Abstract
Gonatocerus morrilli (Howard) (Hymenoptera: Mymaridae) is an egg parasitoid used in California, USA to control glassy-winged sharpshooter (GWSS), Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae). Virgin GWSS females deposit non-fertilized eggs and mated females can exhaust sperm reserves for egg fertilization. However, nothing is known about Gonatocerus spp. performance when using non-fertilized GWSS eggs as hosts. Host age preference for oviposition and suitability of non-fertilized GWSS eggs as hosts for G. morrilli reproduction were investigated to determine whether non-fertilized eggs on sentinel plants could be used to monitor egg parasitoid populations. Gonatocerus morrilli parasitized all ages of GWSS eggs (1–8 days old) regardless if the host egg was fertilized or not. In choice tests (fertilized versus non-fertilized eggs), parasitoids failed to emerge as adults from non-fertilized eggs more often than from fertilized eggs. The results indicate that non-fertilized eggs were accepted by G. morrilli as suitable hosts for oviposition, but were less suitable for immature development compared to fertilized eggs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The glassy-winged sharpshooter (GWSS), Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae), is a key vector of Xylella fastidiosa Wells et al., the bacterium that causes Pierce’s disease of grapevines (Davis et al. 1978) and other diseases of high-valued crops in the Americas (Hopkins and Purcell 2002). GWSS arrived in California, USA in ~1988 (Stenger et al. 2010) and was detected following a period of rapid population growth and range expansion (Sorensen and Gill 1996). GWSS is a polyphagous insect (Turner and Pollard 1959) that was responsible for spreading X. fastidiosa among vineyards in the southern San Joaquin and Temecula valleys of California (Blua et al. 1999; Tubajika et al. 2004), which triggered the establishment of state quarantines to keep GWSS from spreading to other regions of the state. Current control measures for GWSS in California include an area-wide insecticide application program and release of mass-produced solitary egg parasitoids, including the mymarids Gonatocerus ashmeadi Girault, G. morrilli (Howard), G. triguttatus Girault, and G. morgani Triapitsyn (CDFA 2010). Despite such efforts, the geographic distribution of GWSS continues to expand.
Chemical control of GWSS in urban areas, organic farms, and farming operations under integrated pest management programs is problematic because insecticides are ineffective, not used, or incompatible with existing practices, respectively. Thus, suppression of GWSS populations in these areas relies heavily on natural mortality caused by predators and parasitoids. Gonatocerus spp. exhibit a high potential for suppressing GWSS populations during summer months (Lytle and Morse 2012). In California, Gonatocerus spp. can also parasitize eggs of H. liturata Ball (Hemiptera: Cicadellidae) (Boyd and Hoddle 2007), but this species is substantially less common than GWSS in areas under the biological control program (Al-Wahaibi and Morse 2010). Therefore, the scarcity of GWSS egg masses during late fall to early spring (Krugner et al. 2009a), the absence of alternative insect hosts (Boyd and Hoddle 2007), and the unfavorable low winter temperatures in California (Chen et al. 2006; Pilkington and Hoddle 2006a, b; Son et al. 2012) impair their rapid increase in numbers and performance in suppressing GWSS populations during the first (spring) generation.
GWSS has two to the three broods per year (spring, summer, and late fall). Hummel et al. (2006) suggested that mating may occur throughout the summer and into fall, but more likely occurs in June, October, and December, when there is a peak in newly emerged previtellogenic females. The spring generation results from oviposition by overwintering GWSS females. However, it is not known whether overwintering GWSS females re-mate in spring, use sperm acquired before or during the winter, or both. Research by Krugner (2010) demonstrated that GWSS females can exhaust sperm reserves and that polyandry may be necessary for GWSS to successfully produce viable progeny. Thus, mating disruption and/or insect sterilization strategies may have the potential to reduce population growth by reducing egg fertilization rates and simultaneously maintaining pressure by egg parasitoid populations, if parasitoids can use non-fertilized eggs for reproduction. Laboratory studies have demonstrated that parasitoids can attack all GWSS egg ages, but most efficiently use young eggs (Irvin and Hoddle 2005; Krugner et al. 2009b; Lytle et al. 2012). However, it is not known whether Gonatocerus spp. would attack and successfully use non-fertilized GWSS eggs for reproduction.
