Abstract
The outbreak species, Orgyia trigotephras caused significant defoliation in northeastern Tunisia in 2005. This work aims to elucidate the population cycle of this pest by testing a wide range of variation of fecundity and population growth. The recorded fecundity was at its highest peak with an average of 153 eggs/egg batch during outbreaks. The action of the complex of egg parasitoid/predator associated with O. trigotephras varies over time. The action of Aprostocetus sp. and Coccidiphila rungsella was at its utmost during the collapse phase of the insect in 2007 and 2014. Only these two species were recorded over our 17-year study. A low proportion of dried eggs versus a high proportion of unfertilized eggs were observed due to the poor quality of foliage consumed by the mother larva during its development. The correlation between fecundity and unfertilized eggs was highly significant. Fecundity change indicators of O. trigotephras are indirect, namely defoliation by high densities of larvae reducing leaf quality for the next generation. The abundance of the host species makes it easily found by parasitoids or vulnerable to parasitism. This strategy is used by O. trigotephras since the action of natural enemies is very low. Yet, the insect was not observed since 2018. It will be important to conduct large research in the region to see whether the insect is still present in the localities or relocate to other ones near the studied site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population cycles are determined by biotic/abiotic or by intrinsic/extrinsic factors (Elton 1927; Rossiter 1994; Myers and Cory 2013). Natural enemies are reported as the main driving force in limiting and regulating the pest insect (Münster-Swendsen 1985; Berryman 1996; Wyckhuys and O’Neil 2006), suppressing incipient outbreaks and limiting the spatial spread of existing outbreaks (Maron et al. 2001; Maron and Harrison 1997). Along with multispecies models involving mixes of parasitoids, predators, and pathogens (May and Hassell 1988), other researchers believe that parasitoids do not cause sufficient mortality to limit the growth of populations (Campbell et al. 1977; Ticehurst et al. 1978). Forest structure may also affect the parasitism rates and survival of forest defoliators (Cappuccino et al. 1998). Likewise, maternal effects (e.g. development rate and final size of offspring) are related to egg size and the order of laying (Rossiter 1994). Long-time series studies on outbreak pests were mostly conducted on larval parasitoids (Münster-Swendsen 1985; Montgomery and Wallner 1988). The goal of this research is to identify factors responsible for maintaining the population dynamic of the outbreak pest, O. trigotephras at low densities at the egg stage. This negative density-dependent could explain the decline of population on the following years. This Erebidae caused significant defoliation of maquis in northern Tunisia in 2005 (Ezzine et al. 2010) and on Pistacia lentiscus in Italy in 2010 (Bella et al. 2011). In this context, this work aims to elucidate the population cycle of O. trigotephras by testing (i) a wide range of variation of fecundity and population growth; (ii) the interaction between parasitism and predation of eggs of O. trigotephras and another intrinsic effect of eggs mortality as unfertilized and eggs containing dead larva by measuring long-term average levels (population abundance) from 2005 to 2021.
Materials and methods
Sampling
Investigations were carried out in northeastern Tunisia (36°849′N; 10°779′; alt. 410 m), in the forest of Jebel Beni Oulid (2670 ha) from 2005 to 2021. The region is under a sub-humid climate with a warm winter, characterized by Mediterranean vegetation, mainly the cork oak series represented by the Quercus coccifera, Erica arborea, and Lavandula stoechas group. Average annual rainfall varies between 450 mm and more than 600 mm (DGF 1995).
Females of O. trigotephras lay eggs in late May-early June. Egg batches can be oviposited in leaves of evergreen plants, namely Callicotome villosa, Erica arborea, E. multiflora, and Phillyrea media, and mainly on Quercus coccifera and Pistacia lentiscus (Ezzine 2016). Thereby, egg batches (n ≤ 30) were firstly collected on P. lentiscus from 2005 to 2015. Since 2016, egg batches were only observed on Q. cocciferaon which we collected the detected ones. Once in the laboratory, these latter were kept individually in plastic boxes at ambient temperature (25 ± 2 °C) until the emergence of natural enemies (Ezzine et al. 2010, 2015).
Laboratory trials
As the eggs of Orgyia trigotephras are protected inside a cocoon, egg batches were rubbed with a stiff bristle brush against a strainer over a bowl. The mesh of the strainer is less than 1 mm (size of the egg) to retain eggs and let the bristle (Ezzine et al. 2010). Eggs were then placed in a petri dish (9 cm ⌀) and observed under a binocular microscope to distinguish different types of eggs. Natural enemies were kept in the Eppendorf tube, in ethanol (96%), or killed with ether before morphological identification (Ezzine et al. 2015).
