Abstract
The growth and morphophysiological responses of wild watermelon (C. lanatus var. citroide) M20 and Chinese domesticated watermelon (C. lanatus var. lanatus) Y34 to drought stress and subsequent re-watering were compared. Wild watermelon is drought-tolerant, whereas the domesticated watermelon is susceptible. Irrigation was withheld from seedlings for 10 days and the seedlings were then allowed to recover for 1 day. Drought treatment resulted in the wilting and yellowing of leaves in both genotypes, but symptoms occurred earlier and more visibly in Y34. Drought stress inhibited the growth of both genotypes but increased the root/shoot ratio more pronouncedly in M20 than in Y34. Under drought conditions, M20 maintained a higher leaf water status than Y34 due to its denser trichomes and more sensitive stomatal control, which minimized the transpiration rate. Y34 was more vulnerable to drought, resulting in larger decreases in photosystem II efficiency, initial Rubisco activity and chlorophyll concentration. H2O2, O2 −, and MDA contents were significantly increased in both genotypes; however, these increases were smaller in M20, possibly due to a greater enhancement of antioxidant enzyme activities and related-gene expression levels. Moreover, M20 accumulated soluble sugars and proline to greater levels to counter reduced soil moisture. These adaptive mechanisms enabled M20 to recover more rapidly after re-watering. Our findings provide guidance for improving the drought tolerance of Chinese watermelon cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is among the most important factors for normal plant growth and development. Particularly in arid and semiarid regions, plants are often challenged with periods of soil and atmospheric water deficits (drought stress) during their life cycles; these periods are known to hamper growth and productivity by affecting a variety of vital biochemical and physiological processes (Talbi et al. 2015). Due to recent climate change, drought stress is an increasingly serious environmental constraint that is predicted to worsen (Posch and Bennett 2009), even affecting drought-resistant species. To survive under such harsh conditions, plants will initiate several self-protection responses at the molecular, cellular or even whole organism levels. Plants differ in their ability to withstand drought stress, and the induced responses vary among and within species (Jaleel et al. 2009). Understanding the various adaptive strategies that enable plants to address stress is of great importance for improving water management and clarifying the breeding goals for improving intolerant cultivars.
In higher plants, photosynthesis is among the physiological processes that are most drought-sensitive (Zhang et al. 2013). When water availability is reduced, many plants suffer a reduced photosynthetic rate (P n). It has been concluded that reduced photosynthetic CO2 assimilation results from the low CO2 availability caused by limited diffusion through the stomata and mesophyll or from alterations in metabolism (Hu et al. 2010). Under early or mild water deficits, stomatal limitation is generally considered the main cause for reduced photosynthesis; however, under late, more severe conditions, metabolic and biochemical limitations become the predominant driver of the decline in photosynthesis, a process involving pigment loss, the deactivation of photosynthesis-related enzymes (Zhang et al. 2013), and inhibition of the functional activity of photosystem II (PSII) (Posch and Bennett 2009). Chlorophyll fluorescence kinetics analysis is a rapid and nonintrusive probe that can be used to monitor and quantify the changes that are induced in the photosynthetic apparatus by drought and provide information about the extent to which PSII uses the energy absorbed by chlorophyll and the extent to which it is damaged by excess light (Maxwell and Johnson 2000). This technique, coupled with gas-exchange measurements, has been widely employed in photosynthetic research on different plant species under drought (Zhang et al. 2015), flooding (Du et al. 2012), salt (Hunsche et al. 2010) and many other environmental stresses.
Exposure of plants to drought usually triggers the enhanced generation of reactive oxygen species (ROS) and other oxidizing agents, resulting in oxidative stress (Liu et al. 2012). To minimize the effects of induced peroxidation damage, plants have evolved an highly efficient detoxification system comprising enzymatic and non-enzymatic antioxidants (Boaretto et al. 2014). Increased antioxidant enzyme activity is a well-known adaptive mechanism for responding to drought (Wang et al. 2012), and drought-tolerant cultivars have a much higher constitutive or induced antioxidant capacity than sensitive cultivars, as shown for sugarcane (Boaretto et al. 2014), apple (Wang et al. 2012) and maize hybrids (de Souza et al. 2014).
Watermelon [Citrullus lanatus (thunb.) Matsum. & Nakai] is an economically important fruit crop worldwide (Zhang et al. 2011). In China, approximately 1,839,750 hectares were planted with watermelon in 2013, rendering it the top watermelon producer worldwide (FAO 2015). However, most Chinese watermelon cultivars are intolerant to drought stress due to traditional selection and breeding pressure for productivity and quality under irrigated field conditions (Zhang et al. 2011). Wild germplasms from dry desert areas are attractive sources of useful genetic traits for improving the drought resistance of domesticated watermelons because these plants exhibit considerable ability to combat drought stress (Kawasaki et al. 2000; Si et al. 2009). Several studies have described the responses of wild watermelon to drought stress (e.g., electron transport regulation, citrulline accumulation, and changes in the proteome and transcriptome) (Kawasaki et al. 2000; Sanda et al. 2011; Si et al. 2009); however, no reports have compared the behaviour of wild and domesticated watermelons under conditions of reduced water supply and their capacity to recover from water deficit. In our previous work, genotypic variation regarding drought resistance was examined among 12 watermelon varieties; the wild watermelon germplasm (C. lanatus var. citroide, M20) was identified as the most tolerant genotype, and the Chinese domesticated watermelon (C. lanatus var. lanatus, Y34) was identified as the most susceptible genotype. In this study, we compared the effects of growth and morphophysiological changes on the drought stress response and subsequent recovery of the two genotypes. The aims of this study were (1) to evaluate the harmful effects of drought stress, particularly on the photosynthesis and ROS metabolism of watermelon plants, and (2) to elucidate genotypic differences in the mechanisms that affect the tolerance of both genotypes to drought and the degree of recovery upon rehydration.