Monitoring of GWSS egg parasitoid populations relies on information obtained from field collected host eggs. Collection of GWSS egg masses is labor intensive and scourged by difficulties of access, particularly in urban residential areas and landscapes composed of relatively tall trees. In both urban and agricultural settings, collection of sufficient numbers of egg masses is important to infer whether parasitoids are present, but finding egg masses in areas with low GWSS population density and/or sparse distribution can be a challenge. Therefore, field collected egg masses may have limited use in assessing establishment, dispersal, reproductive success, and overwintering behavior of parasitoids and are a poor proxy for measuring parasitoid effectiveness during some periods and in some locations. One alternative to field collecting egg masses is to use sentinel plants infested with GWSS eggs. Sentinels are plants that were caged in the greenhouse or laboratory for oviposition by GWSS and then transferred to select locations in the field for monitoring of parasitoid populations or used in experiments. Currently, use of sentinel plants in California is limited due to regulations designed to prevent unintended introduction of GWSS lifestages into areas under the eradication program. Sentinel plants harboring non-fertilized GWSS eggs would pose little risk of accidental introduction and could prove to be a useful tool. The objectives of this study were to: (1) describe and quantify G. morrilli host age preference for oviposition, (2) compare the suitability of fertilized versus non-fertilized GWSS eggs for G. morrilli development, and (3) determine under local field conditions whether G. morrilli can overwinter using non-fertilized GWSS eggs.
Materials and methods
Plants for GWSS rearing
Five species of plants were used in GWSS rearing: Japanese spindle (Euonymus japonica Thunb.), okra (Abelmoschus esculentus (L.) Moench cv. ‘Cajun delight’), cowpea (Vigna unguiculata L. Walp. cv. ‘Blackeye’) (both from Vermont Bean Seed Co., Randolph, WI, USA), basil (Ocimum basilicum L. ‘Genovese’), and sunflower (Helianthus annuus L. ‘American Giant Hybrid’) (both from Ferry-Morse Seed Co., Fulton, KY, USA). Plants for insect rearing were grown using Sunshine Soil Mix 1 (Sun Gro Horticulture, Bellevue, WA, USA) in 0.5 l pots (cowpea, okra, and sunflower) and 3.8 l containers (basil).
Establishment and maintenance of insect colonies
GWSS colonies were established from egg masses collected March to April 2012 in Bakersfield, California, USA. Groups of 10–15 field collected egg masses were placed in 10-cm Petri dishes lined with moist paper towel. As eggs hatched, newly emerged nymphs were transferred to cages (~100 nymphs per cage) (Bug Dorm-2®, BioQuip Products, Rancho Dominguez, CA, USA). Each cage contained four cowpea plants, four okra plants, one basil plant, and three sunflower plants. Plants were replaced every seven days. The insect rearing room was maintained at 24–27 °C, 22–25 % RH, and 16:8 [L:D] h using artificial light from high-pressure sodium vapor bulbs.
Colonies of G. morrilli were established from adults provided by Dr. Youngsoo Son (California Department of Food and Agriculture, GWSS Biological Control Program, Arvin, CA, USA). The G. morrilli colonies were maintained in rectangular, acrylic cages (10 × 10 × 15 cm) with fine mesh screening on the sides. Each week, parasitoids were provided with 5–10 E. japonica leaves that harbored GWSS egg masses. Leaves harboring egg masses were inserted into foam sheets (9.7 × 9.7 cm) that were floated on 3 cm of water within the bottom of the cage to avoid leaf desiccation. To refill the water within the cages, the bottom of the cage was perforated and cages were kept in water-filled trays. These cages were used in all studies described below unless otherwise stated. Honey was streaked on the cage wall(s) as a food source for parasitoids. Cages were held under constant conditions (26.7 °C, 65 % RH, and 16:8 [L:D] h) in an environmental chamber. Parasitoid development from egg to adult was completed within ~12 days, and the rearing process was repeated following adult eclosion.
