergy tests find extremely broad IgE sensitizations to food, this may be based on a reaction to panallergens such as profilins – as in skin testing – or CCD. The combination in particular of positive in vitro IgE detection and a negative SPT can point to an in vitro reaction to nonhistamine-releasing CCD in the SPT [5]. In the case of severe anaphylactic reactions, the possible presence of mastocytosis should be investigated by measuring serum tryptase. The measurement of food-specific IgG or IgG4 levels is considered diagnostically unhelpful and, as such, should not be performed [57].
Oral provocation testing
The detection of sensitization in skin testing or in vitro should not be equated with clinical relevance. Therefore, in the case of an unclear patient history, oral provocation is the only option to confirm a food allergy and may be highly beneficial prior to prescribing an elimination diet (Table 3; [2,3,4]). Disadvantages include the time requirements, the costs, and the complexity of producing exposure meals using native material [2,3,4, 58]. There are no commercially available provocation meals as yet. Attention should be paid here to fluctuating allergen contents depending on where the plants come from and on their growing and storage conditions [59]. Possible allergen interaction with blinding materials should also be borne in mind. In addition, the shelf-life of provocation meals in terms of stability and microbiological aspects needs to be considered [60]. Altogether, it has been difficult up to now to compare test results from different time points and different hospitals; this is due to insufficient standardization in the organizational performance and evaluation of tests [2,3,4, 58]. The possibility of component-resolved oral provocation using recombinant Mal d 1 was recently investigated for the first time [61].
Measuring quality of life
The possible effects of a pollen-related food allergy on health-related quality of life can be measured using the Food Allergy Quality of Life Questionnaire (FAQLQ) [18, 62].
Therapeutic consequences
Dietary measures
An elimination diet should only be implemented if sensitization has been shown to be of clear clinical relevance. Particularly in the case of atopic individuals, it is important to ensure that no strict diets are adhered to purely on the basis of positive allergy tests [2,3,4]. Moreover, an elimination diet should only cover the clinically relevant food allergens and not be based solely on a list of known and possibly cross-reactive foods [4]. Individual dietary counseling is required to this end [2,3,4, 12]. The following food allergens capable of causing pollen-related symptoms are declarable in Europe: peanut, soybeans, edible nuts (almond, pistachio, Brazil/macadamia nuts, and hazelnuts), celery, and mustard [2,3,4].
In addition to complete avoidance, some patients may be able to consume products low in allergens where appropriate. Studies have shown for apple that the cultivars Elise and Santana cause clinical oropharyngeal symptoms more rarely compared with other apple cultivars, whereas Renate and Cortland do so more frequently [19, 63]. Storage period and conditions also affect Mal d 1 content [59]. Using genetic engineering techniques, it was possible to reduce the Mal d 1 content in – and thereby the allergenicity of – apples [64], as well as lower the Lyc c 1 content in tomatoes [65]. However, these foods are not commercially available as yet. It is possible, by means of heating or processing, to reduce the allergenicity of Bet v 1 homologs and profilins [2,3,4,5].
Emergency medication
The treatment of affected individuals with emergency medication, including an adrenaline autoinjector, needs to be weighed up taking into consideration the expected clinical reaction (local or systemic), the risk of exposure, as well as other possible comorbidities and cofactors [66]. In the case of only mild oropharyngeal symptoms upon consumption of foods that are otherwise generally easy to avoid, the recommendation to use oral antihistamines as required appears adequate for accidental exposure [2,3,4].
Allergen-specific immunotherapy
Allergen-specific immunotherapy (ASIT) against cross-reactive pollen allergens appears to confer a benefit in terms of concomitant food allergy in some patients [67,68,69,70]. As yet, however, study data have not been sufficiently unequivocal to justify deeming food allergy alone as an indication to initiate ASIT. Therefore, in accordance with the guidelines, pollen ASIT should only be performed if the indication is made on the basis of concomitant respiratory symptoms [2]. A recent study showed, both in vitro and in a mouse model, that vaccination with a hybrid molecule directed against the three relevant T‑cell epitopes (Bet v 1 as well as cross-reactive apple and hazelnut epitopes) is capable of inducing protective antibodies in pollen-related food allergy [71]. Potential clinical application in humans remains to be seen.