Materials and methods
Plant materials, growth conditions and treatments
Two watermelon germplasms were studied: ‘M20’, a drought-resistant variety, and ‘Y34’, a drought-sensitive inbred line; these germplasms were provided by the Watermelon and Melon Research Group at Northwest A&F University, Yangling, Shaanxi, China.
The experiment was conducted in a greenhouse at Northwest A&F University (34°283′N, 108°067′E). Seeds were sterilized with 2 % (v/v) sodium hypochlorite for 10 min, rinsed with distilled water and soaked for 4–6 h, then being put in a dark growth chamberat at 30 °C for germination. Uniformly germinated seeds were directly sown in plastic pots (10 cm × 8.5 cm × 7 cm), which were filled with a homogenous mixture of sand and a commercial peat-based compost (1:1 by volume), one seed per pot. All pots initially contained the same weight of dry growth media (353.18 g). Seedlings were pre-cultured in the greenhouse under natural light at a temperature ranging from 18.0 to 36.0 °C and under a relative humidity range of 65–80 %. Before the experiments began, all plants were well watered each day and fertilized weekly with 1/2 Hoagland’s solution (pH 6.5). When the plants reached the 4- to 5-leaf stage, morphologically uniform healthy seedlings of each genotype were randomly assigned (50:50) to control and treatment groups. The control plants were well-watered to 75 ± 5 % field capacity (FC) every evening on the basis of weight, whereas the treated plants were unwatered for 10 days until the leaves of Y34 showed extreme wilting, at which point they were re-watered to the control level for 1 day (recovery). Samples were harvested on days 0, 2, 4, 6, 8, and 10 during the drought stress and on day 11 (1 day post-drought). Leaf sampling was randomly conducted: the second topmost fully expanded leaves from four plants per treatment were sampled in each biological sample, and at least three biological replicates were used.
Leaf water status and soil water status
Five pots were randomly selected per treatment, and the leaf relative water content (RWC) and soil water availability (SWA) were recorded. Leaf RWC was determined according to Barrs and Weatherley (1962), and SWA was determined according to Sanda et al. (2011). Soil water availability was calculated using the following equation: SWA (%FC) = (soil weight − dried soil weight)/(fully-watered soil weight − dried soil weight) × 100 (%).
Growth measurements
After 10 days of drought treatment, plant heights were measured (ten plants per treatment). Then, all seedlings were harvested, washed carefully and divided into shoots and roots. Root length was recorded, and the samples were oven-dried for 72 h at 80 °C to determine the dry weights. The root/shoot ratio was calculated on the basis of dry mass.
Scanning electron microscopy (SEM) observation of epidermal trichome and stomata
Four leaves were collected per genotype per treatment on days 0, 2, 4, and 8 of drought treatment for SEM processing. Specimens were prepared as described by Wu et al. (2014). The surface of the lower leaf epidermis was observed and photographed under a JSM-6360LV microscope (JEOL Ltd., Tokyo, Japan). Epidermal trichomes and stomata were counted in 15 randomly selected microscope visual fields, and stomatal apertures were measured from 15 randomly selected stomata on the same specimen using Image J software.
Gas exchange and chlorophyll fluorescence measurements
All measurements were performed on the second fully expanded intact leaves obtained from five plants per treatment. Leaf gas exchange parameters were measured using an LI-6400 portable photosynthesis system (Li-6400; Li-Cor, Lincoln, NE, USA) equipped with an LED red/blue light source (6400-02B). The photosynthetic photon flux density was set at 500 μmol m−2 s−1. Photosynthesis rate, intercellular CO2 concentration (C i), transpiration rate (T r) and stomatal conductance (G s) were recorded automatically. Chlorophyll fluorescence was measured using a portable PAM-2500 fluorometer (Walz, Germany) equipped with computer-operated PAM-control software (PAMWin 3.0). The photochemical quenching (qP = (F′m − F s)/(F′m − F′0), nonphotochemical quenching (NPQ = (F m − F′m)/F′m), maximum photochemical efficiency of PSII (F v/F m, F v = F m − F 0), actual photochemical efficiency of PSII (ΦPSII = (F′m − F s)/F′m) and electron transport rate [ETR, ETR = ΦPSII × 0.5 × PPFD × 0.84] were calculated according to Maxwell and Johnson (2000).
Chlorophyll concentration and analysis of initial Rubisco activity
The total chlorophyll content (Chl a + b) was assayed spectrophotometrically according to Lichtenthaler and Wellburn (1983). Initial Rubisco activity was quantified using a modified version of the method described by Zhang et al. (2013).