Source of fertilized and non-fertilized GWSS eggs
To generate groups of virgin GWSS females that served as a source of non-fertilized eggs, fourth and fifth instar GWSS nymphs were separated by gender. Groups of 100 female nymphs were placed in a cage and reared to the adult stage using the methods described above. To generate groups of mated females that served as a source of fertilized eggs, about 50 male and 50 female nymphs were mixed in cages and reared to the adult stage as described above. At 30 days after adult molt, females from both colonies were transferred individually from the rearing cages to cylindrical plastic cages (10 cm diameter × 35 cm height) and kept on potted cowpea plants for a ten-day oviposition period. After the ten-day oviposition period, cowpea plants were replaced and plants hosting eggs were transferred to a new cylindrical plastic cage. Eggs deposited during the oviposition period in cylindrical plastic cages were held for another 14 days and checked for signs of hatching or embryo development to confirm that eggs deposited by test females were either fertilized or not fertilized. After female fertility was determined, females were transferred back into the respective rearing cages in groups and held for oviposition using three sunflower plants and one E. japonica plant per cage. Euonymus japonica was used because, under the conditions described below in the host preference study, E. japonica leaves are more durable than leaves from the other plant species used in GWSS colony maintenance.
Host preference for oviposition
To determine host egg ages that were preferred for oviposition, mated G. morrilli confined in acrylic cages were provided with fertilized and non-fertilized GWSS eggs of various ages (1–8 days old) in choice and no-choice arenas. GWSS egg masses of specific age were obtained as follows. Groups of virgin and mated GWSS females were provided with E. japonica plants as an oviposition substrate for ten days. Each day at 09:00 h, plants were inspected for newly laid egg masses. Egg masses were date-marked and left on the plants to avoid any changes in egg development that might be caused by leaf removal. Egg masses of the appropriate age for the experiments were removed from plants and number of eggs per egg mass was recorded.
In choice tests for non-fertilized host eggs of different ages, one egg mass of each age, with a similar number of eggs, was exposed to two-day old G. morrilli females in acrylic cages. A 1:10 (female parasitoid:host egg) ratio in each cage was used to maintain uniform parasitism rates across cages and repetitions. Female parasitoids were allowed to oviposit in GWSS egg masses for 24 h. Choice test assemblages (one to eight-day old eggs) were replicated in four cages and repeated on three different days under constant conditions (26.7 °C, 65 % RH, and 16:8 [L:D] h) in an environmental chamber. After the 24-h ovipositional period, females were discarded and cages and leaves rinsed with water to ensure that all parasitoids were removed. Leaves containing egg masses were kept for 21 days to evaluate parasitism rates. After all G. morrilli individuals had enclosed, egg masses were dissected to record the number of parasitoids emerged, number of partially and fully developed parasitoids that failed to emerge, and the number of non-hatched GWSS eggs and nymphs. The total number of eggs parasitized per egg mass was determined by the sum of the number of eggs from which parasitoids had emerged and the number of eggs with detectable signs of parasitism (i.e., presence of parasitoid larva or pupa). Lack of sufficient egg masses prevented the execution of one repetition of no-choice tests. Therefore, no-choice tests were performed simultaneously only with the first and third choice test repetitions using the same methodology described above, except that G. morrilli were exposed to eight GWSS egg masses of the same age (i.e., same egg ages as described above) in a cage.
