Abstract
Salinity is a major abiotic stress, limiting plant growth and agriculture productivity worldwide. Salicylic acid is known to alleviate the negative effects of salinity. The present study demonstrated the impact of SA on sorghum, a moderately salt-tolerant crop, grown for food, fodder, fiber, and fuel. A screen house experiment was conducted using sorghum genotypes Haryana Jowar HJ 513 and HJ 541 under 4 salt levels (0, 5.0, 7.5, and 10.0 dS m−1 NaCl) and 3 SA (0, 25, and 50 mg dm−3) levels with 12 combinations. The leaves were assayed for electrolyte leakage percentage (ELP), i.e., 88.7 % in HJ 541 and 87.2 % in HJ 513, and osmolyte content. Proline content, total soluble carbohydrate content, and glycine betaine content increased considerably. Photosynthetic rate, transpiration rate, and stomatal conductance declined at higher salt levels. The specific enzymatic activities of SOD, CAT, and POX increased 41.1 %, 122.0 %, and 72.8 %, respectively, in HJ 513 under salt stress. Combinations of salt treatment and SA decreased ELP and enhanced osmolyte concentration, rates of gaseous exchange attributes, and also the antioxidant enzymatic activity in salt-stressed leaves. The study established that the specific activity of antioxidative enzymes is enhanced further by addition of SA which may protect the cells from oxidative damage under salt stress, thus mitigating salt stress and enhancing the yield of sorghum. SA can ameliorate the salt stress in plants by affecting the metabolic or physiological frameworks. SA application is an effective management strategy towards mitigating salt stress in order to meet agricultural production and sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The major producers of sorghum are India, America, and Nigeria. India contributes 9.45 % of the world’s sorghum production with 5.82 million hectare area (M ha) and 5.39 million tonnes of total production [1]. The major constraints reducing sorghum productivity are abiotic and biotic stresses. Salt stress is one of the major agricultural abiotic stresses and a worldwide eco-environmental problem. Salinity is a major threat to the agriculture industry worldwide and is associated with morphological, physiological, and biochemical responses [2].
According to Liang et al. [3], about 1/3 of the world irrigated land is affected by salinity. Area under salinity is increasing due to inappropriate management practices; therefore, crop varieties tolerant to salinity are required to meet fodder and bioenergy requirements. In India, salinity causes adverse alteration of 6.73 M ha of the irrigated land area [4]. In Haryana alone, 0.50 M ha area is under salinity [5]. The saline area in Haryana has grown by 35% to 80,000 ha in 20 years, as per a 2016 study by the ICAR-Central Soil Salinity Research Institute (CSSRI), Karnal. The electrical conductance of soil ranges from 1.23 to 138.9 dS m−1 with a mean of 28.34 dS m−1 indicating that soil is moderate to high saline [6]. Short-term effects of salinity include reduction of growth by salt due to osmotic effects. Misra et al. [7] observed that excessive uptake of salt stress causes plants to ionic stress leading to reduction of plant growth and their productivity due to the premature ageing due to reduction in photosynthetic enzymatic activity of Calvin cycle. Ion toxicity is the major factor for the plant growth and productivity with excessive application of mainly Na+ as well as due to imbalanced mineral nutrients. Under salt stress, reactive oxygen species (ROS) concentration increases beyond the optimum limit due to which some osmoprotectants are synthesized which help in osmotic adjustments for which proper enzymatic activity is required under maintained levels of K+ and Mg2+. The contents of osmolytes such as glycine betaine, proline, sugars, and polyols tend to increase in salt-tolerant species [8]. Due to salt stress, many physiological changes were observed in plants like reduction in photosynthetic activity, enzyme activity, increase of oxidative stress, dysfunction of membrane as well as other morphological and anatomical alterations [9]. Adverse environmental conditions cannot be tolerated by plants since they are not able to move from one place to another, so for survival, plants do some metabolic changes under stress conditions like synthesis of some organic metabolites or some growth regulators. According to Iqbal and Ashraf [10], stress tolerance is achieved by application of these growth regulators in plants. Amelioration of salt stress can be achieved by overcoming the irregularities in plant physiological mechanisms by some of the plant growth regulators and nutrients.
Salicylic acid is a phenolic endogenous plant growth hormone, which regulates the plant growth and acts as a barrier against various environmental stresses like salt stress [11]. Photosynthesis, chlorophyll content, stomatal regulation, and seed yield are known to be influenced by application of salicylic acid [12, 13]. High rate of nutrient uptake, water potential value, photosynthetic rate, and enhancement in growth results due to the application of salicylic acid under abiotic stresses. SA is endogenous hormone which improves the rate of germination under salt stress or non-stress conditions and foliar application of SA enhances the plant growth which ultimately leads to high yield of crop [14]. SA protects the plants from stress-caused oxidative damage, growth reduction, and loss of photosynthesis efficiency [15]. Salicylic acid–mediated improvement of growth traits and elevated levels of antioxidants and osmoprotectants to overcome the adverse effects of salt stress have been reported by Yadu et al. [16].
Sorghum, being a multi-purpose crop grown for food, fodder, fiber, and fuel, has been used in the present study. Sorghum is a moderately salt-tolerant C4 crop and its high production under salinity is found in rainfed arid and semi-arid regions. Its salinity tolerance threshold is 6.86 dS m−1 with a slope of 16% per dS m−1 of irrigation water [17]. Mechanism of osmoregulation under saline conditions has an effective role in reducing the negative effects of salinity on sorghum. SA also plays a positive role in alleviation of salinity stress in sorghum [18, 19]. It has been observed that there is an increase in the Na+ concentration and a decrease in the K+ concentration in sorghum during salt stress which lead to decreased levels of photosynthesis and nutrient transport [20, 21], hindering the growth of plants [22]. Application of SA results in selective absorption of Na and Cl under saline conditions. SA maintains cell redox balance, thus preventing ROS-induced oxidative damage to plants [23]. However, not much information is available on the effects of spraying SA on sorghum plants under saline and non-saline conditions.
The present research was conducted to understand the correlation between salicylic acid and salt stress in sorghum and to elucidate the physiological responses like electrolyte leakage percent, osmolytes values, attributes of gaseous exchange, and specific activity of antioxidative enzymes under salt stress following foliar application of different doses of salicylic acid in sorghum.
