Abstract
The polyphagous larvae of Orgyia trigotephras cause considerable damages to the maquis. The aim of this work is to study the influence of host plants on larval growth, pupal development, female fecundity of O. trigotephras, and the natural enemies community on Quercus coccifera L. and Pistacia lentiscus L. in two Tunisian forests during a post-outbreak phase of the pest from 2013 to 2021. Eggs, larvae, and pupae were collected and reared until the emergence of parasitoids. Results showed that densities of egg batches, larvae, and pupae varied significantly among years, between forests and host plants. Fecundity of females varied between years and host species, but not between forests. Egg mortality was significantly different only between forests. However, there was a significant variation in larval mortality between host plants over years. General pupal mortality was significantly different only among years; 23.4% of pupae were dead, and the remaining ones (76.5%) become adults. The action of natural enemies on eggs and larvae was highly significant over years and differed between forests. Pupal parasitism varied between years and host plants but not between forests. Host plant and parasitoids allowed us to understand the mechanisms involved in the interaction between defoliating insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to trophic resources, natural enemies have an important impact on the population dynamics of phytophagous insects (Villemant 1989; Hammami et al. 2016). Each development stage (eggs, larvae, or pupae) of insects has a specific parasitoid complex (Villemant 2003). Moreover, higher temperatures endanger both, survival of insects and ecosystems (Parmesan et al. 2000), and result in changes primarily in insect populations, either directly by acting on insect behavior or indirectly via effects on hosts, competitors, or natural enemies (Orazio et al. 2014). Together, all these factors may influence the distribution, abundance, growth, development, fecundity and survival of the insect species. Extreme temperatures have been shown to be a major factor influencing forest pest populations in various ways. It is a determining factor in the dynamics of Lepidopteran populations and may have a negative impact on them (Brown et al. 2004; Bourougaaoui et al. 2021). Works of Fraval (1986) on dynamics of the Erebidae Lymantria dispar (Linnaeus, 1758) and of Ezzine et al. (2015a, b) on the host use by Orgyia trigotephras Boisduval, 1828 during outbreaks showed that host plant is among the most important factors of regulation of the pest populations. As a generalist species, O. trigotephras encounters spatial and temporal variation in food quality (Ezzine et al. 2010, 2015a, b; Bella et al. 2011). Larvae feed on oak (El Hassani et al. 1994; Ezzine et al. 2010) and shrub species, mainly Pistacia lentiscus, Erica arborea, and Erica multiflora (Ezzine et al. 2010, 2014; Bella et al. 2011). It caused considerable damage to kermes oak in Tunisia (Ezzine et al. 2010, 2015a, b) and to lentisk in Italy (Bella et al. 2011).
In Tunisia, O. trigotephras develops two generations per year (Chénour 1955; Ezzine et al. 2014). The pupal stage lasts 10–14 days. Upon emerging, flightless females attract males to their cocoons (Ezzine 2013). After mating, the female lays on average 140 (up to 347) eggs in a single egg mass covered with scales from its abdomen and never leaves its pupation cocoon (Ezzine et al. 2010, 2015a, b). The dispersal of the pest occurs by ballooning of the first instar larvae; older larvae are able to walk as single individuals quickly to other plants (Ezzine et al. 2015a, b).
In 2005, an outbreak of O. trigotephras was observed in northeastern Tunisia (Ezzine et al. 2010). Its population density tends to oscillate over time and, outbreaks occur every five years (Ezzine et al. 2015a, b). At the beginning of April 2010, severe defoliation of shrub-forest was observed in northwestern Tunisia. Since 2013, the population density of O. trigotephras decreased in number and went into the collapse phase (Ezzine et al. 2015a, b). Despite O. trigotephras larvae compete with other lepidopterans for the same trophic resources (kermes oak) in order to survive (Ezzine et al. 2015a, b), they are also vulnerable to parasitoids (Hammami et al. 2019). In the case of several phytophagous insects, the level of predation and parasitism can influence the dynamics of forest pests (Bernays and Graham 1988; Moon and Stiling 2006). In this context, the aim of this work is to assess the densities of immature stages (eggs, larvae, and pupae) of O. trigotephras, as well as its mortality and incidence of different parasitoids in two Tunisian forests during a long-term period from 2013 to 2021. Field data and laboratory experiments were combined to study (i) host plant infestation; (ii) factors causing egg, larval and pupal mortality in O. trigotephras populations in Tunisia.