In choice tests for fertilized versus non-fertilized eggs, G. morrilli were exposed to three fertilized and three non-fertilized GWSS egg masses of the same age in a cage. This experiment was repeated on three different days and evaluated as described above. For each repetition of choice and no-choice tests for oviposition in non-fertilized eggs and choice test for oviposition in fertilized versus non-fertilized GWSS eggs, groups of four egg masses of each age and fertility status (fertilized and non-fertilized) were placed in acrylic cages without female parasitoids to serve as a control.
Parasitoid overwintering in non-fertilized host eggs
To generate sentinel plants with non-fertilized eggs, virgin GWSS females were provided with ten E. japonica plants for a 48-h oviposition period. After the oviposition period, the number of eggs per egg mass per plant was recorded and the plant was covered with the cylindrical plastic cage described above. Three-day old male and female G. morrilli were introduced into each cage in a 2:1:10 (female parasitoid:male parasitoid:host egg) ratio. Honey was streaked on cage walls to serve as a food source for the parasitoid. Host eggs were exposed to parasitoids for 24 h under constant conditions (26.7 °C, 65 % RH, and 16:8 [L:D] h) in an environmental chamber. At the end of the parasitism period, parasitoids were removed and the plant was rinsed with water. The plant was covered with a mesh screen bag to protect egg masses from predators and transported immediately to the campus of the California State University, Fresno, CA. During the 2012–2013 winter season, a total of nine, 27, 18, 13, ten, and seven GWSS egg masses deposited on E. japonica plants were placed in the field on December 12 and 26, January 14 and 28, and February 11 and 25, respectively. Egg masses were checked weekly for parasitoid emergence. In mid April 2013, all plants were taken to the laboratory and eggs were dissected to determine number of parasitoids emerged and number of eggs parasitized.
Data analysis
The proportion of eggs parasitized was calculated using the total number of eggs per egg mass. The proportion of parasitoids emerged was calculated using the total number of eggs parasitized per egg mass. ANOVA was used to test the effect of egg age on G. morrilli oviposition preference and successful development in choice and non-choice tests using non-fertilized GWSS eggs. Data were arcsine transformed prior to ANOVA to normalize distribution. If a significant treatment effect was observed, treatment means were separated using Tukey’s HSD test (α = 0.05). Results from choice tests (fertilized vs. non-fertilized eggs) were analyzed using t-tests, provided the results were normally distributed. If results were not normally distributed, a Mann–Whitney sum rank test was performed.
Results
Host age preference for oviposition
Choice and no-choice tests for oviposition in non-fertilized host eggs
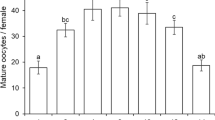
In tests for G. morrilli oviposition rates in non-fertilized GWSS eggs, the proportion of eggs parasitized was different across GWSS egg ages in choice (F 7,131 = 21.040, P < 0.0001) (Fig. 1a) and no-choice (F 7,49 = 13.218, P < 0.0001) tests (Fig. 1b). In choice tests, the proportion of parasitized GWSS eggs was higher in one to five-day old egg masses than in six to eight-day old egg masses (Fig. 1a). In no-choice tests, parasitism was higher in one and five-day old egg masses than six to eight-day old egg masses and intermediate in two to four-day old egg masses when compared to the other egg mass ages tested (Fig. 1b). Results obtained from GWSS egg dissections after parasitoid emergence revealed the presence of partially or fully developed parasitoids that failed to emerge from GWSS eggs. In choice tests, the proportion of parasitoids that successfully emerged from GWSS egg masses was different among the egg ages tested (F 7,94 = 3.059, P = 0.006). Specifically, significantly more parasitoids successfully emerged from one, two, three, five and six-day old egg masses than from seven-day old (Fig. 1a) egg masses. In no-choice tests, the proportion of parasitoids that successfully emerged from parasitized host eggs was not different among the egg ages tested (F 6,33 = 1.083, P = 0.393). Parasitoids did not emerge from parasitized eight-day old host eggs (Fig. 1b).