Lipid peroxidation and H2O2 and O2 − accumulation assays
Lipid peroxidation was estimated as equivalents of malondialdehyde (MDA) according to Guo et al. (2012). Two milliliter crude extract was mixed with the same volume of a 0.5 % (w/v) thiobarbituric acid (TBA) solution containing 5 % (w/v) trichloroacetic acid. The mixture was heated at 99 °C for 15 min, cooled quickly, and centrifuged at 10,000 rpm for 10 min. The supernatant was used to measure the absorbance at 532, 600, and 450 nm. Hydrogen peroxide (H2O2) and superoxide anion radical (O2 −) generation was measured according to Bai et al. (2010). Leaves were homogenized in cold (−20 °C) acetone at a ratio of 1.0 g sample to 2 mL acetone and centrifuged at 12,000g for 10 min at 4 °C. One milliliter extract supernatant was mixed with 0.1 mL 2 % TiSO4 and 0.2 mL of 17 mM ammonia solution. After a second centrifugation, the precipitate was washed five times with cold acetone to disappear the pigment, then drained and dissolved in 3 mL of 2 M H2SO4. Absorbance of the solution was measured at 410 nm against blanks, and the amount of H2O2 production in leaves was calculated based on an H2O2 solution-derived standard curve. For the determination of O2 − generation rate, 0.2 g leaf tissue was ground with 4 mL 50 mM cold phosphate buffer solution (PBS; pH 7.8) and centrifuged at 12,000g for 10 min. Afterwards, 0.9 mL 50 mM PBS (pH 7.8) and 0.1 mL 10 mM hydroxylamine hydrochloride were added into 1 mL of extract. Mixture then incubated at room temperature for 20 min. After the following addition of 2 mL 17 mM sulfanilamide and 2 mL 7 mM a-anaphthylamine, the resulting mixture was incubated for 20 min again. Then, light absorbance was measured at 530 nm, and a standard curve with nitrogen dioxide radical (NO2 −) was used to calculate the production rate of O2 −.
Antioxidant enzyme extraction and activity assay
Lyophilized leaf samples (0.5 g each) were pulverised and suspended in 8 mL ice-cold 50 mM PBS (pH 7.8) containing 0.1 mM EDTA and 1 % (m/v) polyvinylpolypyrrolidone (PVPP) in a chilled mortar. The homogenates were centrifuged at 12,000g for 20 min at 4 °C, and the resulting supernatants were used to assay the enzyme activities.
Superoxide dismustase (SOD) activity was measured as described by Bai et al. (2010). A 3-mL reaction mixture contained 50 mM Na-phosphate buffer (pH 7.3), 13 mM methionine, 75 mM NBT, 0.1 mM EDTA, 4 mM riboflavin, and 0.02 mL of enzyme extract. All of the reaction mixtures in the glass test tubes were mixed well in the dark, then being irradiated for 15 min at 200 µmol m−2 s−1 photon flux density. Absorbance was read at 560 nm. Blanks or controls were run in the same manner but without illumination or enzyme, respectively. One unit of SOD was defined as the amount of enzyme that inhibit the reduction of NBT by 50 % under assay conditions. Catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) activities were determined according to Guo et al. (2012). CAT activity was evaluated by monitoring the decrease in absorbance at 240 nm owing to decomposition of H2O2 (extinction coefficient of 39.4 mM−1 cm−1). A 1-mL reaction mixture contained 50 mM PBS (pH 7.0), 10 mM H2O2 and 20 µL supernatant. The reaction was initiated by adding H2O2. APX activity was determined by tracking the oxidation of ASA at 290 nm (extinction coefficient of 2.8 mM−1 cm−1). The 1 mL reaction mixture contained 50 mM Hepes–KOH (pH 7.6), 0.1 mM EDTA-Na2, 0.5 mM ascorbate (ASA), 1 mM H2O2 and 20 µL enzyme extract. The reaction was initiated by adding H2O2. GR activity was estimated from the absorbance change at 340 nm caused by NADPH oxidation (extinction coefficient of 6.2 mM−1 cm−1). A 1 mL reaction mixture contained 100 mM Tris–HCl buffer (pH 7.5), 1 mM EDTA-Na2, 0.1 mM NADPH, 0.25 mM oxidized glutathione (GSSG) and 20 µL enzyme extract. The reaction was initiated by the addition of NADPH.
AsA and GSH determination
Reduced AsA and dehydroascorbate (DHA) concentrations were measured according to Logan et al. (1998). Reduced glutathione (GSH) and GSSG contents were determined as described by Guo et al. (2012). DHA and GSH contents were estimated from the differences between total AsA and AsA and between total GSH and GSSG, respectively.
Proline and soluble sugar content determination
Total soluble sugar content was determined according to the anthrone sulphuric acid method, and free proline content was estimated using the ninhydrin method according to Gao (2000).