Statistical analysis
Statistical analyses were performed using the SPSS-10.0 software package for Windows. The average number of eggs/egg batch (fecundity), parasitized, predated, “unfertilized, and dried eggs” were calculated and reported as mean ± standard error of the mean (MSE). Differences in egg categories among years were tested with analysis of variance (ANOVA) followed by multiple comparisons of means using the Duncan test.
Dispersion index (DI = Var (X)/Average (X)) values were estimated for both parasitized and predated egg numbers. The correlation between predated and parasitized eggs was calculated using the Pearson correlation coefficient.
Results
Egg categories
Sorting eggs allowed us to identify five types of eggs; (i) parasitized eggs present a circular and regular exit hole. The interior emptied contains often waste black (rest of larva); (ii) predated eggs, in general, are fragments derived chorions eggs predation; (iii) dried eggs are mostly flat, “brown, black or orange”, and contain a dry larva; (iv) flattened eggs are unfertilized; and (v) hatched eggs recognizable through the large and reniform or circular exit hole of the larva.
Female fecundity
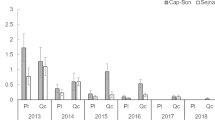
The number of eggs/egg batches (fecundity) varied significantly among years (ANOVA, F(16, 314) = 15.768, P < 0.001). In 2005 and 2014, fecundity was higher on average (153 eggs/egg batch). It was lower in 2007 with an average of 45 ± 5.63 eggs/ egg batch. Yet, for the other years, the fecundity average varied between 60 (2017) and 133 (2015) eggs/ egg batches (Fig. 1). The maximum number of eggs was 238 eggs (2010), however, the minimum was 5 eggs (2006).
Egg mortality
Egg mortality includes dried eggs, flattened/unfertilized eggs, “predated eggs, and parasitized eggs”. Percentage of each category varied significantly among years (P < 0.001). According to multiple comparisons of means Duncan, six groups of unfertilized eggs were identified. In 2015, we recorded the highest mean percentage (88.73 ± 3.07%) and the lowest in 2014 (14.43 ± 2.6%) (Fig. 2A). The maximum percentage of unfertilized eggs was 100% (2005, 2006, 2008, and 2009), however, the minimum was 0.00% (2006, 2010, 2014, and 2017).
The highest mean percentage of dried eggs was recorded in 2017 (12.86 ± 3.19%). It was lower than the other years and it does not exceed 4% (Fig. 2B). The maximum percentage of dried eggs was 90.43% (2009), however, the minimum was 0.00% throughout the study period.
One parasitoid, the Hymenoptera, Eulophid Aprostocetus sp. parasitized the eggs. Even, predation was caused by the larvae of the Cosmopterigidae, Coccidiphila rungsella Nel and Brusseaux, 1997 (Ezzine et al. 2015). The mean percentage of parasitized eggs varied between 1.08 ± 0.25 (2009) and 22.09 ± 5.4 (2014) (Fig. 2C). The maximum was 72.27% and the minimum was 00%. On the other side, predation varied between 00% (2012) and 5.33 ± 2.16 (2016) (Fig. 2D), with a maximum of 36.84% (2016).
The dispersion index (DI) was well above 1.0 in all studied years (except 2011 and 2012 for predated eggs). It shows the gathering distribution of parasitoid and predator attacks (Table 1).
The number of parasitized or dried eggs was not significantly correlated with Fecundity. Yet, the number of unfertilized or predated eggs was significantly correlated with fecundity. On the other hand, parasitized eggs were significantly correlated with dried and predated eggs. Unfertilized eggs were significantly correlated with predated eggs. Parasitized and predated eggs were correlated significantly (Table 2).
Figure 3 shows the simulation between fecundity, parasitized, and predated eggs. Two highest peaks of fecundity (> 153 eggs/egg batch) were observed in 2005 and 2012, and a relatively low one in 2015 (134 eggs). The highest peak of parasitism was observed in 2014 (22%) and a relatively low one in 2007 (16%). The same results were observedfor predation, a peak in 2016 (5.33%) and two relatively low ones in 2013 (2.41%) and 2014 (2.78%).