Methods
Study area description
Experiments were performed in northeastern (Cap-Bon; altitude 432 m; 36°52’N, 10°48´E) and northwestern (Sejnane; alt. 48 m; 37°11’N, 9°11´E) Tunisia, from 2013 to 2021. The vegetation consists of Mediterranean maquis with shrubs reaching up to two meters in height. In Cap-Bon, the predominant plant species are Calicotome villosa, Cistus crispus, Cistus monspeliensis, Erica arborea, Erica multiflora, Quercus coccifera, Pistacia lentiscus, and Phillyrea media (Ezzine et al. 2015a, b). Similarly, in Sejnane the predominant plant species are Daphne gnidium, and Halimium halimifolium, Q. coccifera, P. lentiscus ((Ezzine et al. 2015a). In each forest, research was conducted on a plot of approximately 78,400 m2. Crown defoliation of the host plant was assessed visually using six number classes according to Ezzine et al. (2015a, b): C1 (0%); C2 (1 to 20%); C3 (> 20 to 40%); C4 (> 40 to 60%); C5 (> 60 to 80%) and C6 (> 80 to 100%); the mid-point of the respective class was assigned to each plant in that category, respectively, 0, 10, 30, 50, 70 and 90% (Table 1). The most extreme temperatures (T max and T min) in February, March, and April were recorded in both forests using the freely available and accessible website https://tn.freemeteo.com (Table 2).
Sampling and monitoring
For field evaluations, the plant used for oviposition, larval feeding and pupation of O. trigotephras were investigated by calculating the occurrence and counting egg batches, larvae and pupae per plant on Q. coccifera (n = 30 Sejnane, n = 50 Cap-Bon) and P. lentiscus (n = 30 Sejnane, n = 50 Cap-Bon) individuals from 2013 to 2021 (Table 1). Assay conditions in laboratory experiments were identical to natural ones (25 ± 2 °C and a light regime of 12:12 L: D (light: dark) hours). At the end of June of each study year, egg batches (n = 195) were randomly collected, individually placed in a plastic box (8 cm length × 3 cm width) and monitored until the emergence of parasitoids.
From mid-April to early June of each study year, larvae of the 3rd, 4th and 5th instars were collected (n = 989), classified, placed individually in vials and reared on fresh leaves of kermes oak and lentisk until pupation or emergence of parasitoids (Table 1). At the end of May of each study year, twigs of kermes oak and lentisk holding pupae were cut off (n = 175). Leaves were removed and pupae were placed separately in plastic boxes at 25 ± 2 °C until the emergence of pupal parasitoids and/or O. trigotephras adults.
Eggs sorting
To sort and count different types of eggs, we used the method described by Villemant (1993) and Ezzine et al. (2010). To remove the scale layer, the silky cocoon was removed, and the egg batch was brushed through a 1 mm mesh strainer.
Then, eggs were placed in a Petri dish, observed under a binocular microscope (Leica, S42), sorted, and counted according to the categories listed below: (i) viable egg; (ii) hatched egg; (iii) parasitized egg, distinguished by the presence of a parasitoid exit hole; (iv) preyed egg, with distinct chorion fragments; (v) dried or brown egg containing a dry larva; (vi) unfertilized egg, characterized by the flat shape of a deflated balloon. Viable eggs were dissected under a binocular microscope to identify eggs holding non emerged larvae or non-emerged parasitoids. An estimate of fecundity from all collected egg batches was calculated based on the overall number of eggs per batch. Analysis of batches allowed the estimation of the average fertility of the female (the total number of eggs per batch), egg mortality, the rate of parasitism and predation.