Mean (+SE) proportion of GWSS eggs parasitized by Gonatocerus morrilli and proportion of parasitoids emerged in choice (a) and no-choice (b) tests for oviposition in non-fertilized GWSS eggs of different ages. Data on parasitism and parasitoid emergence across egg ages were subjected to ANOVA and means were separated using Tukey’s HSD test. Mean proportion of eggs parasitized indicated by bars followed by the same lower-case letter are not significantly different (P = 0.05). Mean proportion of parasitoids emerged indicated by bars followed by the same upper-case letter are not significantly different (P = 0.05)
Choice test for oviposition in fertilized versus non-fertilized host eggs
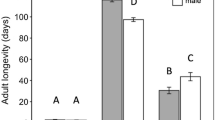
Gonatocerus morrilli parasitized GWSS eggs of all ages (one to eight-day old eggs) and fertility status (fertilized and non-fertilized) (Fig. 2). The exception was lack of detectable parasitism in seven-day old non-fertilized GWSS eggs (Fig. 2a). There were no significant differences in the proportion of parasitism between fertilized and non-fertilized eggs in egg masses ages one (t = −0.0227, df = 8, P = 0.982), two (U = 23.5, P = 0.613), three (t = 1.773, df = 8, P = 0.114), four (t = 1.064, df = 10, P = 0.312), and five-day old (U = 8.0, P = 0.247) (Fig. 2a). Non-fertilized eggs that were six (t = 9.058, df = 8, P < 0.0001), seven, and eight days old (t = 6.400, df = 6, P < 0.0001) were less preferred by G. morrilli females than fertilized eggs of the same age (Fig. 2a).
Mean (+SE) proportion of GWSS eggs parasitized by Gonatocerus morrilli (a) and proportion of parasitoids emerged (b) in choice tests for oviposition in fertilized versus non-fertilized GWSS eggs of different ages. t-tests and the Mann–Whitney Sum Rank Test were used within egg age categories to compare parameters (i.e., proportion of eggs parasitized and parasitoids emerged) between fertilized and non-fertilized host eggs. An asterisk above bars indicates a significant difference between the two parameters. “ns” above bars indicates a non-significant difference between the two parameters
In general, the proportion of parasitoids that successfully emerged from parasitized host eggs were lower in non-fertilized than fertilized eggs (Fig. 2b). The proportion of parasitoids emerged from non-fertilized GWSS egg masses was lower than in fertilized eggs ages two (t = 5.290, df = 11, P < 0.0001), three (t = 5.0, df = 7, P = 0.0016), four (t = 80.864, df = 6, P < 0.0001), five (t = 2.873, df = 7, P = 0.024), and eight-day old (t = 7.333, df = 4, P = 0.0018) (Fig. 2b). There were no differences in the proportion of parasitoids emerged from non-fertilized to fertilized GWSS egg ages one (t = 1.047, df = 7, P = 0.330) and six-day old (t = 1.026, df = 5, P = 0.352) (Fig. 2b).
Parasitoid overwintering in non-fertilized host eggs
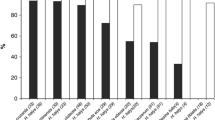
There were no visible signs of parasitoid development in host eggs that were placed in the field on December 12 and 26, 2012 (Fig. 3). Partial parasitoid development was detected in host eggs that were placed in the field on January 14 and 28, 2013, but all individuals failed to emerge from the host eggs. Successful adult parasitoid emergence was detected only from egg masses that were placed in the field on February 11 and 25, 2013 (Fig. 3). Adult parasitoids emerged in the field from late March to early April. In general, the proportion of eggs with detectable parasitoid development and successful emergence increased from mid to late winter.