Total RNA extraction and gene expression analysis
Eight genes including WRKY70-like, MYB96-like, pheide a oxygenase (PAO), pheophytin pheophorbide hydrolase (PPH), Cu–Zn SOD, CAT, APX and GR were searched from the Watermelon Genome Database (http://www.icugi.org) for time-course gene expression analysis. The primers are listed in Table S1. Total RNA from leaves was isolated with the TRIzol Reagent (Invitrogen, USA). Dnase-treated RNA (1 µg) was used for reverse transcription. qRT-PCR was performed with an iCycler iQ TM Multicolor PCR Detection System (Bio-Rad, Hercules, CA, USA) using the SYBR Premix ExTaq II (2×) Kit (Takara). The PCR conditions consisted of pre-denaturing at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 30 s. Melting-curve analysis of the products was conducted at the end of each PCR cycle to verify that a single product was amplified. These qRT-PCR experiments were repeated three times, based on three separate RNA extracts from three samples. The relative gene expressions were analyzed using the 2−ΔΔCT method (Livak and Schmittgen 2001). Watermelon β-actin gene (ClACT) was used as the internal control (Kong et al. 2014).
Statistical analysis
Statistical analysis was conducted using the PASW Statistics 18.0 program. All data were analysed using a one-way analysis of variance (ANOVA), and differences between means were assessed using Duncan multiple range tests (P < 0.05). The results are presented as the mean ± standard deviation (SD) of 3–10 independent biological replicates.
Results
Changes in soil water availability, leaf relative water content and plant growth parameters
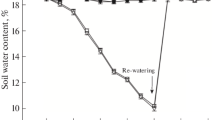
Watermelon seedlings were normally watered (control) or subjected to drought stress for 10 days followed by a 1-day re-watering. Soil water availability in the well-watered controls remained constant at 74.4–76.45 %, while those in pots with stressed plants declined over time to the minimum value by day 10 (Fig. 1a). After re-watering, the SWA of both genotypes returned to the control levels. Drought stress progressively decreased leaf RWC (Fig. 1b). Compared with M20, the RWC decrease was more marked in Y34. On days 4, 6, and 10, RWC had decreased by 2.80, 19.23, and 48.71 %, respectively, for Y34; these values compare with 1.65, 10.13, and 16.13 %, respectively, for M20. Following the re-watering, leaf RWC in M20 plants exhibited an almost complete recovery; however, leaf RWC in Y34 plants was only partially restored.
Soil water availability (SWA), leaf relative water content (RWC) and growth performance in M20 (M) and Y34 (Y) grown under well-watered conditions (CK) and drought stress conditions for 10 days, followed by a 1-day re-watering (DS). a Soil water availability, b leaf relative water content, c plant height, d root length, e total dry weight, f ratio of root/shoot. SWA and RWC were measured on days 0, 2, 4, 6, 8, 10, and 11 of the treatment; data represent mean ± SD for five replicates. Growth parameters were measured at the end of the stress period (day 10); data represent mean ± SD of 10 individual plants; different letters represent significant differences at P < 0.05 according to Duncan’s multiple-range test
Watermelon seedling growth was significantly inhibited by drought stress, and dramatic decreases in plant height, root length and biomass production were observed for both genotypes at final harvest; however, root/shoot ratios were significantly greater in stressed plants than in non-stressed plants (Fig. 1c–f). Both in control and stressed plants, no significant differences in dry weight were found between the genotypes; nonetheless, M20 exhibited a much higher root/shoot ratio and also a much longer root length, indicating that the root system was more developed in M20 than in Y34.
Lower leaf epidermal morphology and stomatal behaviour
The lower epidermal surfaces of M20 and Y34 leaves were scanned after exposure of the seedlings to 0, 4, 6 and 8 days of drought treatment. Tissues from M20 plants had greater trichome densities but lower stomata numbers than thoes from Y34 plants (Fig. 2a, b). During drought progression, both genotypes showed similarities in general stomatal behaviour, including sinking of the stomata below the epidermal surface, narrowing of stomatal aperture, and increasing closed stomata numbers (Fig. S1); however, these behaviours differed slightly between the genotypes. As expected, M20 presented a smaller stomatal aperture in response to drought stress than Y34, and the closed stomata ratio was also higher in M20 than in Y34; though the differences were only significant during early drought stress (day 2) (Fig. 2c, d).
Changes in lower leaf surface morphology in M20 and Y34 after exposure to 0, 2, 4, and 8 days of drought stress. a Epidermal trichome density, b stomatal density, c stomatal aperture, d ratio of closed stomata. Data represent the mean ± SD of 40 images. Different letters represent significant differences at P < 0.05 according to Duncan’s multiple-range test
Gas exchange response and chlorophyll fluorescence index
Drought significantly decreased net photosynthetic rate, stomatal conductance and leaf transpiration rate (Fig. 3a–c). The pattern of the change in P n over the experiment was similar in both genotypes; however, G s and T r decreased more rapidly in M20 than in Y34 during the first 2 days. After re-watering, the value of P n in M20 recovered to 45.36 % of that seen in the control, whereas the value of P n in Y34 was restored to only 12.71 % of that seen in the control.