Abbreviations
- ASIT:
-
Allergen-specific immunotherapy
- CCD:
-
Cross-reactive carbohydrate determinants
- DEGS:
-
Study on Adult Health in Germany Studie zur Gesundheit Erwachsener in Deutschland
- FAQL:
-
Food allergy quality of life (questionnaire)
- SPT:
-
Skin prick test
- IgE:
-
Immunoglobulin E
- IgG:
-
Immunoglobulin G
- CRD:
-
Component-resolved diagnosis
- KDa:
-
Kilodalton
- LTP:
-
Lipid transfer proteins
- FA:
-
Food allergy
- OAS:
-
Oropharyngeal contact urticaria (formerly oral allergy syndrome)
- PR:
-
Family of pathogenesis-related proteins
- SIgE:
-
Specific immunoglobulin E
- TLP:
-
Thaumatin-like proteins
References
Haftenberger M, Laußmann D, Ellert U, Kalcklösch M, Langen U, et al. Prevalence of sensitisation to aeraoallergens and food allergens: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:687–97.
Worm M, Reese I, Ballmer-Weber B, Beyer K, Bischoff SC, Classen M, et al. Guidelines on the management of IgE-mediated food allergies: S2k-Guidelines of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Medical Association of Allergologists (AeDA), the German Professional Association of Pediatricians (BVKJ), the German Allergy and Asthma Association (DAAB), German Dermatological Society (DDG), the German Society for Nutrition (DGE), the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS), the German Society for Oto-Rhino-Laryngology, Head and Neck Surgery, the German Society for Pediatric and Adolescent Medicine (DGKJ), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Society for Pneumology (DGP), the German Society for Pediatric Gastroenterology and Nutrition (GPGE), German Contact Allergy Group (DKG), the Austrian Society for Allergology and Immunology (Æ-GAI), German Professional Association of Nutritional Sciences (VDOE) and the Association of the Scientific Medical Societies Germany (AWMF). Allergo J Int. 2015;24:256–93.
Worm M, Jappe U, Kleine-Tebbe J, Schäfer C, Reese I, Saloga J, et al. Food allergies resulting from immunological cross-reactivity with inhalant allergens: Guidelines from the German Society for Allergology and Clinical Immunology (DGAKI), the German Dermatology Society (DDG), the Association of German Allergologists (AeDA) and the Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J Int. 2014;23:1–16.
Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015;70:1079–90.
Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250.
Datema MR, Zuidmeer-Jongejan L, Asero R, Barreales L, Belohlavkova S, de Blay F, et al. Hazelnut allergy across Europe dissected molecularly: a EuroPrevall outpatient clinic survey. J Allergy Clin Immunol. 2015;136:382–91.
Treudler R, Reuter A, Engin AM, Simon JC. A case of anaphylaxis after garlic ingestion: is alliinase the only culprit allergen? J Investig Allergol Clin Immunol. 2015;25:374–5.
Gebhardt C, Vieths S, Gubesch M, Averbeck M, Simon JC, Treudler R. 10 kDa lipid transfer protein: the main allergenic structure in a German patient with anaphylaxis to blueberry. Allergy. 2009;64:498–9.
Renner R, Hipler C, Treudler R, Harth W, Süss A, Simon JC. Identification of a 27 kDa protein in patients with anaphylactic reactions to mango. J Investig Allergol Clin Immunol. 2008;18:476–81.
Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013;13:110–7.
Treudler R. Update on in vitro allergy diagnostics. J Dtsch Dermatol Ges. 2012;10:89–97.
Treudler R, Kramer S, Kleine-Tebbe J, Simon JC. Steigende Popularität von Soja: Wie werden Birkenpollenallergiker richtig beraten? Allergo J. 2010;19:243–50.
Treudler R, Werner M, Thiery J, Kramer S, Gebhardt C, Averbeck M, et al. High risk of immediate-type reactions to soy drinks in 50 patients with birch pollinosis. J Investig Allergol Clin Immunol. 2008;18:483–4.
Kleine-Tebbe J, Vogel L, Crowell DN, Haustein U, Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1‑related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002;110:797–804.
Turner PJ, Baumert JL, Beyer K, Boyle RJ, Chan CH, Clark AT, et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71:1241–55.
Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491–9.
Mahdavinia M, Bishehsari F, Hayat W, Elhassan A, Tobin MC, Ditto AM. Association of eosinophilic esophagitis and food pollen allergy syndrome. Ann Allergy Asthma Immunol. 2017;118:116–7.