Identification of parasitoids
Morphological identification of larval parasitoids of Hymenoptera was done at both genus and species levels using the keys of Mason (1981), Schmiedeknecht (1902), Horstmann (1968), Van Achterberg (1993), Papp (1959, 1960, 1979), Gibson (1989), Graham (1987), Nixon (1970) and Stigenberg and Ronquist (2011). Parasitoids from Diptera order were identified using Herting and Tschorsnig (1994) key.
Data analysis
Generalized linear models (GLMs) were applied to the following dependent variables: (1) number of egg batches; (2) number of eggs per female (i.e., egg load); (3) number of each egg category (hatched, dried, unfertilized, parasitized and predated); (4) number of larvae and pupae. Three explanatory categorical variables were tested: year, host species and forests. The best distribution model was chosen according to the deviance of the degrees of freedom (df) criterion. A Poisson distribution model best fitted (1) the egg batches density; (2) the egg load data; (3) collected larvae and pupa per tree. For the number of eggs in each category the Negative Binomial distribution model best fitted the data.
The proportion of observed dead larvae and pupae was analyzed by GLM using UNIANOVA, considering the factors year*host plant*site. Results are presented in the form of the Wald’s chi-square test value (χ2), parameter estimates and the respective p- value. Correlation between larvae density and defoliation index was tested by the Pearson correlation test.
Results
Egg batches, larval and pupal densities
Statistical analysis showed that the number of egg batches, caterpillars and pupae varied significantly for the three tested variables, respectively, among years (p < 0.001), host plants (p = 0.001, p < 0.001, p = 0.003) and between forests (p = 0.021, p < 0.001, p < 0.001). The interaction term (year*host plant) was significant for egg batches (p = 0.023) and larvae (p < 0.001).
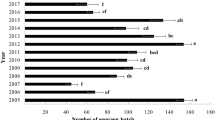
Egg batch density was high in 2013 on both host plants in Cap-Bon, while in Sejnane it was high on kermes oak. Between 2018 and 2021, it diminished progressively until it vanished altogether (Fig. 1). Furthermore, from 2013 to 2015, the larval and pupal densities varied. Orgyia trigotephras larvae attacked the two host plants with low defoliation intensity (C2 and C3) in the two forests. Pupae were observed on both host plants in both forests. In Cap-Bon, larvae and pupae were found in high densities on kermes oak with low defoliation intensity (C2), although Pearson correlation was highly significant (r = 0.928, p < 0.001) between larval density and defoliation index. Nonetheless, no larvae or pupae were found on either host species in the two study forests between 2018 and 2021 (Table 1).
Female fecundity
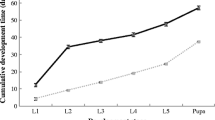
Fecundity or the number of eggs/egg batch produced by females varied considerably across host species (p < 0.001), and years (p < 0.001), but not between forests. Interaction terms were significant (p < 0.001). In 2013 and 2014, fecundity in female was high (> 100 eggs/egg batch) on both host plants. Yet, in 2015, it becomes low (> 50 eggs/egg batch). In 2016 and 2017, egg batches were collected on kermes oak and female fecundity does not exceed 90 eggs/egg batch (Fig. 2).
Egg mortality
Egg mortality included dry, flattened, predated and parasitized eggs. GLMs analysis showed that egg mortality significantly differed between forests (p < 0.001), but not significantly between years and host plants. Thus, the interaction was significant. In Cap-Bon, from 2013 to 2015, egg mortality was quite important on both host plants. However, in Sejnane (2013) it was lower on lentisk than on kermes oak. In 2016 and 2017, egg mortality was detected on kermes oak in both forests (Fig. 3).