Changes in leaf photosynthetic parameters and leaf chlorophyll fluorescence parameters in M20 (M) and Y34 (Y) grown under well-watered conditions (CK) and subjected to drought stress for 10 days, followed by a 1-day re-watering (DS). a Photosynthetic rate (P n); b stomatal conductance (G s); c leaf transpiration rate (T r); d maximum photochemical efficiency of PSII (F v/F m); e actual photochemical efficiency of PSII (ΦPSII); f electron transport rate (ETR); g photochemical quenching (qP); h nonphotochemical quenching (NPQ). Values represent mean ± SD (n = 5)
Leaf F v/F m under drought treatment were similar to those observed in the control plants during the first 4 days and then declined in both genotypes, although the values in M20 decreased to a lesser extent (Fig. 3d). ΦPSII was also negatively affected by drought stress, declining gradually and showing reductions of 51.86 and 64.08 % in M20 and Y34 plants, respectively, at the end of the drought stress (Fig. 3e). Identical patterns of decrease were observed for ETR and qP (Fig. 3f, g). On day 11, F v/F m, ΦPSII, ETR and qP were restored to 97.77, 79.04, 79.71 and 87.02 % of the background levels in M20, respectively, values that were much higher than those found in Y34 (91.56, 58.58, 58.55 and 74.055 %, respectively) (Fig. 3e–g). NPQ was significantly increased by drought stress and was higher in M20 than in Y34 from day 6 to day 10 (Fig. 3h).
Production of H2O2 and O2 − and lipid peroxidation
In both genotypes, levels of O2 −, H2O2 and MDA remained stable and low under the control conditions (Fig. 4). The imposition of water deprivation resulted in obvious O2 −, H2O2 and MDA accumulation in the leaves in both genotypes, but the extent of this accumulation was much greater in Y34 than in M20. By day 10, O2 −, H2O2 and MDA contents in Y34 were 1.62, 1.37 and 1.39 times thoes contents in M20, respectively.
Changes in a superoxide anion radical (O2 −) and b hydrogen peroxide (H2O2) generation and c malondialdehyde (MDA) content in leaves of M20 (M) and Y34 (Y) grown under well-watered conditions (CK) and subjected to drought stress for 10 days, followed by a 1-day re-watering (DS). Data represent mean ± SD for three replicates
Antioxidant enzyme activities
Antioxidant enzyme activities changed significantly during the period of water withholding (Fig. 5a–d). SOD activity increased steadily in both genotypes and reached maximum levels on day 8 (Fig. 6a). CAT activity was greater in stressed M20 than in stressed Y34 throughout the experimental period (Fig. 5b). APX activity increased rapidly in M20, but this increase was more delayed and gradual in Y34 (Fig. 5c). GR activity increased from day 4 to a maximum on day 6 in Y34 and from day 4 to a maximum on day 8 in M20 (Fig. 5d).
Changes in the activities of a superoxide dismutase (SOD), b catalase (CAT), c ascorbate peroxidase (APX), and d glutathione reductase (GR) in leaves of M20 (M) and Y34 (Y) grown under well-watered conditions (CK) and subjected to drought stress for 10 days, followed by a 1-day re-watering (DS). Data represent mean ± SD for three replicates
Relative expression levels of a WRKY70-like, b MYB96-like, c PAO, d PPH, e Cu–Zn SOD, f CAT, g APX and h GR genes in M20 (M) and Y34 (Y) grown under well-watered conditions (CK) and subjected to drought stress for 0, 2, 6, and 10 days, followed by a 1-day re-watering (DS). Expression level of gene in Y34 at day 0 was used as the control with a nominal value of 1. Data represent mean ± SD for three replicates
Expression profiles of drought responsive genes
To provide more insights into the molecular regulations controlling the drought tolerance in wild watermelon, the relative expressions of drought tolerance-related transcription factors (WRKY70-like and MYB96-like), chlorophyll degradation related genes (PAO and PPH) and ROS scavenging systems related genes (Cu–Zn SOD, CAT, APX and GR) were determined by qRT-PCR. As shown in Fig. 6a, b, drought stress down-regulated WRKY70-like but up-regulated MYB96-like transcript levels in both genotypes. The responses of the two genes were earlier and more marked in M20 than in Y34. Drought stress induced the transcripts of PAO and PPH genes, with the effects more pronounced in the sensitive genotype (Fig. 6c, d). Transcriptions of antioxidative enzyme genes were also up-regulated after drought treatment, but those were soon down-regulated by prolonged water deficit, particularly for Y34 (Fig. 6e–h). Following the re-watering, transcript levels of all tested genes were partially or fully restored to the control levels (Fig. 6).
Discussion
Two watermelon genotypes of contrasting drought-stress tolerance were used to characterize the morphophysiological changes that occur in response to progressive water restriction and subsequent re-watering. The wild watermelon M20 presented less leaf damage during and after drought stress than the domesticated watermelon Y34 (Fig. S3). Drought treatment had reduced the growth of both genotypes by the end of the experiment; however, the root to shoot ratios had increased (Fig. 1c–f). Plants will react to water deprivation by increasing the flow of assimilates to their roots, leading to an increased proportion of root mass (Bray 1997). Such a redistribution of photoassimilates is considered an efficient adaptive mechanism that enables the minimization of the evaporative canopy surface area (Diaz-Lopez et al. 2012) while improving water uptake from dry soil (Bray 1997). In this study, the biomass allocation was more distinct in the drought-stressed M20 plants than in the drought-stressed Y34 plants. The better phenotypic performance together with the allocation of a greater portion of the dry weight to the roots but less to the shoots suggests that the variety M20 exhibits a higher adaptive capacity to drought stress than the variety Y34. This was further demonstrated by the extent of the rate of water loss from the leaves, in that a large decline in RWC was observed in Y34, but a much lower decline was observed in M20 under the same SWA (Fig. 1a, b).