Beyer S, Franke A, Simon JC, Treudler R. Measurement of health-related quality of life in adult patients with birch pollen-associated food allergy. J Dtsch Dermatol Ges. 2016;14:397–404.
Wagner A, Szwed A, Buczyłko K, Wagner W. Allergy to apple cultivars among patients with birch pollinosis and oral allergy syndrome. Ann Allergy Asthma Immunol. 2016;117:399–404.
Mastrorilli C, Tripodi S, Caffarelli C, Perna S, Di Rienzo-Businco A, Sfika I, et al. Endotypes of pollen-food syndrome in children with seasonal allergic rhinoconjunctivitis: a molecular classification. Allergy. 2016;71:1181–91.
Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–9.
Hansen KS, Ballmer-Weber BK, Lüttkopf D, Skov PS, Wüthrich B, Bindslev-Jensen C, et al. Roasted hazelnuts – allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy. 2003;58:132–8.
Worm M, Hompes S, Fiedler E, Illner A, Zuberbier T, Vieths S. Impact of native, heat-processed and encapsulated hazelnuts on the allergic response in hazelnut-allergic patients. Clin Exp Allergy. 2009;39:159–66.
Ballmer-Weber BK, Hoffmann A, Wüthrich B, Lüttkopf D, Pompei C, Wangorsch A, et al. Influence of food processing on the allergenicity of celery: DBPCFC with celery spice and cooked celery in patients with celery allergy. Allergy. 2002;57:228–35.
Zuidmeer L, van Ree R. Lipid transfer protein allergy: primary food allergy or pollen/food syndrome in some cases. Curr Opin Allergy Clin Immunol. 2007;7:269–73.
Pascal M, Muñoz-Cano R, Reina Z, Palacín A, Vilella R, Picado C, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012;42:1529–39.
Asero R, Monsalve R, Barber D. Profilin sensitization detected in the office by skin prick test: a study of prevalence and clinical relevance of profilin as a plant food allergen. Clin Exp Allergy. 2008;38:1033–7.
San Nicoló M, Braun T, Eder K, Berghaus A, Gröger M. Clinical relevance of IgE to profilin and/or polcalcin in pollen-sensitized patients. Int Arch Allergy Immunol. 2016;169:101–7.
Wallner M, Ferreira F, Hofer H, Hauser M, Mahler V, Kleine-Tebbe J. Das Konzept der Pollen-Panallergene. In: Kleine-Tebbe J, Jakob T, editors. Molekulare Allergiediagnostik. Berlin: Springer; 2015. pp. 33–42.
Asero R, Tripodi S, Dondi A, Di Rienzo Businco A, Sfika I, Bianchi A, Candelotti P, et al. Italian Pediatric Allergy Network (I-PAN). Prevalence and clinical relevance of IgE sensitization to profilin in childhood: a multicenter study. Int Arch Allergy Immunol. 2015;168:25–31.
Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006;61:461–76.
Palacín A, Rivas LA, Gómez-Casado C, Aguirre J, Tordesillas L, Bartra J, Blanco C, et al. The involvement of thaumatin-like proteins in plant food cross-reactivity: a multicenter study using a specific protein microarray. PLOS ONE. 2012;7:e44088.
Lukschal A, Wallmann J, Bublin M, Hofstetter G, Mothes-Luksch N, Breiteneder H, et al. Mimotopes for Api g 5, a relevant cross-reactive allergen, in the Celery-Mugwort-Birch-Spice syndrome. Allergy Asthma Immunol Res. 2016;8:124–31.
Borghesan F, Mistrello G, Amato S, Giuffrida MG, Villalta D, Asero R. Mugwort-fennel-allergy-syndrome associated with sensitization to an allergen homologous to Api g 5. Eur Ann Allergy Clin Immunol. 2013;45:130–7.
Rüger RD, Wagner S, Simon JC, Treudler R. Severe type 1‑allergy to raw bell pepper. Hautarzt. 2010;61:339–42.
Wangorsch A, Jamin A, Foetisch K, Malczyk A, Reuter A, Vierecke S, et al. Identification of Sola l 4 as Bet v 1 homologous pathogenesis related-10 allergen in tomato fruits. Mol Nutr Food Res. 2015;59:582–92.