Discussion and conclusion
Given the long-term available data sets, we studied the female fecundity and the mortality factors of eggs of O. trigotephras and the action of natural enemies. Like other outbreak insects, O. trigotephras occurs in many sites at low densities, and a few sites at very high densities (Harrison 1997; Maron et al. 2001; Ben Jamâa et al. 2002; Ezzine et al. 2021). In 2005, the first year of a large outbreak (pro-gradation phase), larvae density of O. trigotephras was very high and larvae fed on Quercus coccifera, Pistacia lentiscus, Callicotome villosa, Erica arborea, E. multiflora, Arbutus unedo and Cistus Crispus (Ezzine et al. 2010; Ezzine, 2016). The recorded fecundity was at its highest peak with an average of 153 eggs/egg batch (max = 232 eggs). From 2006 to 2012, the insect was present but with a low density (latency phase). In 2012, a second and low outbreak was observed with female fecundity of 153 eggs/ egg batch (max = 227 eggs). During these years, the density of O. trigotephras was very low and it correspond to the retro gradation phase of the insect till 2018 wherein no more egg batch was observed on any host plant in this site. According to Myers and Cory (2013), three factors are required for cyclic population dynamics: the first one is a high fecundity to allow the population to increase by three to six orders of magnitude during the four or five generations of the increase phase; the second one is the density-related, increased mortality factors initiating the decline at peak density; and the last one is delayed density-related mechanisms inducing the decline of the population. A review of Berryman (1996) on cyclic forest Lepidoptera led to conclude that parasitoids invariably kill a consistently high proportion of lepidopteran eggs, larvae, and pupae during the decline phase and relax their effects during the increase phase. According to the data reported in the present paper, the action of the complex of egg parasitoid/predator associated with O. trigotephras varies in time. The action of Aprostocetus sp. and C. rungsella was at its utmost during the collapse phase of the insect in 2007 and 2014. Only very low numbers of Aprostocetus sp. were recovered from O. trigotephras moth eggs and were found to cause a lower percentage of parasitism and did not exceed 22% (2014). Yet, predation caused by larvae of C. rungsella was very low and the percentage did not exceed 5% (2016). Only these two species were recorded over our 17-year study. The deepest study conducted by Myers and Cory (2013) showed that parasitism is a universal mortality factor for forest Lepidoptera. Parasitism by hymenopteran parasitoids leads to a delayed density dependence capable of producing cyclic oscillations in the population dynamics of Lepidoptera (Berryman 1996; Tanhuanpää et al. 2002). Egg parasitism of L. dispar was unimportant as a mortality factor in the examined Slovak populations compared to the other stages (Hoch et al. 2001). The Eulophid parasitoids were reported to be successfully used to control important forest pests (Voegele 1989; Duan et al. 2013; Wang et al. 2021). Generally, parasitoids parasitize fertilized host eggs (Krugner 2014) since it’s important for parasitism and fitness of the parasitoid offspring (Wang et al. 2020). Aprostocetus spp. were used in forestry to control pests (Sampson et al. 2013) and, parasitize the eggs of Lepidoptera (Yang et al. 2015). In Viggiani (2021), it was reported that egg parasitoids, Aprostocetus, Anastatus bifasciatus, and Baryscapus sp. of Phaneroptera nana, oviposit in the host eggs in autumn and complete a generation in the next spring–summer and can also develop another generation on the same host in summer-autumn. Our results showed that the biology of Aprostocetus is synchronized with the egg deposition of O. trigotephras. On the other side, females of other species, as the trichogrammatid oviposit in the host egg in autumn and a long development takes place (Viggiani 2021). Ezzine et al. (2020) elucidate the effect of pupa parasitoids on the outbreak species the Erebidae, Casama innotata which suggests the existence of strong natural regulation mechanisms, by the mortality of more than 93% of the population by parasitoids. In our work, results indicate that natural enemies play a role in maintaining this population dynamic but with a low proportion. Nevertheless, intrinsic factors may play a crucial role in regulating the population. Our findings showed a low proportion of dried eggs (< 13%). Contrariwise, the proportion of unfertilized eggs was very important and ranged between 15% (2014) and 89% (2015). These defective eggs do not have a complete plan for building an embryo or being incapable of further development. This may be due to the poor quality of foliage consumed by the mother larva during its development (Ezzine et al. 2010, 2015). The correlation between fecundity and unfertilized eggs was highly significant. Fecundity change indicators of O. trigotephras are indirect, namely defoliation by high densities of larvae reducing leaf quality for the next generation (Ezzine et al. 2015). Furthermore, Villemant and Fraval (1992), considered that after strong defoliation caused by Lymantria dispar, the attack of the parasitoid was low and it was due to the high proportion of dry eggs resulting from the poor nutrition of parents.
The abundance of the host species makes it easily found by parasitoids or vulnerable to parasitism. Thus, existence at relatively low abundance may provide refuge from parasitism (Barbosa 2004). Maybe, this strategy is used by O. trigotephras since the action of natural enemies is very low. Yet, the insect was not observed since 2018, it’s probably due to the competition that reigns in the studied site since 2013 (Hammami et al. 2019). It will be important to conduct large research in the region to see whether the insect is still present in the localities or relocate to other ones near the studied site.