Leaves from M20 had greater numbers of trichomes than Y34 under both control and drought conditions (Fig. 2a). Similar findings have been reported in the chrysanthemum (Sun et al. 2013). Leaf trichomes are considered effective barriers to the diffusion of CO2 and H2O through their role in increasing the leaf boundary layer resistance (Guerfel et al. 2009), thus a thick trichome covering might act as a drought resistance feature to protect watermelon plants against excessive water loss. WRKY is a gene family which is highly induced by abiotic stresses. It has been reported that WRKY70 and WRKY54 cooperate as negative regulators of stomatal closure in response to osmotic stress in Arabidopsis (Li et al. 2013). In this study, the expression of watermelon WRKY70-like gene (homologous to AtWRKY70) was down-regulated by drought stress, and the transcription levels were lower in M20 than in Y34 over the whole treatment period (Fig. 6a). Seo et al. (2009) found that AtMYB96 mediated abscisic acid (ABA) signaling in drought stress response by reducing stomatal opening. Here, we observed that MYB96-like in both genotypes showed increased expression after drought treatment, but the rise in M20 was more sharp and earlier (Fig. 6b). The gene expression data correlates well with the observation that the M20 genotype exhibited smaller stomatal apertures and more closed stomata in response to drought stress, although the difference was not significant after day 2, as shown in Fig. 2c, d. These results suggest that the higher leaf water status in M20 plants is likely to be related to the greater trichome density and more sensitive stomatal control under drought stress.
Stomatal closure to minimise water loss would lead to the restriction of CO2 diffusion into the leaf photosynthetic parenchyma (Zhang et al. 2013). For this reason, the decline in G s was accompanied by notable decreases in T r and P n (Fig. 3a–c). In general, the drought-induced inhibition of photosynthesis is attributed to two factors, i.e., stomatal and non-stomatal limitation (Zhang et al. 2013); stomatal limitation is characterized by a daily maximum value of G s of greater than 0.05–0.10 mmol H2O m−2 s−1, whereas non-stomatal limitation is characterized by a value of G s of less than that threshold, as suggested by Flexas et al. (2009). In our results, during the initial stages of dehydration (to day 4), G s was ≥0.05 mmol H2O m−2 s−1 in both genotypes (Fig. 3b), and the photosynthetic machinery remained intact because F v/F m did not substantially change (Fig. 3e); thus, the reduction in P n was probably due to stomatal limitation alone. With more severe dehydration, the value of G s continued to decrease to below the threshold level by day 6. F v/F m was also significantly reduced from day 6 on until the end of the stress, suggesting that prolonged drought stress might inhibit photosynthesis through non-stomatal limitations in the watermelon genotypes. It has been claimed that non-stomatal limitations not only inhibit the activity of enzymes related to the Calvin cycle but also destroy the PSII system and adversely affect its efficiency (Sapeta et al. 2013). Consistent with this conclusion, our study showed that drought negatively affected initial Rubisco activity in both genotypes (Fig. S4a). Significant decreases in F v/F m, ΦPSII, ETR and qP were also observed in the genotypes, but the effects were more dramatic in the drought-sensitive variety (Fig. 3e–g), a finding that implies that the metabolic impairment of photosynthesis was more severe in Y34 than in M20. Thermal energy dissipation, as indicated by an increase in NPQ, is a typical response that protects the leaf photosynthetic apparatus from light-induced damage from drought stress (Sheng et al. 2008). In the present study, M20 showed higher increasement in NPQ than Y34 under severe water stress (Fig. 3h), thus dissipating heat at a much greater rate and thereby protecting its leaves better from photodamage. Chlorophyll content is closely related to P n. Under drought stress, the susceptible variety Y34 experienced an earlier and greater loss of leaf chlorophyll (Fig. S4b). PAO and PPH are two key genes in the pathway of chlorophyll breakdown that could be highly up-regulated under drought, osmotic stress, and in response to challenge by several pathogens (Hörtensteiner and Kräutler 2011). In this study, drought treatment induced higher transcripts of PAO and PPH genes in Y34 than in M20 (Fig. 6c, d), indicating that the greater loss of leaf chlorophyll in the susceptible variety might be a consequence of faster chlorophyll degradation.