Material and Methods
Plant Material, Treatment, and Plant Growth Conditions
Seeds of two salt-tolerant sorghum genotypes (Haryana Jowar, HJ 513 and HJ 541) were collected from Forage Section, Department of Genetics and Plant Breeding, Chaudhary Charan Singh Haryana Agricultural University, Hisar (Haryana). Dune sand was taken from Gangwa village. The two genotypes were selected after preliminary screening to salt stress. Haryana Jowar HJ-513 and HJ-541 were developed by CCS HAU, Hisar, and were released for north-west zone of the country in 2004 and 2018, respectively. They are the major genotypes in farmers’ fields. They are recommended for cultivation in Haryana under timely sown/normal fertility/irrigated conditions and are tolerant to major foliar diseases and stem borer [24, 25]. HJ 541 is common forage sorghum and shows higher tolerance to salinity as compared to HJ 513 [5]. These genotypes have similar phenology and are in cultivation in Haryana with an approximate production of 400–500 q ha-1.
Seeds were grown in pots under screen house conditions in July 2017. Each plastic pot was filled with 10 kg sand. Then the seeds of sorghum were sterilized in 1 % sodium hypochlorite (NaOCl) solution for 5 min to avoid contamination before using them for experimentation. Ten seeds of each sorghum genotype were sown in each pot of 25 cm diameter with equal depth and distance containing 10 kg well-washed sand. After germination, seedlings were thinned to three of equal size. Hoagland nutrient solution was used to each pot after every week and moisture content of the sand was maintained daily by adding 200 cm3 distilled water to each pot [26]. Before sowing, pots were saturated with desired levels of salt, i.e., control (0), 5.0, 7.5, and 10.0 dS m−1. Saline soil was artificially prepared by using sodium chloride (NaCl) salt and by putting calculated values of soil EC (electrical conductivity) plus required EC in a quadratic equation [27]. There are significant reductions in biometric parameters of plants with salinity levels higher than 10 dS m−1 [28, 29]. Soil was checked for EC at regular intervals of time and accordingly irrigated with saline water to maintain salinity. The control pots were irrigated with canal water. Salicylic acid (15 cm3 of the concentrations 0, 25, and 50 mg dm−3) was applied exogenously with the help of manual sprayer after 30 DAS. Arrangement of the experiment was completely randomized design with three replicates. The sampling was done at vegetative stage at 40 DAS.
Growth/Morphological Attributes
Plant Height (cm)
Plant height was measured with the help of meter scale from surface of soil to the tip of the apical shoot at 40 DAS. Average plant height was calculated.
Fresh Mass—Root, Stem, and Leaves (g plant−1)
Fresh mass of root, stem, and leaves were measured immediately after taken from pots on weighing machine.
Dry Mass—Root, Stem, and Leaves (g plant−1)
Dry mass of root, stem, and leaves were measured after taken from pots on weighing machine and after that samples were dried in an oven at 80°C for 48 h.
Number of Leaves per Plant
The number of leaves per plant was counted and the data were expressed on average number of leaves per plant basis.
Leaf Area per Plant (cm2 plant−1)
The leaf area of the detached leaves of each sample was measured with the help of leaf area meter (Model LI 3000, LI COR Ltd., Nebraska, USA) and expressed as cm2 plant−1.
Physiological and Biochemical Attributes
Membrane Permeability (Electrolyte Leakage)
Electrolyte leakage was assessed as described by Sullivan and Ross [30], to understand the membrane stability and thereby on the relative ion content in the apoplastic space. Leaf samples were collected from control as well as treated plants. One hundred milligrams of leaf tissue was taken separately in 20 cm3 test tube containing 10 cm3 of de-ionized water. These samples were incubated for 3–4 h at room temperature. The conductance of decanted liquid containing effluxed electrolytes was determined with a conductivity meter and designated as ECa (before boiling). Then the samples were subjected to heating at 100 °C in a water bath for 30 min. After cooling, the electrical conductivity of the solutions was measured. The electrolyte leakage was expressed by the following formula:
Determination of Compatible Osmolyte Content
Proline content of cell free extract was estimated by the method of Bates et al. [31]. Three hundred milligrams of leaf tissue was homogenized in 3 cm3 of 3 % aqueous sulpho-salicylic acid and centrifuged at 12,000 g for 10 min, then 2 cm3 of the supernatant was taken, and then 2 cm3 acid-ninhydrin and 2 cm3 of glacial acetic acid were added in a test tube. Test tubes were kept in water bath for 1 h at 100 °C and the reaction is terminated in an ice bath. The reaction mixture was extracted with 4 cm3 of toluene, mixed vigorously, and left at room temperature for 30 min until separation of the two phases. The chromophore-containing toluene (upper phase) was warmed to room temperature and its optical density was measured at 520 nm using toluene as a blank. The proline concentration was determined from a standard curve using L-proline.
Total soluble carbohydrates were determined with the method of Yemm and Willis [32], using anthrone reagent. An aliquot from the 80 % ethanolic extract, measuring 0.2 cm3, was evaporated to dryness in a test tube in a boiling water bath. On cooling, the residue left in the tube was dissolved in 1 cm3 of distilled water and mixed with 4.0 cm3 anthrone reagent. The mixture was heated in a water bath for 10 min. After cooling, absorbance was recorded at 620 nm using visible spectrophotometer (UV-1900 UV-VIS, Shimadzu). Standard curve was prepared using graded concentration (20–100 μg cm−3) of D-Glucose and the data was expressed as mg g−1 (d. m.).
Glycine betaine was estimated according to the method of Grieve and Grattan [33]. Leaf extract was prepared in 20 cm3 of test tubes by chopping 0.5 g leaves in 5 cm3 of 0.05 % toluene. All the tubes were kept for 24 h at 25 °C. After filtration, 0.5 cm3 of extract was mixed with 1 cm3 of 2 N HCl solution and 0.1 cm3 of potassium tri-iodide solution (containing 7.5 g iodine and 10 g potassium iodide in 100 cm3 of 1 N HCl) was added and shaken in ice cold water bath for 90 min and then 2 cm3 of ice cold water was added after gentle shaking and then 10 cm3 of 1,2-dichloroethane (chilled at −10°C) was poured in it. By passing a continuous stream of air for 1–2 min, two layers were separated, upper aqueous layer was discarded, and optical density of organic layer was recorded at 365 nm. Standard curve was prepared using graded concentration of glycine betaine and the data were expressed as μmol g−1 (d. m.) of the tissue.
Gaseous Exchange Attributes
Photosynthetic rate, transpiration rate, and stomatal conductance of the third fully expanded leaf were measured by infrared gas analyzer (IRGA LCi-SD, ADC Biosciences) at maximum PAR. The system automatically calculated the A, E, and gs on the basis of pre-loaded flow rate and leaf area. Measurements were taken when relative humidity, temperature, photosynthetic photon flux density, and amp; CO2 concentration ranged from 50 to 60 %, 25–35 °C, 1200 μmol (photon) m−1 s−1, and amp; 350–360 μmol dm−3, respectively.