Gao ZS, Yang ZW, Wu SD, Wang HY, Liu ML, Mao WL, et al. Peach allergy in China: a dominant role for mugwort pollen lipid transfer protein as a primary sensitizer. J Allergy Clin Immunol. 2013;131:224–226.e3.
Dölle S, Hompes S, Lange L, Worm M. Cabbage allergy: a rare cause of food-induced anaphylaxis. Acta Derm Venereol. 2013;93:485–6.
Sugita Y, Makino T, Mizawa M, Shimizu T. Mugwort-Mustard allergy syndrome due to broccoli consumption. Case Rep Dermatol Med. 2016;2016:8413767.
Buters J, Alberternst B, Nawrath S, Wimmer M, Traidl-Hoffmann C, Starfinger U, et al. Ambrosia artemisiifolia (ragweed) in Germany – current presence, allergological relevance and containment procedures. Allergo J Int. 2015;24:108–20.
Ruëff F, Przybilla B, Walker A, Gmeiner J, Kramer M, Sabanés-Bové D, et al. Sensitization to common ragweed in southern Bavaria: clinical and geographical risk factors in atopic patients. Int Arch Allergy Immunol. 2012;159:65–74.
Wangorsch A, Larsson H, Messmer M, García-Moral A, Lauer I, Wolfheimer S, et al. Molecular cloning of plane pollen allergen Pla a 3 and its utility as diagnostic marker for peach associated plane pollen allergy. Clin Exp Allergy. 2016;46:764–74.
Scala E, Cecchi L, Abeni D, Guerra EC, Pirrotta L, Locanto M, et al. Pla a 2 and Pla a 3 reactivities identify plane tree-allergic patients with respiratory symptoms or food allergy. Allergy. 2017;72:671–4.
Martínez A, Asturias JA, Monteseirín J, Moreno V, García-Cubillana A, et al. The allergenic relevance of profilin (Ole e 2) from Olea europaea pollen. Allergy. 2002;57(Suppl 71):17–23.
Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;26:31–50.
Herrera-Mozo I, Ferrer B, Luís Rodriguez-Sanchez J, Juarez C. Description of a novel panallergen of cross-reactivity between moulds and foods. Immunol Invest. 2006;35:181–97.
de la Torre Morín F, Sánchez Machín I, García Robaina JC, Fernández-Caldas E, Sánchez Triviño M. Clinical cross-reactivity between Artemisia vulgaris and Matricaria chamomilla (chamomile). J Investig Allergol Clin Immunol. 2001;11:118–22.
Caimmi D, Barber D, Hoffmann-Sommergruber K, Amrane H, Bousquet PJ, et al. Understanding the molecular sensitization for cypress pollen and peach in the Languedoc-Roussillon area. Allergy. 2013;68:249–51.
Henzgen M, Ballmer-Weber BK, Erdmann S, Fuchs T, Kleine-Tebbe J, Lepp U, et al. Hauttestung mit Nahrungsmittelallergenen. Allergo J. 2008;17:401–6.
Asero R, Jimeno L, Barber D. Preliminary results of a skin prick test-based study of the prevalence and clinical impact of hypersensitivity to pollen panallergens (polcalcin and profilin). J Investig Allergol Clin Immunol. 2010;20:35–8.
Asero R, Jimeno L, Barber D. Component-resolved diagnosis of plant food allergy by SPT. Eur Ann Allergy Clin Immunol. 2008;40:115–21.
Kollmann D, Geroldinger-Simic M, Kinaciyan T, Huber H, Ebner C, Lidholm J, et al. Recombinant Mal d 1 is a reliable diagnostic tool for birch pollen allergen-associated apple allergy. J Allergy Clin Immunol. 2013;132:1008–10.
Park KH, Son YW, Lee SC, Jeong K, da Sim W, Park HJ, et al. Clinical significance of component allergens in fagales pollen-sensitized peanut allergy in Korea. Allergy Asthma Immunol Res. 2016;8:505–11.
Beyer K, Grabenhenrich L, Härtl M, Beder A, Kalb B, Ziegert M, et al. Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy. 2015;70:90–8.
Tolkki L, Alanko K, Petman L, Skydtsgaard MB, Milvang PG, Seppälä U, et al. Clinical characterization and IgE profiling of birch (Betula verrucosa) – allergic individuals suffering from allergic reactions to raw fruits and vegetables. J Allergy Clin Immunol Pract. 2013;1:623–31.