The percentage of dried eggs differed significantly between years (p < 0.001), but not between forests or host plants. The interaction term (forest*year) was significant (p < 0.001). Throughout the entire study period, the average of dried eggs was very low (< 0.5%) in both forests and on both host plants (Fig. 4). Nonetheless, the proportion of unfertilized eggs differed significantly between forests (p < 0.001) and years (p = 0.032), but not between host plants. The interaction terms were significant. In 2013, lentisk had a lower percentage of unfertilized eggs (2.49%) than kermes oak in Sejnane. However, this percentage was higher in Cap-Bon on both host species in 2015, and lower in Sejnane. The average number of flattened eggs was quite high in the two forests in 2014, 2016, and 2017 (Fig. 4).
Eggs were parasitized by the Eulophid, Aprostocetus sp. identified by Ezzine et al. (2015a, b) and the scelionid Telenomus sp. identified for the first time in this study. Eggs were predated by the larvae of the cosmopterigid Coccidiphila rungsella Nel and Brusseaux, 1997 formerly identified by Ezzine et al. (2015a, b). The action of these natural enemies was highly significant among years (p < 0.001) and forests (p = 0.05). In 2013, parasitism and predation were lower than in 2014 (Fig. 5). Natural enemies were only observed in Cap-Bon on the two hosts in 2015, and only on kermes oak in 2016 and 2017 (Fig. 5). The GLMs analysis showed that the proportion of parasitized eggs varied significantly among years (p < 0.001) and between forests (p = 0.015). In contrast, predation was not significant for any variables. In 2013 and 2015, parasitism was very low in the two forests and on both host plants and more importantly during 2016 on kermes oak than in 2017 (Fig. 5).
Larval mortality
Larval mortality, caused by parasitoids (emerged adults from larvae during rearing), was observed during molting or during development. GLM analysis showed significant variations in larval mortality between host plants (p = 0.002) and among years (p = 0.001). Furthermore, the interaction term (host plant*year*forest) was highly significant (p < 0.001). In 2013, larval mortality was higher on lentisk (36.8%) than on kermes oak (21.4%) in Cap-Bon, while the opposite was the case for Sejnane (27.2% and 17.5% for kermes oak and lentisk, respectively.
In 2014, larval mortality was high in Sejnane on both host plants (45.8% and 60% for lentisk and kermes oak, respectively) in comparison with Cap-Bon (14% and 32%, for lentisk and kermes oak, respectively). However, in 2015, mortality was higher either on both host plants or in both forests. In 2016, 2017 and 2018, larvae were collected only on kermes oak in Cap-Bon and larval mortality reached 100% in 2018. From 2019 to 2021, no larvae were observed in any forest.
In total, nine larvae parasitoids species belonging to the Hymenoptera and Diptera orders were recorded. Among the Hymenoptera order, four species belonging to the Braconidae family, Aleiodes bicolor Spinola, 1808, Zele sp., Apanteles longicauda (Wesmael, 1837) and Bracon sp., were identified in the present work for the first time in Tunisia. The three other recorded Hymenoptera species, Agrothereutes tunetanus (Habermehl, 1925) (Hym.: Ichneumonidae), Microplitis sofron Nixon, 1970 (Hym.: Braconidae) and Eupelmus sp. (Hym.: Eupelmidae), were identified for the first time by Hammami et al. (2017). Moreover, two Diptera species, Compsilura concinnata (Meigen, 1824) and Exorista segregate (Rondani, 1859) were reported. Diptera species were reported to cause important larval mortality (94.8%) in comparison with Hymenoptera species (5.2%).
GLM analysis showed that parasitism varied significantly among years (p < 0.001) and between forests (p = 0.005). Also, the interaction term (forests*years) was highly significant (p < 0.001). In 2013, parasitism was higher on both host plants in Cap-Bon (92.3% on lentisk and 72.3% on kermes oak). Whereas, in Sejnane, it was higher on kermes oak (41.6%) than on lentisk (14.2%). In 2014, parasitism was greater either on both host plants or in the two forests.
In 2015, parasitoids were observed on both hosts and parasitism was important on lentisk in Cap-Bon (95.2%) and in Sejnane (100%). However, on kermes oak it was higher in Sejnane (78%) than in Cap-Bon (40%). From 2016 to 2018, larvae were collected only in Cap-Bon on kermes oak and were all highly parasitized (80% and 100% of parasitism rate).