The stomatal closure-induced lack of CO2 also promotes photo-oxidative stress in the chloroplasts, leading to the excessive generation of ROS (Posch and Bennett 2009). High ROS levels increase lipid peroxidation, thereby causing oxidative damage that can normally be seen by monitoring changes in MDA concentration (Wang et al. 2012). Our data showed that drought induced more serious oxidative damage in Y34 than in M20, as manifested by its much greater accumulation of MDA upon both water deprivation and rehydration (Fig. 4c). ROS detoxification is important for cellular survival; therefore, plants employ antioxidant defence systems to protect cells from oxidative damage (Gill and Tuteja 2010). This was also supported by our results that SOD, CAT, APX and GR activities were all positively regulated in watermelon seedlings to response to water deprivation; but severe water stress downregulated the elevations in the enzyme activities (Fig. 5). A possible explanation of these trends might be that the increased generation of ROS caused by the early drought stress stimulates the cellular protective enzymes to mitigate damage; however, as ROS production increases further under severe drought, beyond the control of the protective enzymes, ROS might interact with the enzymes, leading to their oxidation and inactivation (Sharma and Dubey 2005). In the control plants, the constitutive activities of the enzymes (except GR) analysed in this study did not significantly differ between the genotypes (Fig. 5). Nonetheless, in the treated plants, these four enzyme activities were all enhanced more markedly in the drought-tolerant genotype than in the drought-sensitive genotype. Moreover, expression levels and accumulation rates of their encoding genes (except APX) were also significantly higher in stressed M20 than in stressed Y34 (Fig. 6e–h). Higher increased enzyme activities under drought stress were associated with a more efficient antioxidant system response, assisting in providing tolerance against drought stress (Boaretto et al. 2014). This might explain why M20 was able to maintain relatively low levels of ROS and MDA induced by drought. The non-enzymatic antioxidants ascorbate and glutathione provide cellular protection by acting as substrates in the AsA–GSH cycle for maintaining the redox status of cells (Sharma and Dubey 2005). Drought induced an increase in the concentrations of AsA and GSH followed by a subsequent decline in both genotypes (Fig. S2a, b). According to Gill and Tuteja (2010) and Sharma and Dubey (2005), such a decline during the late stage of drought stress can be partially attributed to the inhibition of the regeneration ability; here, we found that the observed decrease in GR and APX activities was accompanied by an increasing shift in the cellular AsA and GSH pools toward their oxidized forms (Fig. 5c, d; Fig. S2). Therefore, we conclude that drought stress significantly affected ascorbate and glutathione redox homeostasis and that watermelon Y34 plants grown under drought were more susceptible than M20 plants. Under drought conditions, a significant increase in the total soluble sugar and proline content was observed in both genotypes and higher concentrations accumulated in M20 (Fig. S5a). The increase in soluble sugar and proline content was considered a typical response to water stress in the attempt of plants to quench ROS (Moustakas et al. 2011), stabilize membranes and maintain turgor at low leaf water potential (Ramachandra Reddy et al. 2004).
In conclusion, drought stress caused wilting and leaf chlorosis in watermelon plants and inhibited photosynthesis, finally decreasing growth. The higher tolerance of M20 to water deficit was associated with a greater dry mass allocation to the roots, a greater trichome density and more sensitive stomatal control. Coupled with a better ability to regulate the energy bifurcation between photochemical and non-photochemical events, M20 also exhibited a more efficient antioxidant response and osmoregulation to withstand drought stress. All of these adaptive mechanisms contributed to the rapid recovery of this variety after re-watering. Thus, we consider this wild watermelon genotype very promising for use in the improvement of domesticated watermelons. Further work is encouraged to identify the candidate genes associated with drought tolerance in wild watermelon M20.
Abbreviations
- P n :
-
Photosynthetic rate
- RWC:
-
Relative water content
- SWA:
-
Soil water availability
- C i :
-
Intercellular CO2 concentration
- T r :
-
Transpiration rate
- G s :
-
Stomatal conductance
- qP:
-
Photochemical quenching
- NPQ:
-
Nonphotochemical quenching
- F v/F m :
-
Maximum photochemical efficiency of PSII
- ΦPSII:
-
Actual photochemical efficiency of PSII
- ETR:
-
Electron transport rate
- H2O2 :
-
Hydrogen peroxide
- O2 − :
-
Superoxide anion radical
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismustase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- GR:
-
Glutathione reductase
- AsA:
-
Ascorbate
- DHA:
-
Dehydroascorbate
- GSH:
-
Glutathione
- GSSG:
-
Oxidized glutathione
- PAO:
-
Pheide a oxygenase
- PPH:
-
Pheophytin pheophorbide hydrolase
References
Bai T, Li C, Ma F, Feng F, Shu H (2010) Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil 327(1–2):95–105. doi:10.1007/s11104-009-0034-x
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15(3):413–428
Boaretto LF, Carvalho G, Borgo L, Creste S, Landell MG, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–175
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2(2):48–54. doi:10.1016/s1360-1385(97)82562-9
de Souza TC, Magalhães PC, de Castro EM, Carneiro NP, Padilha FA, Júnior CCG (2014) ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul 73(3):205–217
Diaz-Lopez L, Gimeno V, Simon I, Martinez V, Rodriguez-Ortega WM, Garcia-Sanchez F (2012) Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agric Water Manag 105:48–56. doi:10.1016/j.agwat.2012.01.001
Du K, Xu L, Wu H, Tu B, Zheng B (2012) Ecophysiological and morphological adaption to soil flooding of two poplar clones differing in flood-tolerance. Flora 207(2):96–106