Specific Activity of Antioxidant Enzymes
Superoxide Dismutase (SOD)
Specific activity of SOD was estimated by the method of Giannopolitis and Ries [34] with little modifications. One cubic centimeter of enzyme extract was mixed with 0.5 cm3 of each of methionine, NBT, EDTA, and Na2CO3 and the total volume of 4 cm3 was made with buffer in each set adjusting the pH at 10.2 followed by addition of 0.5 cm3 of riboflavin. The tubes were shaken and placed 30 cm from light source (8 * 20 W fluorescent lamps). The reaction was stopped after 10 min by switching off the light. The tubes were immediately covered with a black cloth. The absorbance was recorded at 560 nm and expressed as unit mg−1 (protein) min−1. One unit of enzyme activity was defined as the amount of enzyme bringing about 50 % inhibition in the reduction rate of NBT detected at 560 nm
Catalase (CAT)
Specific activity of CAT was estimated by the method of Aebi [35]. Enzyme extract was made with 0.1M phosphate buffer (pH 7.0); 500 mm3 of extract was taken and to this added 0.2 cm3 of 0.1 M H2O2 and 1.5 cm3 of 50 mM potassium phosphate buffer. The enzyme sample was added immediately at the time of taking the absorbance and incubated for 3 min. The change in absorbance was recorded at 240 nm at an interval of 15 s for 1.5 min. The specific activity of enzyme was calculated as decrease in absorbance per minute and expressed as unit mg−1 (protein) min−1.
Peroxidase (POX)
The procedure of Siegel and Siegel [36] was followed for estimating POX activity. Three cubic centimeters of reaction mixture contained 0.1 M phosphate buffer (pH 7.0), 0.1 mM guaiacol, and 100 mm3 cell free extract. Reaction was started with the addition of 0.1 mM H2O2 and an increase in absorbance at 470 nm was recorded for 2 min. The activity was calculated using the extinction coefficient value of 26.6 mM cm−1 for guaiacol. One unit of enzyme activity was equivalent to μmol of H2O2 consumed per minute during the reaction.
Statistical Analysis
The data were analyzed statistically for ANOVA using complete randomized design (CRD) by using SPSS 13.0 (Statistical Package for the Social Sciences) (SPSS Inc., Chicago, IL, USA) and OPSTAT statistical package [37] using Tukey’s HSD test at 0.05 significance level and was expressed as mean + standard errors. Treatments were compared with CD values at 5 % level of significance. Pearson’s correlation analysis among the traits was also performed, in order to define possible relationships among variables. A heat map, generated by using the http://biit.cs.ut.ee/clustvis program package with Euclidean distance as the similarity measure and hierarchical clustering with complete linkage heat map, summarized all the plant responses (morphological, biochemical, and physiological) to various treatments.
Results and Discussion
Growth/Morphological Attributes
Plant Height (cm)
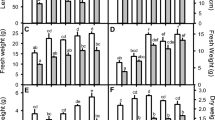
At 40 DAS, plant height decreased significantly with the increasing levels (control to 10 dS m−1) of salt stress in both the genotypes (HJ 513 and HJ 541) (Table 1). Percent decline was higher in HJ 541 (75.3 %) as compared to HJ 513 (74 %) at 10 dS m−1 of salt stress over their respective control. Significant enhancement in both genotypes was seen after foliar spray of salicylic acid (SA), i.e., 25 and 50 mg dm−3, but this increase was on higher side at 50 mg dm−3. Maximum increment was noticed at 5 dS m−1 of salt stress, i.e., 46.9 % and 40 %, respectively, in HJ 513 and HJ 541, after application of 50 mg dm−3 of salicylic acid.
Leaf Number
At 40 DAS, significant reduction in leaf number was observed with the increasing levels of salt stress (control to 10 dS m−1) in both genotypes (Table 1). Percent reduction in leaf number at 10 dS m−1 of salt stress was 50 % in HJ 513 and 57.1 % in HJ 541 over their respective control. After foliar spray of SA in both genotypes under stressed and un-stressed conditions, a slight increase in leaf number was observed. This increase was found 33.4 % in HJ 513 and 33.3 % in HJ 541 at 10 dS m−1 over their respective control.
Fresh Mass of Plant (g)
With the increasing levels of salt stress from control to 10 dS m−1, a significant reduction in fresh weight of plant in both the genotypes, i.e., HJ 513 and HJ 541, was observed (Table 1). Maximum fresh mass was decreased in both genotypes HJ 513 (81 %) and HJ 541 (83 %) at 10 dS m−1 of salt level over their respective control. Application of salicylic acid (25 and 50 mg dm−3) increased the fresh mass of plant, but more increment was observed at 50 mg dm−3 of SA in both genotypes. Maximum enhancement was noticed at 7.5 dS m−1 of salt level, i.e., 45.8 % and 35.2 %, in HJ 513 and HJ 541 over their respective control, at 40 DAS.
Dry Mass—Root, Stem, and Leaves (g plant−1)
At 40 DAS, dry mass of plant (Table 1) decreased with the increasing levels of salt stress from control to 10 dS m−1 in both the genotypes. Percent decline was found maximum at 10 dS m−1 of salt stress, i.e., 87.7 % and 89.5 %, in HJ 513 and HJ 541, respectively, with respect to control. Increment in dry mass was noticed after foliar application of salicylic acid, but maximum enhancement was observed at 50 mg dm−3, over 25 mg dm−3. After application of 50 mg dm−3 of SA, percent increase was on higher side at 7.5 dS m−1 salt level in both genotypes, i.e., HJ 513 (78.7 %) and HJ 541 (63.7 %), with respect to their control.
Leaf Area per Plant (cm2 plant−1)
Table 1 indicates that leaf area per plant was decreased with the increasing levels of salt stress from control to 10 dS m−1. Maximum decline in leaf area was observed at 10 dS m−1 of salt level, but on comparison of both genotypes, HJ 541 (92.7 %) had a maximum decrease in leaf area per plant as compared to HJ 513 (92.2 %) with respect to control. Leaf area per plant was increased after the foliar spray of salicylic acid at 25 and 50 mg dm−3, but maximum increment was observed at 50 mg dm−3.
The results indicated that salinity had negative effects on growth attributes of sorghum. Reduction in fresh weight, dry weight, height, leaf area, and number of leaves of sorghum plants due to salinity has been observed by Sun et al. [38] and Dehnavi et al. [18]. This may be related to a decrease in plant water absorption owing to osmotic stress, Na- and Cl-induced ionic toxicity, and reduction of essential elements [39]. Foliar application of SA increased these attributes under both normal and saline conditions. SA improved the plant growth and tolerance to salinity stress possibly by increasing photosynthetic assimilates, reducing the Na and Cl contents, stimulation of antioxidant system thus protecting cell membranes against oxidative stress, and modification of the absorption of essential nutrients into the plants thereby reducing the harmful effects of salinity as corroborated by Dehnavi et al. [18]. The extent of mitigation of the adverse effects of salinity was proportional to the concentration of SA applied.