Pupal mortality
Pupal mortality was caused by parasitoids (emerged adults from collected pupae) or a poor development of pupae (dried and flattened pupae). About one fourth of pupae (23.4%) were dead, the remaining (76.5%) became adults. GLM analysis showed that general pupal mortality differed significantly among years (F1 = 5.220, p = 0.024) but not between forests and between host plants. The interaction term (forests*years*host plants) was not significant. In 2013, pupal mortality was higher on lentisk (38%) than on kermes oak (15.7%) in Cap-Bon. However, the opposite case was observed in Sejnane (23.5% and 12.5% for kermes oak and lentisk, respectively). In 2014 and 2015, mortality was noticed only in Cap-Bon and was higher on lentisk (62.5% and 40% for 2014 and 2015, respectively) than on kermes oak (16.6% and 8.3% for 2014 and 2015, respectively). In 2016, only three pupae were collected and became adults.
Dried pupae were observed only in 2013 on kermes oak, and their rate was higher in Cap-Bon (14.3%) than in Sejnane (9.7%). Pupae were parasitized by the ichneumonid Pimpla rufipes (Miller, 1759), the torymid Monodontomerus minor (Ratzeburg, 1848) and the chalcidid Brachymeria tibialis (Walker, 1834) which were identified by Hammami et al. (2019). Statistical analysis showed highly significant differences in parasitism among years (p < 0.001) and between host plants (p < 0.001), but never between forests. The interaction term (forests*years*host plants) was significant (p < 0.001). In 2013, parasitism was observed only on lentisk (46% in Cap-Bon and 2% in Sejnane). In 2014 and 2015, parasitism was reported only in Cap-Bon on both host plants. In 2014, it was quite higher on lentisk (12%) in comparison with kermes oak (7%), while in 2015, it was lower on both hosts (4% and 6% for lentisk and kermes oak, respectively). In 2016, no pupae were parasitized neither in both localities nor on both hosts.
Discussion
Northern Tunisia experienced dramatic changes in mean annual temperature throughout the twentieth century, with an average increase of 2.5-3 °C. (Andersen et al. 2019). In fact, measured daily maximum and minimum temperatures from April to June have increased by 2 °C in both studied regions between 2013 and 2021 (Table 2). These fluctuations may impact the population dynamic of the pests and all their development stages. Indeed, the sex ratio (SR) bias at the population level of O. trigotephras tended to be 1:1 from 2013 to 2015, showing that the total reproductive value of the males in the population was approximately equal to the total value of all the females (Fisher 1930). From 2016 to 2018, the population decline seemed to favor males over females, in fact, the bias of the SR presented a preponderance of males in the population (Hammami 2021). In our study, female fecundity of O. trigotephras varied over the years, among host plants but not between forests. It was higher on kermes oak (> 100 eggs/batch) than on lentisk (45 eggs/batch) mainly in 2015. On the other hand, egg mortality was high (> 80%) and the proportion of dried eggs varied greatly over the years on both host plants in both forests. Ezzine et al. (2010) reported a highly significant difference in proportion of parasitized eggs, with 1.2% on kermes oak and 0.2% on lentisk. In our study proportion of parasitized eggs varied significantly among years and between forests, yet egg parasitoids were observed in Cap-Bon on kermes oak.
Moreover, the attack of the two host plant species by O. trigotephras larvae was not intense, and it exceeded 50% only once, in 2013. This may be due to the fact that mature larvae feed on other shrub species (Ezzine 2016). Several years after heavy defoliation, the nutritive quality of the foliage became low thereby larval mortality was high in 2015. Total larval mortality was high and nearly the half of O. trigotephras population (48%) was dead. In contrast, total pupal mortality was low (23%) in the two forests. Dried pupae were observed only in 2013 on kermes oak on both forests which coincide with the retro-gradation phase of O. trigotephras (low density). Larval parasitism rate was higher in 2013 in both forests and on both host plants than in 2014 (collapse phase). This may be due to host plant selection and herbivore performance (Gripenberg et al. 2010; Hammami et al. 2019). Previous studies conducted by Chakir and Fraval (1985), and Villemant (1989) revealed that larval parasitoids have a significant impact in the regulation of L. dispar that was in its ‘retro-gradation phase’ Since no larvae were observed on the two host species in either study forest from 2018 to 2021, it appears that the same behavior was observed for O. trigotephras.