FAO of the United Nations (2015) Food and agricultural commodities production. FAO of the United Nations. http://faostat3.fao.org/download/Q/QC/E.2013
Flexas J, Barón M, Bota J, Ducruet J-M, Gallé A, Galmés J, Jiménez M, Pou A, Ribas-Carbó M, Sajnani C (2009) Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J Exp Bot 60(8):2361–2377
Gao J (2000) Experimental techniques of plant physiology. World Publishing Corporation, Xi’an
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Guerfel M, Baccouri O, Boujnah D, Chaibi W, Zarrouk M (2009) Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci Hortic (Amsterdam) 119(3):257–263. doi:10.1016/j.scienta.2008.08.006
Guo W, Chen R, Gong Z, Yin Y, Ahmedand S, He Y (2012) Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet Mol Res 11(4):4063–4080
Hörtensteiner S, Kräutler B (2011) Chlorophyll breakdown in higher plants. BBA Bioenergetics 1807(8):977–988
Hu LX, Wang ZL, Huang BR (2010) Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C-3 perennial grass species. Physiol Plant 139(1):93–106. doi:10.1111/j.1399-3054.2010.01350.x
Hunsche M, Bürling K, Saied AS, Schmitz-Eiberger M, Sohail M, Gebauer J, Noga G, Buerkert A (2010) Effects of NaCl on surface properties, chlorophyll fluorescence and light remission, and cellular compounds of Grewia tenax (Forssk.) Fiori and Tamarindus indica L. leaves. Plant Growth Regul 61(3):253–263 (211)
Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R (2009) Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol 11(1):100–105
Kawasaki S, Miyake C, Kohchi T, Fujii S, Uchida M, Yokota A (2000) Responses of wild watermelon to drought stress: accumulation of an ArgE homologue and citrulline in leaves during water deficits. Plant Cell Physiol 41(7):864–873
Kong Q, Yuan J, Gao L, Zhao S, Jiang W, Huang Y, Bie Z (2014) Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS One 9(2):e90612
Li J, Besseau S, Törönen P, Sipari N, Kollist H, Holm L, Palva ET (2013) Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol 200(2):457–472
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Liu B, Li M, Cheng L, Liang D, Zou Y, Ma F (2012) Influence of rootstock on antioxidant system in leaves and roots of young apple trees in response to drought stress. Plant Growth Regul 67(3):247–256
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Logan BA, Grace SC, Adams WW III, Demmig-Adams B (1998) Seasonal differences in xanthophyll cycle characteristics and antioxidants in Mahonia repens growing in different light environments. Oecologia 116(1–2):9–17
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668. doi:10.1093/jexbot/51.345.659
Moustakas M, Sperdouli I, Kouna T, Antonopoulou C-I, Therios I (2011) Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul 65(2):315–325. doi:10.1007/s10725-011-9604-z
Posch S, Bennett LT (2009) Photosynthesis, photochemistry and antioxidative defence in response to two drought severities and with re-watering in Allocasuarina luehmannii. Plant Biol 11:83–93. doi:10.1111/j.1438-8677.2009.00245.x
Ramachandra Reddy A, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161(11):1189–1202
Sanda S, Yoshida K, Kuwano M, Kawamura T, Munekage YN, Akashi K, Yokota A (2011) Responses of the photosynthetic electron transport system to excess light energy caused by water deficit in wild watermelon. Physiol Plant 142(3):247–264
Sapeta H, Miguel Costa J, Lourenco T, Maroco J, van der Linde P, Margarida Oliveira M (2013) Drought stress response in Jatropha curcas: growth and physiology. Environ Exp Bot 85:76–84. doi:10.1016/j.envexpbot.2012.08.012
Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM (2009) The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol 151(1):275–289
Sharma P, Dubey R (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46(3):209–221. doi:10.1007/s10725-005-0002-2
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18(6–7):287–296. doi:10.1007/s00572-008-0180-7
Si Y, Zhang C, Meng S, Dane F (2009) Gene expression changes in response to drought stress in Citrullus colocynthis. Plant Cell Rep 28(6):997–1009. doi:10.1007/s00299-009-0703-5
Sun J, Gu J, Zeng J, Han S, Song A, Chen F, Fang W, Jiang J, Chen S (2013) Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci Hortic (Amsterdam) 161:249–258. doi:10.1016/j.scienta.2013.07.015
Talbi S, Romero-Puertas MC, Hernandez A, Terron L, Ferchichi A, Sandalio LM (2015) Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defences. Environ Exp Bot 111:114–126. doi:10.1016/j.envexpbot.2014.11.004
Wang S, Liang D, Li C, Hao Y, Ma F, Shu H (2012) Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol Biochem 51:81–89. doi:10.1016/j.plaphy.2011.10.014
Wu S, Liang D, Ma F (2014) Leaf micromorphology and sugar may contribute to differences in drought tolerance for two apple cultivars. Plant Physiol Biochem 80:249–258
Zhang H, Gong G, Guo S, Ren Y, Xu Y, Ling K-S (2011) Screening the USDA watermelon germplasm collection for drought tolerance at the seedling stage. HortScience 46(9):1245–1248
Zhang L, Zhang L, Sun J, Zhang Z, Ren H, Sui X (2013) Rubisco gene expression and photosynthetic characteristics of cucumber seedlings in response to water deficit. Sci Hortic (Amsterdam) 161:81–87. doi:10.1016/j.scienta.2013.06.029
Zhang M, Jin ZQ, Zhao J, Zhang G, Wu F (2015) Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul 75(2):567–574
Acknowledgments
This study was supported by the Modern Agro-industry Technology Research System of China (CARS-26-18), and Shaanxi Provincial Science and Technology Research and Development Project Fund, China (No. 2015NY091). The authors are grateful to Joshua M. and Mike F. for help in revising the use of English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanling Mo and Ruiping Yang have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mo, Y., Yang, R., Liu, L. et al. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul 79, 229–241 (2016). https://doi.org/10.1007/s10725-015-0128-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0128-9