Biochemical and Physiological Attributes
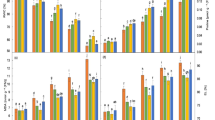
Mitigation of negative effects of salinity through salicylic acid–mediated modulation of different osmoprotectants, antioxidants, and nutrient uptake has been discussed by Sharma et al. [40] and Ahmad et al. [41]. Mitigation of salt stress by SA application has been depicted in Fig. 1.
Membrane Permeability (Electrolyte Leakage)
Maintenance of membrane integrity in leaf is a major component of salt tolerance in plants. Electrolyte leakage can be correlated with physiological parameters acclimatizing the plant responses to stress conditions [42]. Ion specific salt injury causes leakage of electrolytes from plasma membranes. In the present study, a significant increase in electrolyte leakage was observed with increased salt stress, a condition which is strongly associated to a loss of the integrity of the cell membranes. Electrolyte leakage in sorghum genotypes was higher at salt stress treatment as compared to un-stressed condition. Several authors like Kukreja et al. [43] and Rani [44] reported that electrolyte leakage increased consistently with increasing levels of salt stress due to accumulation of H2O2 content. McNeil et al. [45] reported that an increase in electrolytes under salt stress was due to the displacement of Ca2+ membrane protein and change in composition of membrane lipids, which alter membrane permeability. However, exogenous SA applications reduce the leakage of electrolytes as compared to the control under salt stress. Out of two genotypes, HJ 513 showed greater reduction in percent of electrolyte leakage as compared to HJ 541 on the foliar application of 50 mg dm−3 SA under salt stress. Reduction of electrolyte leakage was by 24.30 % in HJ 513 and 12.94 % in HJ 541, respectively, as compared to the control (Table 1). Foliar application of SA decreased the electrolyte leakage under salt stress as well as in control condition. Reduction in electrolyte leakage with application of SA is due to enhanced activity of antioxidant enzymes and increased Ca uptake, leading to higher membrane integrity as mentioned by Sultan et al. [46]. SA plays a role in maintenance of membrane integrity by electrostatic binding with negatively charged phospholipid head, thus causing a decline in electrolyte leakage [47].
Osmolyte Content
Plants accumulate organic osmolytes in response to the salt stress to mediate osmotic adjustment and protect subcellular structures as osmoprotectors. Sugars stabilize membranes and proteins; glycine betaine aids in osmotic adjustment, photosynthetic apparatus protection, and reduction of ROS; and proline protects the protein turnover machinery against stress injury and up-regulates the stress-responsive genes, thus improving the stress tolerance mechanisms of plants.
Sorghum witnessed a significant increase in the proline, glycine betaine, and total soluble carbohydrates content under salt stress as compared to the non-saline conditions. Foliar application of salicylic acid also increased the osmolyte content under salt stress.
An increase in proline content was estimated in stressed as well as in un-stressed conditions after application of SA. Maximum increment was noticed at 50 mg dm−3 of salicylic acid in both genotypes, i.e., value increase from 75.85 to 87.36 in HJ 513 and 57.29 to 64.23 in HJ 541 at 10 dS m−1 of salt level (Table 2). In many plant species, proline accumulation has been correlated with stress tolerance, and a higher concentration of proline has been reported in salt-tolerant than in salt-sensitive plants [48]. The accumulation of proline for osmotic adjustment is an adaptive mechanism under salt stress [49]. Proline is significantly involved in defense reactions of plants under salt stress, and pretreatment with salicylic acid contributes to proline accumulation under stress through maintaining an enhanced level of ABA in seedlings [50].
Glycine betaine values also increased after the foliar application of salicylic acid under stressed as well as in un-stressed plants. However, 50 mg dm−3 of SA brought more increment in glycine betaine values, i.e., 238.36 to 291.61 in HJ 513 and 103.43 to 121.55 in HJ 541 at 10 dS m−1 of salt level (Table 2). The glycine betaine content was found to increase under stress conditions in barley [51] and Radix astragali [52]. Glycine betaine is known to protect the membrane and enzymes and stabilize photosystem II protein pigment complexes under stress [53]. Accumulated glycine betaine may be responsible for intercellular osmotic balance and can be closely correlated with the elevation of osmotic pressure [54].
Application of 50 mg dm−3 SA also caused an increase in total soluble carbohydrates in both genotypes under stressed as well as in un-stressed conditions, but maximum increment was noticed in HJ 513 as compared to HJ 541 at 5 dS m−1 of salt level (Table 2). The increased level of total soluble carbohydrates is due to the breakdown of starch as mentioned by Singh and Pareek [55], Kukreja et al. [43], and Nandwal et al. [56]. An increase in the total soluble sugars has been observed after exogenous application of SA in plants under stress [57, 58]. SA supplementation may activate the metabolic pathways which consume soluble sugars, thus replenishing the growth of stressed plants. SA may also inhibit the polysaccharide hydrolyzing enzymes as corroborated by Sharma et al. [40].