The composition of the parasitoid complex of O. trigotephras varied spatiotemporally and during pro-gradation and retro-gradation phases. In this context, Sisosevic (1979) reported that the Tachinidae family belonging to the Diptera order exert a strong parasitic pressure and dominate during outbreaks of L. dispar. It may be the same behavior of O. trigotephras since parasitism rate by Hymenoptera was low (5.2%) in comparison with parasitism by Diptera (94.8%).
Conclusion
This work present data on metrics of performance (e.g., fecundity, eggs, larval and pupal mortality) of Orgyia trigotephras on two host plants (kermes oak and lentisk). It highlights that insect population dynamics (mainly mortality) is driven more by abiotic (extreme temperature) and other factors than by natural enemies during a collapsing outbreak. Monitoring the parasitism rate over several years provides useful data to contribute to the knowledge of parasitoids.
References
Andersen JC, Havill NP, Mannai Y, Ezzine O, Dhahri S, Ben Jamâa ML, Caccone A, Elkinton JS (2019) Identification of winter moth (Operophtera brumata) refugia in North Africa and the Italian Peninsula during the last glacial maximum. Ecol Evol 24:13931–1394. https://doi.org/10.1002/ece3.5830
Bella S, Longo S, Sidoti A (2011) Indagini su Teia trigotephras defogliatore del lentisco nella sicilia sud-orientale. Dissertation, Atti XXIII Congresso Nazionale Italiano di Entomologia
Bernays EA, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–92. https://doi.org/10.2307/1941237
Bourougaaoui A, Ben Jamâa ML, Robinet C (2021) Has North Africa turned too warm for Mediterranean forest pest because of climate change? Clim Change 165:46. https://doi.org/10.1007/s10584-021-030771
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Towards metabolic theory of ecology. Ecology 85(7):1771–1789. https://doi.org/10.1890/03-9000
Chénour A (1955) Macrolépidoptères de Tunisie (Bombyces). Bull Soc Sci Nat Tunisie 8:257–277
Chakir S, Fraval A (1985) Les ennemis naturels de Lymantria dispar (L.) (Lep. Lymantridae) en forêt de la Mamora (Maroc): étude le long d’un transect (1976–1982). Actes Inst Agro Vét 5(1–2):27–36
de Graham MWR V (1987) A reclassification of the European Tetrastichinae (Hymenoptera: Eulophidae), with a revision of certain genera. Bull Br Mus Nat Hist Entomol 55:1–392
El Hassani A, Graf P, Hamdoui M, Harrachi K, Messaoudi J, Mzibri M, Stiki A (1994) Ravageurs et maladies des forets au Maroc. Guide pratique pour la protection phytosanitaire des forêts. Editions D.P.V.C.T.R.F., Rabat.
Ezzine O (2016) Interactions insectes/plantes-hôtes : cas Orgyia trigotephras Boisduval (1829) (Lepidoptera, Erebidae) en Tunisie. Dissertation. Université El Manar.