Ebo DG, Bridts CH, Verweij MM, De Knop KJ, Hagendorens MM, De Clerck LS, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy. 2010;40:339–47.
Kleine-Tebbe J, Herold DA. Inappropriate test methods in allergy. Hautarzt. 2010;61:961–6.
Treudler R, Franke A, Schmiedeknecht A, Ballmer-Weber BK, Worm M, Werfel T, et al. Standardization of double blind placebo controlled food challenge with soy within a multicentre trial. Clin Transl Allergy. 2016;6:39.
Kiewning D, Schmitz-Eiberger M. Effects of long-term storage on Mal d 1 content of four apple cultivars with initial low Mal d 1 content. J Sci Food Agric. 2014;94:798–802.
Holzhauser T, Franke A, Treudler R, Schmiedeknecht A, Randow S, Becker WM, et al. The BASALIT multicentre trial: Gly m 4 quantification for consistency control of challenge meal batches and towards Gly m 4 threshold data. Mol Nutr Food Res. 2017;61:1600527.
Kinaciyan T, Nagl B, Faustmann S, Kopp S, Wolkersdorfer M, Bohle B. Recombinant Mal d 1 facilitates sublingual challenge tests of birch pollen-allergic patients with apple allergy. Allergy. 2016;71:272–4.
Lange L. Quality of life in the setting of anaphylaxis and food allergy. Allergo J Int. 2014;23:252–60.
Vlieg-Boerstra BJ, van de Weg WE, van der Heide S, Kerkhof M, Arens P, et al. Identification of low allergenic apple cultivars using skin prick tests and oral food challenges. Allergy. 2011;66:491–8.
Dubois AE, Pagliarani G, Brouwer RM, Kollen BJ, Dragsted LO, Eriksen FD, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1‑silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy. 2015;70:1406–12.
Le LQ, Mahler V, Lorenz Y, Scheurer S, Biemelt S, Vieths S, et al. Reduced allergenicity of tomato fruits harvested from Lyc e 1‑silenced transgenic tomato plants. J Allergy Clin Immunol. 2006;118:1176–83.
Katelaris CH. Should patients with pollen fruit syndrome be prescribed an automatic epinephrine injector? Curr Opin Allergy Clin Immunol. 2016;16:370–4.
Treudler R, Simon JC. Schwere Sojaallergie im Erwachsenenalter: Hilft eine spezifische Immuntherapie? Hautarzt. 2012;63:307–12.
van Hoffen E, Peeters KA, van Neerven RJ, van der Tas CW, Zuidmeer L, et al. Effect of birch pollen-specific immunotherapy on birch pollen-related hazelnut allergy. J Allergy Clin Immunol. 2011;127:100–1.
Treudler R, Franke A, Schmiedekneht A, Ballmer-Weber B, Worm M, Werfel T, et al. BASALIT-trial: double-blind placebo-controlled allergen immunotherapy with rBet v 1‑FV in birch related soy allergy. Allergy. 2016; doi:10.1111/all.13112.
Geroldinger-Simic M, Kinaciyan T, Nagl B, Baumgartner-Durchschlag U, et al. Oral exposure to Mal d 1 affects the immune response in patients with birch pollen allergy. J Allergy Clin Immunol. 2013;131:94–102.
Hofer H, Asam C, Hauser M, Nagl B, Laimer J, Himly M, et al. Tackling Bet v 1 and associated food allergies with a single hybrid protein. J Allergy Clin Immunol. 2016; doi:10.1016/j.jaci.2016.09.055.
Wangorsch A, Jamin A, Lidholm J, Gräni N, Lang C, Ballmer-Weber B, et al. Identification and implication of an allergenic PR-10 protein from walnut in birch pollen associated walnut allergy. Mol Nutr Food Res. 2017; doi:10.1002/mnfr.201600902.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Treudler has received fees for consultancy and lectures, as well as travel expenses or study grants, from the following companies: Allergopharma J. Ganzer KG, ALK-Abello, Novartis, Shire. J.-C. Simon declares that he has no competing interests.
Rights and permissions
About this article
Cite this article
Treudler, R., Simon, JC. Pollen-related food allergy: an update. Allergo J Int 26, 273–282 (2017). https://doi.org/10.1007/s40629-017-0022-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-017-0022-2