Gaseous Exchange
Salt stress is known to reduce different gas exchange characteristics, like photosynthetic rate, transpiration rate, and stomatal conductance [42]. In general, a high net photosynthetic rate under salt stress would lead to increased plant growth, implying that the photosynthetic rate will be directly proportional to salt stress tolerance. In the present study, there was a general reduction of photosynthetic rate, transpiration rate, and stomatal conductance in response to increasing salt stress, together with strong correlations among these traits (Fig. 3a, b), suggesting the occurrence of stomatal limitations to photosynthetic processes as corroborated by Mauro et al. [59]. The reduction in transpiration rate, stomatal conductance, and net photosynthetic rate was more pronounced in both the genotypes with an increase in salt levels from control to 10 dS m−1. Percent decline in transpiration rate was observed maximum at 10 dS m−1 of salt level, in HJ 513 (49.2 %) and in HJ 541 (63.4 %), with respect to control. Percent decline in stomatal conductance was observed at 5, 7.5, and 10 dS m−1 of salt level 35 %, 57 %, and 75 %, respectively, in HJ 513 over their respective control. Percent decrease in photosynthetic rate was 58.7 % in HJ 513 and 67.4 % in HJ 541, respectively, at 10 dS m−1 of salt level. A decrease in net photosynthetic rate was due to decrease in leaf water potential, stomatal conductance, and relative water content in the plants and can be explained in the light of contention of several workers like Parimelazhagan and Francis [60] in Vigna radiata, Ali et al. [61] in Sorghum bicolor, and Mohamed et al. [62] in Brassica. Salt stress significantly decreased the net photosynthetic rate, PSII photochemical efficiency, transpiration rate, stomatal conductance, and intercellular CO2 concentration in Sorghum [19, 20]. Foliar application of 50 mg dm−3 SA also enhanced the net rate of photosynthesis in both genotypes. At highest salt level (10 dS m−1), application of SA (50 mg dm−3) showed an increase in transpiration rate, i.e., 41.6 % in HJ 513 and 38.5 % in HJ 541. Foliar application of SA (25 and 50 mg dm−3) leads to an enhancement of stomatal conductance in both genotypes and a maximum increase was observed at 50 mg dm−3. Percent increase was more at 10 dS m−1 of salt level in HJ 513 (72.5 %) and at 5 dS m−1 in HJ 541 (61.2 %) with respect to their control (Table 2). The interesting point emanating from the present studies is that SA caused an overall enhancement in net photosynthetic rate, transpiration rate, and stomatal conductance by improving the plant water status. Chandra and Bhatt [63], Szalai et al. [64], and Majeed et al. [65] have also indicated the increase in chlorophyll content and photochemical quantum yield was responsible for an increased rate of photosynthesis. Hasegawa et al. [66] stated that the higher stomatal conductance value after application of SA might be due to the un-coupler of oxidative phosphorylation which protects the plant under salt stress condition. The plants under salt stress improve their leaf water balance by a decrease in transpiration rate which is associated with a reduction in stomatal conductance, which results in a decrease in water loss and an increase in the photosynthetic rate [67].
Specific Activity of Antioxidant Enzymes
The antioxidant enzyme activity dramatically increased due to salt stress. The lowest and highest activity was observed in control and 10 dS m−1 salt stress treatments, respectively (Table 2).
The activity of SOD significantly increased in stressed plants compared to control plants in both the genotypes. Maximum percent increase was noticed at 10 dS m−1 of salt level, i.e., 41.1 % in HJ 513 and 37.2 % in HJ 541 w.r.t control. Specific activity of CAT (unit mg−1 (protein) min−1) increased with every increment of salt level (from control to 10 dS m−1) in both genotypes. Maximum percent increase in specific activity of CAT was calculated in HJ 513 (122.0 %) as compared to HJ 541 (104.2 %) at 10 dS m−1 of salt level. CAT is a tetrameric heme-containing enzyme that catalyzes the reaction by converting hydrogen peroxide into water and oxygen. Maximum percent increase in POX activity was observed in HJ 513 (72.8 %) as compared to HJ 541 (63.4 %) at 10 dS m−1 of salt level with respect to control. A similar increase in activity of POX was seen under salt stress in chickpea by Kukreja et al. [43]. Various authors like Meloni and Martínez [68], Chernane et al. [69], Mickky and Aldesuquy [70], and Shakeri et al. [71] have also advocated the increase in specific activity of all the enzymes (SOD, CAT, and POX) under salt stress.
Our results indicate that the salt stress in sorghum enhances the antioxidant system and resists the secondary oxidative stress caused by salt stress as corroborated by Sui et al. [20] and Yang et al. [21]. Salt stress can induce the accumulation of ROS, which could greatly affect plant photosynthesis, metabolism, signal transduction, and other physiological and biochemical processes. The antioxidants scavenge ROS and free radicals to prevent damages caused by ROS [20, 72]. Salt stress–induced expression of ROS-associated antioxidant enzymes help to remove ROS in plants and increase plant resistance, allowing the maintenance of physiological growth and development [21].
Application of salicylic acid further enhanced the specific activity of SOD, CAT, and POX under salt stress which is clearly seen in our research findings. Foliar spray of SA (25 and 50 mg dm−3) brought considerable increment in the specific activity of SOD in both genotypes, but more increment was observed at 50 mg dm−3 in HJ 513 (12.8 %) as compared to HJ 541 (11.1 %) with respect to their control at 10 dS m−1 of salt level. Exogenous application of SA significantly enhanced the specific activity of CAT under stressed and un-stressed conditions, but this increase was more towards 50 mg dm−3 of SA under stressed conditions. Treatment with SA also enhanced the specific activity of POX, enhancement was more pronounced at 50 mg dm−3 of SA in stressed as well as in un-stressed conditions, and this increase was on higher side at 7.5 dS m−1 of salt level in both genotypes. Specific activity of all the enzymes increased after application of SA (Table 3). The present results suggested that SA may stimulate the antioxidant mechanism pathway in salt-stressed plants by stimulating antioxidant enzymes activities as reported by El-Esawi et al. [2], Ebrahimian and Bybordi [73], and He and Zhu [74]. SA supplementation improves the growth and yield under salt stress conditions and mitigates the negative effects of salinity stress (Fig. 2).
Heat map analysis combined with hierarchical clusters analysis of the all the plant responses (morphological, biochemical, and physiological) of the two genotypes HJ 513 (a) and HJ 541 (b), separately and combined (c), treated with different concentrations of salt (0, 5.0, 7.5, and 10.0 dS m–1 NaCl) and salicylic acid (0, 25, and 50 mg dm–3)
The present study suggests the SA treatment is an economical and simple method to ameliorate the negative effect of salinity on the growth of sorghum. This may solve sorghum production problems in aridisols caused by high salinity. However, further studies are required in order to determine the efficiency of these materials under natural field conditions.
Interaction Effects and Heat Map Analysis
Correlations and interaction effects between the genotypes and treatments based on different traits were determined using Pearson correlation coefficients (PCCs). Statistical analysis showed a significant interaction effect between genotypes and treatments on various traits analyzed. Traits related to growth correlated well with each other and with the osmolyte content, whereas they showed negative correlation with physiological and biochemical traits. Electrolyte leakage correlated negatively with growth traits but showed significant positive correlation with physiological and biochemical traits (Fig. 3a, 3b). Strong correlation was observed among all the physiological traits, photosynthetic rate, transpiration rate, and stomatal conductance (Fig. 4).
Soil salinity determined a significant reduction in growth traits of sorghum genotypes. Under no SA addition, the strongest salinity (salt 10 dS m−1/SA 0) resulted in around −75% plant height, −50% leaf number, and –90% dry weight with respect to the control (Salt 0/SA 0). Addition of SA determined a slight improvement of all growth traits under no salinity and a significant improvement under salinity implying that salinity and SA exhibited significant interactions in major growth traits. In these interactions, SA always mitigated the negative effects of salinity. The content of osmolytes and antioxidant enzymes also exhibited significant salinity × SA interactions. The antioxidant enzymes were doubly enhanced by salinity and SA; thus, the low initial values under control conditions increased in parallel with salinity and SA levels in both the genotypes. The rates of photosynthesis and transpiration and stomatal conductance were also benefitted by salinity × SA interactions.