Ezzine O, Hausmann A, Branco M, Mannai Y, Dhahri S, Nouira S, Ben Jamâa ML (2014) Genetic patterns, host use and larval morphology in tunisian populations of Orgyia trigotephras. Bull Insectol 67:73–79. https://doi.org/10.1007/s13595-015-0484-7
Ezzine O, Branco M, Villemant C, Schmidt S, Nouira S, Ben Jamâa ML (2015b) Host use in Orgyia trigotephras (Erebidae, Lymantriinae) during outbreak: effects on larval performance and egg mortality. Ann For Sci 72(5):561–568. https://doi.org/10.1007/s13595-015-0484-7
Ezzine O, Ben Jamâa ML, Mnara S, Nouira S (2010) Bio-écologie d’Orgyia trigotephras (Boisduval, 1829) (Lepidoptera, Lymantriidae) à Jebel Abderrahmane, Cap Bon (Nord Est de la Tunisie). IOBC-WPRS Bull 57:123–127
Ezzine O, Hammami S, Hausmann A, Nouira S, Ben Jamâa ML (2015a) First report of Anacampsis scintillela on Halimium halimifolium in Sejnane (Bizerte, Tunisia). TJPP 10:63–68
Ezzine O (2013) Contribution à l’étude bioécologique d’Orgyia trigotephras Boisduval, 1829 au Jebel Abderrahmane (Cap-Bon, Tunisie). Editions universitaires européennes, Tunisia
Fisher RA (1930) The Genetical Theory of Natural selection. Clarendon Press, Oxford. https://doi.org/10.5962/bhl.title.27468
Fraval A (1986) La régulation des populations de Lymantria dispar (Lep., Lymantriidae) en Subéraie marocaine atlantique: effets du climat, des entomophages et des facteurs anthropiques. J Appl Entomol 102:38–52. https://doi.org/10.1111/j.1439-0418.1986.tb00891.x
Gibson GAP (1989) Phylogeny and classification of Eupelmidae, with revision of the world genera of Calosotinae and Metapelmatinae (Hymenoptera: Chalcidoidea). Mem Entomol Soc Can 148:3–121. https://doi.org/10.4039/entm121149fv
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference performance relationships in phytophagous insects. Ecol Lett 13: 383–393. https://doi.org10.1111/j.1461-0248.2009.01433. x
Habermehl H (1925) Beiträge zur Kenntnis der Cryptinengattungen Spilocryptus und Hoplocryptus C.G. Thoms. (Hym. Ichneum.). Neue beitr syst insektenkunde 3(11/12) :101–111.
Hammami S (2021) Study of the population dynamics of defoliating Lepidoptera on two species of the maquis (Pistacia lentiscus and Quercus coccifera) in northern of Tunisia. Dissertation, Faculty of Sciences of Bizerte
Hammami S, Ezzine O, Dhahri S, Villemant C, Schmidt S, Ben Jamâa ML (2019) Pupae mortality of Orgyia trigotephras Boisduval 1829 (Erebidae, Lymantriinae) in Tunisia. Redia 102:107–111. https://doi.org/10.19263/REDIA-102.19.16
Hammami S, Ezzine O, Dhahri S, Ben Jamâa ML (2016) Natural enemies of Orgyia trigotephras (Boisduval 1829) (Lepidoptera, Erebidae, Lymantriinae) in Tunisia. Turk J For 17:58–61. https://doi.org/10.18182/tjf.86221
Horstmann K (1968) Revision einiger Arten der Gattungen Mesostenus Gravenhorst, Agrothereutes Foerster und Ischnus Gravenhorst (Hymenoptera, Ichneumonidae). Entomophaga 13(2):121–133. https://doi.org/10.1007/BF02371782
Linnaeus CV (1758) Systema Naturae per Regna Tria Naturae : Secundum Classes, Ordines, Genrea, Species, Cum Characteribus, Differentiis, Synonymis, Locis. Decima reformata. Laurnetii Salvii, Holmiae Vienna. https://doi.org/10.5962/bhl.title.559.
Mason WRM (1981) The polyphyletic nature of Apantales Förster (Hymenoptera, Braconidae) a phylogeny and reclassification of Microgastrinae. Mem Entomol Soc Can 115:1–147. https://doi.org/10.4039/entm113115fv
Meigen, JW (1824) Systematische Beschreibung der bekannten europäischen zweiflügeligen Insekten. Hamm. German. https://doi.org/10.5962/bhl.title.12464.
Miller J (1759) Engravings of insects with descriptions [*Miller, J. : Engravings of insects]. London.