Discussion
The laboratory assays reported in this study revealed that G. morrilli readily parasitized all GWSS egg ages tested regardless if the host egg was fertilized or not fertilized. However, parasitoid oviposition preference and suitability of GWSS eggs for parasitoid development were affected by the age and fertilization status of the GWSS eggs. Specifically, detectable parasitism was reduced in older (>five-day old) egg masses in choice and no-choice tests for non-fertilized GWSS eggs. In choice tests for fertilized versus non-fertilized eggs, there were no differences in the proportion of eggs parasitized in egg masses ages one to five-day old, which suggest that young fertilized and non-fertilized GWSS eggs are equally recognized and accepted as hosts for oviposition by G. morrilli. However, at egg ages two–five, and eight-day old, more parasitoids failed to complete development on non-fertilized eggs compared to fertilized eggs. Thus, non-fertilized eggs are recognized and accepted by G. morrilli as suitable hosts for oviposition, but are less suitable for parasitoid development compared to fertilized eggs.
Parasitoid development time, larval survival, adult size and fecundity are influenced by the quantity and quality of resources available during immature development (Godfray 1994). Variation in host suitability for GWSS egg parasitoids has been shown to depend on egg age (Irvin and Hoddle 2005; Krugner et al. 2009b; Lytle et al. 2012), presumably because of nutritional characteristics associated with GWSS eggs as they age. For example, young eggs have less differentiated tissues of the embryonic GWSS compared to older eggs. At 25 °C, a fertilized GWSS egg has six distinct developmental stages including an initial undifferentiated stage at 0–90 h post oviposition, when the egg is uniformly opaque, white-yellowish colored, and without apparent structures (Al-Wahaibi and Morse 2009). During this period, non-fertilized GWSS eggs appeared the same when observed under a dissecting microscope, perhaps explaining greater parasitoid acceptance of young eggs. However, parasitoid survival on non-fertilized GWSS eggs was lower compared to survival on fertilized eggs, suggesting that non-fertilized GWSS eggs lack the critical resources found in fertilized eggs. One possible explanation is a difference in nutritional value between fertilized and non-fertilized eggs resulted from lack of GWSS embryo development. However, young (<24-h old) GWSS eggs presumably fertilized and terminated by chilling at 2 °C were fully acceptable and suitable for development of the mymarid egg parasitoid G. ashmeadi Girault (Chen and Leopold 2007), which indicates that host eggs do not need to be alive or physiologically active to support growth of the immature parasitoid.
Ribosomes isolated from fertilized eggs of houseflies, Musca domestica L. (Diptera: Muscidae) had a greater capacity to initiate protein synthesis than those from non-fertilized eggs (Gadallah et al. 1970). Therefore, protein synthesis may be reduced in non-fertilized GWSS eggs resulting in a less nutritious host egg for the parasitoid. Alternatively, it is possible that egg fertilization and/or incorporation of male secondary ejaculatory components into eggs by female GWSS provide additional resources for the developing parasitoid larvae. In the cicadellid Bothrogonia ferrugiana (F.), injection of dyed proteins into the female bursa resulted in the production of dyed eggs in the ovaries, suggesting that females may incorporate proteinaceous material from male spermatophores into the oocytes (Hayashi and Kamimura 2002).
Knowledge of the biology of GWSS egg parasitoids may be obtained through laboratory studies, but field studies are needed to optimize the use of parasitoids in a biological control program. Obtaining permission from regulatory agencies to conduct field studies with GWSS in areas under the eradication program is a challenge because of the risks involved with accidental releases of GWSS from experimental cages. Although non-fertilized GWSS eggs were not as suitable for parasitoid development as fertilized eggs, deployment of non-fertilized eggs in sentinel plants could be used to study parasitoid populations. Under field conditions, G. morrilli successfully developed and emerged as adults when host eggs were parasitized in mid to late winter. Immature parasitoids did not survive when host eggs were parasitized in early winter. Based on results from laboratory experiments, it is expected that immature parasitoids would perform better under field conditions if using fertilized versus non-fertilized host eggs. However, further studies are needed to test this hypothesis. To some extent non-fertilized eggs may be useful in experiments that do not require parasitoids to become fully developed or successfully emerge. For example, Cooksey et al. (2012) developed a polymerase chain reaction assay to identify egg parasitoids located in GWSS eggs. The technique may facilitate monitoring programs by eliminating the process of incubating field collected egg masses for parasitoid emergence and identification in the laboratory. After parasitoid emergence from the host egg, the assay may be used to identify the parasitoid species by analyzing the parasitoid pupal casing left in the host egg.