To investigate the relationships between various traits with salt and SA treatments, correlation analysis and hierarchical clustering were conducted on the combined data sets. The aggregated data heat map analysis (Fig. 2), summarizing all the plant responses (morphological, biochemical, and physiological) of both the genotypes (HJ 513 and HJ 541) to various treatments, identified two clusters corresponding to low and high salt concentrations. Two separate sub-clusters could be defined under each salt treatment, which basically depended on the treatment with salicylic acid. SOD and GB content showed the strongest positive correlation with higher salt and SA concentrations in both genotypes. The hierarchical clustering and heat map provided a comprehensive perspective to reflect the changes of biochemical and physiological changes caused by different salt and salicylic acid treatments.
Conclusions
Soil salinization affects and interferes with the environment and sustainable development of agriculture, globally. Haryana Jowar HJ-513 and HJ-541 are the major genotypes in farmers’ fields in Haryana where vast area of land is affected by salinity. Salicylic acid has the potential to effectively mitigate the negative effects of salt stress. The present research work was carried out to mitigate the adverse effects of salt on electrolyte leakage, osmolyte concentration, gas exchange attributes, and activity of antioxidant enzymes in two sorghum genotypes by the foliar application of salicylic acid.
The salt stress hindered the growth, morpho-physiological, and biochemical attributes leading ultimately to reduction in productivity in the two genotypes of sorghum. Reduction was calculated more in HJ 541 as compared to HJ 513, based upon higher electrolyte leakage, lower accumulation of osmolytes, and increased antioxidative activities with increasing levels of salt stress from control to 10 dS m–1. Deleterious effects were more pronounced at higher levels of salinity (10 dS m–1) stress. The growth inhibition caused by salt treatment of sorghum seedlings was partly improved by SA supplementation. Ameliorating effect of SA was more prominent at 50 mg dm–3 of SA based on the lower values of electrolyte leakage (%), higher values of gaseous exchange attributes, and higher accumulation of osmolytes and further increment in antioxidative activities. The present study established that sorghum genotype HJ 513 performed relatively better under salt stress and showed better response in mitigating salt stress with foliar application of 50 mg dm–3 SA. SA application overcame the adverse impact of salt stress and displayed a better tolerance capacity and improved the growth of sorghum genotypes and consequently mitigated the impact of salinity in both the genotypes studied. Exogenous salicylic acid can act as a potential growth regulator and can effectively counteract the delirious effects of salt stress on sorghum. The salt-tolerant genotypes can be used as targets for breeding programs that aim to improve salinity tolerance in the future in order to meet agricultural production and sustainability.
Data Availability
No separate data available with the authors.
Change history
18 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12010-022-03915-7
Abbreviations
- ANOVA:
-
Analysis of variance
- CAT:
-
Catalase
- CRD:
-
Complete randomized design
- DAS:
-
Days after sowing
- EC:
-
Electrical conductivity
- ELP:
-
Electrolyte leakage percentage
- H2O2 :
-
Hydrogen peroxide
- NUE:
-
Nutrient use efficiency
- POX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
- SPSS:
-
Statistical Package for the Social Sciences
References
Gite, A. G., Kute, N. S., & Patil, V. R. (2015). Heterosis studies for yield and component traits in rabi sorghum [Sorghum bicolor (L.) Moench. J. Global Biosc., 4(8), 3207–3219.
El-Esawi, M. A., Elansary, H. O., El-Shanhorey, N. A., Abdel-Hamid, A. M., Ali, H. M., & Elshikh, M. S. (2017). Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol., 8, 716.
Liang, Y., Shen, Q., Shen, Z., & Ma, T. (1996). Effects of silicon on salinity tolerance of two barley cultivars. J. Pl. Nutr., 19(1), 173–183.
Mandal, A. K., & Paron, P. (2016). Mapping and characterization of salt-affected and waterlogged soils in the Gangetic plain of central Haryana (India) for reclamation and management. Cogent. Geosci., 2, 1.
Devi, S., Talwar, H., Singh, S., Ramprakash Goyal, V., Goyal, M., & Kumar, N. (2018). Physiological variability of sorghum (Sorghum bicolour L. Moench) under salt stress. Forage Res., 44, 101–104.
Chinchmalatpure, Anil & Sethi, Madhurama & Kumar, Parveen & Meena, Murli & Surya, Jaya & Khurana, Maulik & Bishnoi, Sita Ram & Jangra, Sunil & Yadav, Anil & Yadav, Rajender. (2018). Assessment and Mapping of Salt Affected Soils using Remote Sensing and GIS in Southern Districts of Haryana State.
Misra, A. N., Sahu, S. M., Misra, M., Singh, P., Meera, I., Das, N., Kar, M., & Shau, P. (1997). Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant., 39, 257–262.
Saxena, R., Kumar, M., & Tomar, R. S. (2019). Plant responses and resilience towards drought and salinity stress. Plant Archives, 12(2), 50–58.
Parida, A. K., & Das, A. B. (2005). Salt tolerance and salinity effect on plants. Ecotox. Environ. Safe, 60(3), 324–349.
Iqbal, M., & Ashraf, M. (2007). Seed treatment with auxins modulates growth and ion partitioning in salt stressed wheat plants. J. Integr. Pl. Biol., 49(7), 1003–1015.
Kaya, C., Kirnak, H., Higgs, D., & Saltali, K. (2002). Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high salinity. Scientia Horticulturae, 93, 65–74.
Khodary, S. E. A. (2004). Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. & Biol., 6, 5–8.
Yildirim, E., Turan, M., & Guvenc, I. (2008). Effect of foliar salicylic acid applications on growth, chlorophyll and mineral content of cucumber (Cucumis sativus L.) grown under salt stress. J. Pl. Nutr., 31, 593–612.
Kováˇcik, J., Grúz, J., Baˇckor, M., Strnad, M., & Repˇcák, M. (2009). Salicylic acid induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Pl. Cell Rep., 28, 135–143.
Jogawat, A. (2019) Osmolytes and their role in abiotic stress tolerance in plants, In. Molecular PlantAbiotic Stress: Biology and Biotechnology Chapter 5 John Wiley & Sons Ltd
Yadu, S., Dewangan, T. L., Chandrakar, V., & Keshavkant, S. (2017). Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol. Mol. Biol. Pl., 23(1), 43–58.
Soni, P. G., Basak, N., Rai, A. K., et al. (2021). Deficit saline water irrigation under reduced tillage and residue mulch improves soil health in sorghum-wheat cropping system in semi-arid region. Sci Rep, 11, 1880.