Moon DC, Stiling P (2006) Trade-off in oviposition strategy: choosing poor quality host plants reduce mortality from natural enemies for a salt marsh planthopper. Ecol Entomol 31:236–241. https://doi.org/10.1111/j.1365-2311.2006.00785.x
Nel J, Brusseaux G (1997) Coccidiphila rungsella n. sp. (Momphidae). Notes sur la répartition de quelques Gelechioidea (Lepidoptera). Alexanor 19(8):455–459
Nixon GEJ (1970) A revision of the northwestern European species of Microplitis Förster (Hymenoptera: Braconidae). Bull Brit Mus (Nat Hist) Entomol 25:1–30
Orazio C, Stojnic S, Stojanović D, Gartzia N, Hayes S (2014) Influence du changement climatique sur les forêts européennes et sur le secteur forestier. www.rokfor.eu. Accessed 7 January 2019
Papp J (1959) The Microgaster Latr., Microplitis Förster and Hygroplitis Thoms. species of the Carpathian Basin (Hymenoptera, Braconidae). Annls Hist Nat Mus Natn Hung 51:388–413
Papp J (1960) Zur Kenntnis der Microgaster Latr. und Microplitis Först. Arten Österreichs (Hym Braconidae) Zeitschr Arbeitsgemein Österr Entomol 12:117–128
Papp J (1979) Braconidae (Hymenoptera) from Tunisia. Folia Entomol Hung 32(2):175–187
Parmesan C, Root TL, Willig MR (2000) Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc 81:443–450. https://doi.org/10.1175/1520-0477(2000)081<0443:IOEWAC>2.3.CO;2
Ratzeburg JTC (1848) Die Ichneumonen der Forstinsekten in entomologischer und forstlicher Beziehung. Berlin.
Rondani C (1859) Dipterologiae Italicae Prodromus. Parma. Italy.
Schmiedeknecht O (1902) Opuscula Ichneumologica. Blankenburg i Thür, Germany. https://doi.org/10.5962/bhl.title.10486
Spinola M (1808) Insectorum Liguriae species novae aut rariores, quas in agro Ligustico nuper detexit, descripsit, et iconibus illustravit (Hymenoptera). Genua. Italy.
Stigenberg J, Ronquist F (2011) Revision of the western Palearctic Meteorini (Hymenoptera, Braconidae), with a molecular characterization of hidden fennoscandian species diversity. Zootaxa 3084:1–95. https://doi.org/10.11646/zootaxa.3084.1.1
Tschorsnig HP, Herting B (1994) The tachinids (Diptera: Tachinidae) of Central Europe: identification keys for the species and data on distribution and ecology. Stuttg Beitr Naturkd 506:47–90
Van Achterberg C (1993) Illustrated Key to the subfamilies of the Braconidae (Hymenoptera: Ichneumonoidae). Zool Verh Leiden 283:1–189
Villemant C (1989) Ennemis des chenilles et des chrysalides. In: Fraval A (ed) Lymantria dispar, Coll Doc. Sci Techn, Actes Editions, Rabat, pp 125–143
Villemant C (2003) Le Bombyx disparate en corse. Insects 130(3):5–10
Walker F (1834) Monographia Chalciditum. Entomological Magazine 2:13–39. https://www.biodiversitylibrary.org/item/35922
Wesmael C (1837) Monographie des Braconides de Belgique. Nouv Mem Acad R Sci Bruxelles 10 :5–68
Acknowledgements
Thanks to Claire Villemant (MNHN in Paris, France) for her help for the identification of the parasitoids. We wish to thank Emna Darghouthi (mannouta@msn.com) for her assistance with the language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hammami, S., Ezzine, O., Bystrowski, C. et al. Understanding mortality factors of Orgyia trigotephras (Lepidoptera: Erebidae) during its collapse phase: host plant and parasitic complex. Biologia 78, 3143–3152 (2023). https://doi.org/10.1007/s11756-023-01434-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01434-2