To conclude, biological control involves the mutualism among natural enemies affecting the same host (May and Hassell 1988). The broad objective of this study is to look for biological control as it reduces the average abundance of a pest by using one or more populations of natural enemies, and in so doing reduce insect outbreaks. These results caution us against formulating biological control strategies purely in terms of two-species systems. The cyclic dynamics can be affected by environmental disturbances. It’s so important to emphasize other ways for future studies to expand cyclic population dynamics mechanisms, considering the effect of climate change and phenology in elucidating patterns of synchrony among populations and outbreaks.
Data availability
We admit that the results in the paper will be archived in an appropriate public repository.
References
Bella S, Longo S, Sidoti A (2011) Indagini su Teia trigotephras defogliatore del lentisco nella Sicilia sud-orientale. Atti XXIII Congresso Nazionale Italiano di Entomologia Genova, 13–16-giugno: 107.
Ben Jamâa ML, M’nara S, Villemant C, Khaldi A, (2002) Lymantria dispar L. (Lepidoptera, Lymantriidae) en Tunisie: état actuel des connaissances et perspectives de recherche. IOBC-WPRS Bulletin 25:101–108
Berryman AA (1996) What causes population cycles of forest Lepidoptera? Trends Ecol Evol 11:28–32. https://doi.org/10.1016/0169-5347(96)81066-4
Campbell RW, Sloan RJ, Biazak CE (1977) Sources of mortality among late instar gypsy moth larvae in sparse populations. Environ Entomol 6:865–871
Cappuccino N, Lavertu D, Bergeron Y, Regniere R (1998) Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 114:236–242
Direction Générale des Forêts (1995) Forêt domaniale De Béni Oulid: Plan d’aménagement 1996–2015. SOGET Maghreb, Tunisia
Duan JJ, Bauer LS, Abell KJ, Lelito JP, Van Driesche R (2013) Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J Eco Entomol 106:1145–1154. https://doi.org/10.1603/EC13047
Elton C (1927) Animal ecology. Sidgwick and Jackson, London
Ezzine O, Ben Jamâa ML, M’nara S, Nouira S (2010) Bioécologie d’Orgyia trigotephras (Boisduval, 1829), (Lepidoptera, Lymantriidae) à Jebel Abderrahmane (Nord Est, Tunisie). IOBC/WPRS Bull 57:123–127
Ezzine O, Branco M, Villemant C, Schmidt S, Nouira S, BenJamâa ML (2015) Host use in Orgyia trigotephras (Erebidae, Lymantriinae) during the outbreak: effects on larval performance and egg mortality. Anna Forest Sci AFS72:561–568. https://doi.org/10.1007/s13595-015-0484-7
Ezzine O, Dhahri S, Hammami S, LaajimiO MS, Ben Jamâa ML (2020) Pupa parasitoids of Casama innotata (Lepidoptera, Erebidae), defoliator of Acacia horrida in Tunisia. IOBC-WPRS Bull 151:83–88
Ezzine O, Dhahri S, Hammami S, Bourouguaoui A, Ben Jamâa ML (2021) Occurrence of a new pest Casama innotata (Walker 1855) (Lepidoptera, Erebidae) on a nonnative host plant in an arid environment. J Arid Environ 188:104450. https://doi.org/10.1016/j.jaridenv.2021.104450
Ezzine O (2016) Interactions insectes/plantes-hôtes: cas d’Orgyia trigotephras Boisduval (1829) (Lepidoptera, Erebidae) en Tunisie. PhD thesis, Faculté des Sciences Mathématiques, Physiques et Naturelles de Tunis.