Dehnavi, A. R., Zahedi, M., Razmjoo, J., & Eshghizadeh, H. (2019). Effect of exogenous application of salicylic acid on salt-stressed sorghum growth and nutrient contents. J. Pl. Nutr., 42(11-12), 1333–1349.
Nimir, N. E. A., Zhou, G., Guo, W., Ma, B., Lu, S., & Wang, Y. (2017). Effect of foliar application of GA3, kinetin, and salicylic acid on ions content, membrane permeability, and photosynthesis stress of sweet sorghum [Sorghum bicolor (L.) Moench]. Canad. J. Pl. Sci., 97(3), 525–535.
Sui, N., Yang, Z., Liu, M., & Wang, B. (2015). Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genomics, 16, 534. https://doi.org/10.1186/s12864-015-1760-5.
Yang, Z., Zheng, H., Wei, X., Song, J., Wang, B., & Sui, N. (2018). Transcriptome analysis of sweet Sorghum inbred lines differing in salt tolerance provides novel insights into salt exclusion by roots. Plant Soil, 430, 423–439. https://doi.org/10.1007/s11104-018-3736-0.
Almodares, A., Hadi, M. R., Kholdebarin, B., Samedani, B., & Kharazian, Z. A. (2014). The response of sweet sorghum cultivars to salt stress and accumulation of Na+, Cl– and K+ ions in relation to salinity. J. Environ. Biol, 35, 733–739.
Husen, A., Iqbal, M., Sohrab, S. S., et al. (2018). Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata. Br.). Agric. & Food Security, 7, 44. https://doi.org/10.1186/s40066-018-0194-0.
Jindal, Y., Sehrawat, S. K., Chhabra, A. K., Kumar, N., Kumar, S., Kumar, S., Yadav, S. S., Dahiya, M., & Niwas, R. (Eds.). (2021). Varieties of CCSHAU: Continued efforts towards food security (pp. 152). University publication No. CCSHAU/PUB#21-058. Dorex Offset Printers.
Pandey, K. C., & Roy, A. K. (2011). Forage Crops Varieties. IGFRI Jhansi (India).
Hoagland, D. R., & Arnon, D. I. (1950). The water culture method for growing plants without soil. - California Agricultural Experimental Station Circular (pp. 1–32). University of California.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. - In Agriculture Handbook; USDA -Washington DC (p. 60).
Guimarães, M. J. M., Simões Welson, L., Oliveira Anderson, R., de, A., Gherman, G. L., de, S., et al. (2019). Biometrics and grain yield of sorghum varieties irrigated with salt water. Revista Brasileirade Engenharia Agrícolae Ambiental, 23(4), 285–290. https://doi.org/10.1590/1807-1929/agriambi.v23n4p285-290.
Tabatabaei, S. A., & Anagholi, A. (2012). Effects of salinity on some characteristics of forage sorghum genotypes at germination stage. - Int. J. Agric. & Crop Sci., 4(14), 979–983. http://ijagcs.com/wpcontent/uploads/2012/09/979-983.pdf.
Sullivan, C. Y., & Ross, W. M. (1979). Selecting for drought and heat resistance in grain sorghum. In H. Mussell & R. C. Staples (Eds.), Stress physiology in crop plants (pp. 263–281). John Wiley and Sons.
Bates, L., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrate in the plant extract by anthrone reagent. J. Biochem., 57, 508–514.
Grieve, C. M., & Grattan, S. R. (1983). Rapid Assay for Determination of Water Soluble Quaternary Ammonium Compounds. Plant and Soil, 70, 303–307.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Pl. Physiol., 59, 309–314.
Aebi, H.E. (1983) Methods of Enzymatic Analysis. Verlagsgesellschaft GmbH - In: Bergmeyer, H.U., Bergmeyer, J., Grabi, M. (eds.) - Germany, pp. 273-282
Siegel, B.Z. and Siegel, S.M.(1986)Peroxidase activity and stress factors: a complex relationship - In: Molecular and Physiological Aspects of Plant Peroxidases, eds. Greppin, H., Penel, C. and Gaspar, T.H.,-University of Geneva- Geneva, pp. 427-431
Sheoran, O.P. (1995) Statistical Package for Agricultural Scientists (OPSTAT) - CCSHAU, Hisar. http://www.202.141.47.5/opstat/index.aspPapageorgiou, G.C. and Morata, N.
Sun, Y., Niu, G., Osuna, P., Zhao, L., Ganjehunte, G., Peterson, G., & Gardea-Torresdey, J. L. (2014). Variability in salt tolerance of Sorghum bicolor L. Agril. Sci., 2(1), 9.
Gupta, B. & Huang, B. (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. - Int. J. Genomics, 1-18
Sharma, A., Kumar, V., Shahzad, B., et al. (2020). Photosynthetic response of plants under different abiotic stresses: A Review. J. Pl. Gr. Regul., 39, 509–531.
Ahmad, P., Alyemeni, M. N., Ahanger, M. A., et al. (2018). Salicylic Acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in Faba Bean (Vicia faba L.) seedlings under NaCl toxicity. - Russ. J. Pl. Physiol., 65, 104–114.
Mahlooji, M., Sharifi, R. S., Razmjoo, J., Sabzalian, M. R., & Sedghi, M. (2018). Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica, 56(2), 549–556.
Kukreja, S., Nandwal, A. S., Kumar, N., Sharma, S. K., Sharma, S. K., Kundu, B. S., Unvi, V., & Sharma, P. K. (2006). Response of chickpea roots to short-term salinization and desalinization: Plant water status, ethylene evolution, antioxidant activity and membrane integrity. Physiol. Mol. Biol. Pl., 12, 67.
Rani, K.(2004)Effect of salinity on morphological anatomical and reproductive aspectsin mungbean (Vigna radiata L. Wilczek) and their hybrids (Doctoral dissertation, CCS HAU, Hisar),
McNeil, S. D., Nuccio, M. L., & Hanson, A. D. (1999). Betaines and related osmoprotectants targets for metabolic engineering of stress resistance. Pl. Physiol., 120, 945–949.
Sultan, I., Khan, I., Chattha, M. U., Hassan, M. U., Barbanti, L., Calone, R., Ali, M., Majid, S., Ghani, M. A., Batool, M., Izzat, W., & Usman, S. (2021) Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Italian Journal of Agronomy, 16(3). https://doi.org/10.4081/ija.2021.1810
Clarke, S. M., Mur, L. A., Wood, J. E., & Scott, I. M. (2004). Salicylic acid dependent signaling promotes basal thermo-tolerance but is not essential for acquired thermo-tolerance in Arabidopsis thaliana. The Pl. J., 38, 432–447.