In conclusion, non-fertilized GWSS eggs may be used to study Gonatocerus spp. in the environment of GWSS. However, sampling and monitoring egg parasitoids may be complicated by factors such as unstable sex ratios, movement, weather factors, and asynchrony with host populations (Elzen and King 1999). Therefore, further research is needed to determine effects of host egg fertilization status on parasitoid sex allocation and progeny fitness in specific locations and conditions. Due to reduced parasitoid emergence from non-fertilized host eggs, fertilized eggs should be used for mass production of parasitoids to increase rates of parasitoid emergence and perhaps fitness of emerged individuals. Understanding the role of the different egg parasitoid species in the current GWSS range and newly infested habitats will lead to better suppression of GWSS populations.
References
Al-Wahaibi AK, Morse JG (2009) Egg morphology and stages of embryonic development of the glassy-winged sharpshooter (Hemiptera: Cicadellidae). Ann Entomol Soc Am 102:241–248
Al-Wahaibi AK, Morse JG (2010) Temporal patterns in Homalodisca spp. (Hemiptera: Cicadellidae) oviposition on southern California citrus and jojoba. Environ Entomol 39:15–30
Blua MJ, Phillips PA, Redak RA (1999) A new sharpshooter threatens both crops and ornamentals. Calif Agric 53:22–25
Boyd EA, Hoddle MS (2007) Host specificity testing of Gonatocerus spp. egg-parasitoids used in a classical biological control program against Homalodisca vitripennis: a retrospective analysis for non-target impacts in southern California. Biol Control 43:56–70
CDFA (2010) Pierce’s Disease Control Program, Report to the Legislature, 2010. California Department of Food and Agriculture, Sacramento, USA
Chen WL, Leopold RA (2007) Progeny quality of Gonatocerus ashmeadi (Hymenoptera: Mymaridae) reared on stored eggs of Homalodisca coagulata (Hemiptera: Cicadellidae). J Econ Entomol 100:685–694
Chen WL, Leopold RA, Morgan DJW, Harris MO (2006) Development and reproduction of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), as a function of temperature. Environ Entomol 35:1178–1187
Cooksey DA, Morgan D, LeVesque C (2012) Development of effective monitoring techniques for sharpshooters and their parasitoids. In: Esser T (ed) Pierce’s disease research progress reports. California Department of Food and Agriculture, Sacramento, USA, pp 15–19 Available at http://www.cdfa.ca.gov/pdcp/Research.html. Cited June 26, 2013
Davis MJ, Purcell AH, Thompson SV (1978) Pierce’s disease of grapevines: isolation of the causal bacterium. Science 199:75–77
Elzen GW, King EG (1999) Periodic release and manipulation of natural enemies. In: Bellows TS, Fisher TW (eds) Handbook of biological control. Academic Press, San Diego, USA, pp 253–270
Gadallah AI, Kilgore WW, Marei N, Painter RR (1970) Protein synthesis by ribosomes from fertilized and unfertilized eggs of houseflies, Musca domestica. Insect Biochem 1:385–390
Godfray HCJ (1994) Behavioral and Evolutionary Ecology. In: Krebs JR, Clutton-Brock T (eds) Parasitoids. Princeton University Press, Princeton, USA
Hayashi F, Kamimura Y (2002) The potential for incorporation of male derived proteins into developing eggs in the leafhopper Bothrogonia ferruginea. J Insect Physiol 48:153–159
Hopkins DL, Purcell AH (2002) Xylella fastidiosa: cause of Pierce’s disease of grapevine and other emergent diseases. Plant Dis 86:1056–1066