Hammami S, Ezzine O, Dhahri S, Villemant C, Schmidt S, Ben Jamâa ML (2019) Pupae mortality of Orgyia trigotephras Boisduval 1829 (Erebidae, Lymantriinae) in Tunisia. Redia 102:107–111. https://doi.org/10.19263/REDIA-102.19.16
Harrison S (1997) Persistent, localized outbreaks in the western tussock moth Orgyia vetusta: the roles of resource quality, predation, and poor dispersal. Ecol Entomol 22:158–16. https://doi.org/10.1046/j.1365-2311.1997.00053.x
Hoch G, Zubrik M, Novotny J, Schopf A (2001) The natural enemy complex of the gypsy moth, Lymantria dispar (Lep., Lymantriidae) in different phases of its population dynamics in eastern Austria and Slovakia- a comparative study. J Appl Entomol 125:217–227. https://doi.org/10.1046/j.1439-0418.2001.00540.x
Krugner R (2014) Suitability of non-fertilized eggs of Homalodisca vitripennis for the egg parasitoid Gonatocerus morrilli. BioControl 59:167–174. https://doi.org/10.1007/s10526-014-9562-2
Maron JL, Harrison S (1997) Spatial pattern formation in an insect host-parasitoid system. Science 278:1619–1621. https://doi.org/10.1126/science.278.5343.1619
Maron JL, Harrison S, Greaves M (2001) Origin of an insect outbreak: escape in space or time from natural enemies? Oecologia 126:595–602. https://doi.org/10.1007/S004420000558
May RM, Hassell MP (1988) Population dynamics and biological control. R Soc 318:129–169
Montgomery ME, Wallner WE (1988) The gypsy moth: a westward migrant. In: Berryman AA (ed) Dynamics of forest insect populations: patterns, causes, implications. Plenum Press, New York, pp 353–376
Münster-Swendsen M (1985) A simulation study of primary-, clepto and hyper-parasitism in Epinotia tedella (Lepidoptera: Tortricidae). J Anim Ecol 54:683–695
Myers JH, Cory JS (2013) Population cycles in forest lepidoptera revisited. Annu Rev Ecol Evol Syst 44:565–592. https://doi.org/10.1146/annurev-ecolsys-110512-135858
Rossiter MC (1994) Maternal effects hypothesis of herbivore outbreak. BioScience 44:752–63. https://doi.org/10.2307/1312584
Sampson BJ, Roubos CR, Stringer SJ, Marshall D, Liburd OE (2013) Biology and efficacy of Aprostocetus (Eulophidae: Hymenoptera) as a parasitoid of the blueberry gall midge complex: Dasineura oxycoccana and Prodiplosis vaccinii (Diptera: Cecidomyiidae). J Econ Entomol 106:73–79. https://doi.org/10.1603/ec12404
Tanhuanpää M, Ruohomäki K, Turchin P, Ayres MP, Bylund H, Kaitaniemi P, Tammaru T, Haukioja E (2002) Population cycles of the autumnal moth in Fennoscandia. In: Berryman AA (ed) Population cycles: the case for trophic interactions. Oxford University Press, New York, pp 142–164
Ticehurst M, Fusco RA, Kling RP, Unger J (1978) Observations on parasites of gypsy moth in first cycle infestations in Pennsylvania from 1974–1977. Environ Entomol 7:355–358
Viggiani G (2021) Biological notes on some egg parasitoids of Phaneroptera nana Fieber, 1853 (Orthoptera, Tettigoniidae) with a description of a new species of Aprostocetus Westwood, 1833 (Hymenoptera, Eulophidae) from Italy. Biodivers J 12:289–295. https://doi.org/10.31396/Biodiv.Jour.2021.12.2.289.295
Villemant C, Fraval A (1992) Les ennemis des œufs de Porthetria dispar (L.) (Lép.: Lymantriidae) au Maroc: inventaire et problèmes relatifs à l’évaluation de leur impact. Bulletin De L’institut Scientifique Rabat 16:160–172
Voegele JM (1989) Biological control of Brontispa longissima in Western Samoa: an ecological and economic evaluation. Agric Ecosyst Environ 27:315–329
Wang Y, Zou ZP, Hou YY, Yang XB, Wang S, Dai HJ, Xu YY, Zang LS (2020) Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol Gen 40:397–406. https://doi.org/10.1127/entomologia/2020/1060
Wang J, Chen YM, Yang XB, Lv RE, Desneux N, Zang LS (2021) Parasitism and suitability of Aprostocetus brevipedicellus on Chinese Oak silkworm, Antheraea pernyi, a dominant factitious host. Insects 12:694. https://doi.org/10.3390/insects12080694
Wyckhuys KAG, O’Neil RJ (2006) Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot 25:1180–1190. https://doi.org/10.1016/j.cropro.2006.03.003
Yang ZQ, Yao YX, Cao LM (2015) Chalcidoidea parasitizing forest defoliators (Hymenoptera). Science Press, Beijing, China
Acknowledgements
Thanks to our forest technicians for their valuable help in the field. We wish to thank Emna DARGHOUTHI (mannouta@msn.com) for her assistance with the language editing.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ezzine, O., Hammami, S. & Ben Jamâa, M.L. The role of natural enemies in regulating the population of the outbreak species Orgyia trigotephras (Lepidoptera: Erebidae) in North Africa. Arthropod-Plant Interactions 16, 469–475 (2022). https://doi.org/10.1007/s11829-022-09915-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-022-09915-y