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline underchanging environments: a review. Pl. Signal Behav., 7(11), 1456–1466.
Inzé, D., & Van Montagu, M. (1995). Oxidative stress in plants. Curr. Op. Biotech., 6, 153–158.
Kuznetsov, V. V., & Shevyakova, N. I. (1999). Proline under stress: biological role, metabolism and regulation. Russ. J. Pl. Physiol., 46(2), 274–287.
Nakamura, T., Nomura, M., Mori, H., Jagendorf, A. T., Ueda, A., & Takabe, T. (2001). An isozyme of betaine aldehyde dehydrogenase in barley. Pl. Cell Physiol., 42, 1088–1092.
Tan, Y., Liang, Z., Shao, H., & Du, F. (2006). Effect of water deficits on the activity of anti-oxidative enzymes and osmoregulation among three different genotypes of Radix astragali at seeding stage. Colloids Surf. B: Biointerfaces, 49, 60–65.
Papageorgiou, G. C., & Morata, N. (1995). The usually strong stabilizing effects of glycine betaine on the structure and function in the oxygen evolving photosystem-II complex. Photosynth. Res., 44, 243–252.
Kavi Kishor, P. B., Hong, Z., Miao, G. H., Hu, C. A., & Verma, D. P. S. (1995). Over expression of delta- pyrroline 5 carboxylase synthetase increases proline production and confers osmotolerance in transgenic plants. Pl. Physiol., 108(4), 1387–1394. https://doi.org/10.1104/pp.108.4.1387.
Singh, B., & Pareek, R. G. (2003). Effect of phosphorus and biofertilizers on growth and yield of mungbean. Ind. J. Pulses Res, 16(1), 31–33.
Nandwal, A. S., Kukreja, S., Kumar, N., Sharma, P. K., Jain, M., Mann, A., & Singh, S. (2007). Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J. Pl. Physiol., 164, 1161–1169.
Ahmad, M. A., Murali, P. V., & Marimuthu, G. (2014). Impact of salicylic acid on growth, photosynthesis and compatible solute accumulation in Allium cepa L. subjected to drought stress. Int. J. Res. Agric. Food Sci., 4, 22–30.
Kaur, G, Atwal, AK, Sangha, MK, Kaur, G and Banga, SS (2011) Response of Brassica juncea genotypes to heat stress and role of salicylic acid and abscisic acid in thermo tolerance. - In: Rang et al. (eds.) Proc. Int. Conf. Preparing Agriculture for Climate Change, 6-8 February, 2011,- Ludhiana, India - Crop Improv. 3:159,
Mauro, R. P., Agnello, M., Distefano, M., Sabatino, L., San Bautista Primo, A., Leonardi, C., & Giuffrida, F. (2020). Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy, 10(1), 137. https://doi.org/10.3390/agronomy10010137.
Parimelazhagan, T., & Francis, K. (1996). Effect of water stress and salinity on photochemical activity of green gram. Bioved, 7(1), 47–52.
Ali, M., Usman, M., & Ahsan, T. (2012). Sodium sulphate induced modulation in some key morphophysiological characteristics in Sorghum bicolor L. World J. Agric. Res, 8, 381–384.
Mohamed, I., Shalby, N., Bai, C., Qin, M., Agami, R. A., Jie, K., Wang, B., & Zhou, G. (2020). Stomatal and photosynthetic traits are associated with investigating sodium chloride tolerance Brassica napus L. cultivars. Plants Basel - Switzerland, 9(1), 62.
Chandra, A., & Bhatt, R. K. (1998). Biochemical and physiological response to salicylic acid in relation to the systemic acquired resistance. Photosynthetica, 35, 255–258.
Szalai, G., Paldi, E., & Janda, T. (2005). Effect of salt stress on the endogenous salicylic acid content in maize (Zea mays L.) plants. Acta Biologica Szegediensis, 49(1), 47–48.
Majeed, S., Akram, M., Latif, M., Ijaz, M., & Hussain, M. (2016). Mitigation of drought stress by foliar application of salicylic acid and potassium in mung bean (Vigna radiata L.). - Leg. Res, 39, 208–214.
Hasegawa, P. M., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. - Ann. Rev. Pl. Biol., 51, 463–499.
Acosta-Motos, J. R., Ortuño, M. F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M. J., & Hernandez, J. A. (2017). Plant responses to salt stress: adaptive mechanisms. Agronomy, 7, 1–38.
Meloni, D. A., & Martínez, C. A. (2009). Glycine betaine improves salt tolerance in vinal (Prosopisruscifolia Griesbach) seedlings. Braz. J. Pl. Physiol., 21, 233–241.
Chernane, H., Latique, S., Mansori, M., & ElKaoua, M. (2015). Salt stress tolerance andantioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. J. Agric. Vet. Sc., 8, 36–44.
Mickky, B. M., & Aldesuquy, H. S. (2017). Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt. J. Basic & Appl. Sc., 4, 47–54.
Shakeri, E., Emam, Y., Pessarakli, M., & Tabatabaei, S. A. (2020). Biochemical traits associated with growing sorghum genotypes with saline water in the field. J. Pl. Nutr., 43(8), 1136–1153.
Xue, X., Zhang, Q., & Wu, J. (2013). Research of reactive oxygen species in plants and its application on stress tolerance. - Biotechnol. Bulletin, 36, 6–11.
Ebrahimian, E., & Bybordi, A. (2012). Effect of salinity, salicylic acid, silicium and ascorbic acid on lipid peroxidation, antioxidant enzyme activity and fatty acid content of sunflower. Afr. J. Agril. Res, 7, 3685–3694.
He, Y., & Zhu, Z. (2008). Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidative enzyme activity in Lycopersicon esculentum. Biol. Plant., 52, 792–795.
Author information
Authors and Affiliations
Contributions
SD and VG conceived the idea and MJ and S carried out experimental and initial data analysis, and prepared initial draft of manuscript. SM performed major data analysis and subsequent discussion of results and revised the draft of manuscript. VG assisted in data analysis and figure/graphs preparation. SM was involved in correspondence with the journal and subsequent revisions of the manuscript according to reviewers’ comments. All authors read and consented on the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Study requires no ethical approvals. Manuscript is in compliance with ethical standards. An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised. The names of the authors should be spelled out which should read as: Manish Jangra, Sarita Devi, Satpal, Neeraj Kumar, Vinod Goyal and Shweta Mehrotra.
Rights and permissions
About this article
Cite this article
Jangra, M., Devi, S., Satpal et al. Amelioration Effect of Salicylic Acid Under Salt Stress in Sorghum bicolor L.. Appl Biochem Biotechnol 194, 4400–4423 (2022). https://doi.org/10.1007/s12010-022-03853-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03853-4