Hummel NA, Zalom FG, Peng CYS (2006) Anatomy and histology of reproductive organs of female Homalodisca coagulata (Hemiptera: Cicadellidae: Proconiini), with special emphasis on categorization of vitellogenic oocytes. Ann Entomol Soc Am 99:920–932
Irvin NA, Hoddle MS (2005) The competitive ability of three mymarid egg parasitoids (Gonatocerus spp.) for glassy-winged sharpshooter (Homalodisca coagulata) eggs. Biol Control 34:204–214
Krugner R (2010) Differential reproductive maturity between geographically separated populations of Homalodisca vitripennis (Germar) in California. Crop Prot 29:1521–1528
Krugner R, Groves RL, Johnson MW, Flores AP, Hagler JR, Morse JG (2009a) Seasonal population dynamics of Homalodisca vitripennis (Hemiptera: Cicadellidae) in sweet orange trees maintained under continuous deficit irrigation. J Econ Entomol 102:960–973
Krugner R, Johnson MW, Morgan DJW, Morse JG (2009b) Production of Anagrus epos Girault (Hymenoptera: Mymaridae) on Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) eggs. Biol Control 51:122–129
Lytle J, Morse JG (2012) Distribution of several species of parasitoids of the glassy-winged sharpshooter (Hemiptera: Cicadellidae) in southern California. Pan-Pac Entomol 88:1–7
Lytle J, Morse JG, Triapitsyn SV (2012) Biology and host specificity of Gonatocerus deleoni (Hymenoptera: mymaridae), a potential biocontrol agent of Homalodisca vitripennis (Hemiptera: Cicadellidae) in California, USA. BioControl 57:61–69
Pilkington LJ, Hoddle MS (2006a) Reproductive and developmental biology of Gonatocerus ashmeadi (Hymenoptera: Mymaridae), an egg parasitoid of Homalodisca coagulata (Hemiptera: Cicadellidae). Biol Control 37:266–275
Pilkington LJ, Hoddle MS (2006b) Use of life statistics and degree-day values to predict the invasion success of Gonatocerus ashmeadi (Hymenoptera: Mymaridae), an egg parasitoid of Homalodisca coagulata (Hemiptera: Cicadellidae), in California. Biol Control 37:276–283
Son Y, Nadel H, Baek S, Johnson MW, Morgan DJW (2012) Estimation of developmental parameters for adult emergence of Gonatocerus morgani, a novel egg parasitoid of the glassy-winged sharpshooter, and development of a degree-day model. Biol Control 60:233–240
Sorensen JT, Gill RJ (1996) A range extension of Homalodisca coagulata (Say) (Hemiptera: Clypeorrhyncha: Cicadellidae) to southern California. Pan Pac Entomol 72:160–161
Stenger DC, Sisterson MS, French R (2010) Population genetics of Homalodisca vitripennis reovirus validates timing and limited introduction to California of its invasive insect host, the glassy-winged sharpshooter. Virology 407:53–59
Tubajika KM, Civerolo EL, Ciomperlik MA, Luvisi DA, Hashim JM (2004) Analysis of the spatial patterns of Pierce’s disease incidence in the lower San Joaquin valley in California. Phytopathology 94:1136–1144
Turner WF, Pollard HN (1959) Life histories and behavior of five insect vectors of phony peach disease. U. S. Dep Agric Tech Bull 1188:1–28
Acknowledgments
I thank Theresa de la Torre, Aaron J. Salyers, and Angelina Cazares for technical assistance and Drs. Mark Sisterson and James Hagler for comments on an earlier version of this manuscript. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Torsten Meiners.
Rights and permissions
About this article
Cite this article
Krugner, R. Suitability of non-fertilized eggs of Homalodisca vitripennis for the egg parasitoid Gonatocerus morrilli . BioControl 59, 167–174 (2014). https://doi.org/10.1007/s10526-014-9562-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-014-9562-2