Abstract
Despite the plethora of published reports on ameliorative effects of exogenously applied salicylic acid (SA) to plants under salt stress, a critical role of SA in redox balance, photosynthetic and electron transport in mediating salt tolerance in plants is still ignored. The present study was aimed to assess the beneficial effects of SA on photosynthetic electron transport in five radish cultivars during salt stress which may translate into protection from salt-induced oxidative damage. Seeds of five radish cultivars (Red Neck Purple, Early Long White, Minnu Radish, Radish 40-day and Gong Swang Radish) were allowed to germinate for five days after which seedlings were sprayed with different concentrations of SA (0, 2 and 5 mM). After 48 h of SA treatment, seedlings were subjected to salt stress (0, 100, and 200 mM NaCl). Salt stress cause reduction in biomass, chlorophyll contents and PSII photochemistry in all five radish cultivars. However, salt stress causes an increase in proline content, lipid peroxidation, ROS content and antioxidant enzymatic activity. Foliar spray of SA downregulated the absorption, trapping and photosynthetic electron transport fluxes while it increased dissipation flux consequently improving photochemistry of most of the radish cultivars under salt stress. This Improved photochemistry and enhanced antioxidant enzymes activity of radish cultivars by SA application resulted in lower ROS generation and membrane damage. Greater accumulation of compatible solutes due to foliar spray of SA might have additional protective effects on photosynthetic machinery by lowering oxidative stress. Moreover, ameliorative effects of SA were cultivar specific. The study suggested that SA could mitigate the detrimental effects of salt stress by regulating physiological and biochemical mechanisms in radish plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the most devastating environmental stresses that reduce crop productivity. Salt stress reduces plant growth by osmotic effect and ionic effect that disturb ion homeostasis (Athar and Ashraf 2009; Zafar et al. 2017). A number of studies suggested that growth yield reduction under saline conditions were associated with secondary effects of salt stress such as oxidative stress, reduction in photosynthesis (Athar et al. 2015; Munns and Tester 2008). Salt-induced osmotic stress caused biosynthesis of ABA and subsequently causes stomatal closure (Munns 2002). At high salt stress, salt may accumulate in appoplast or in cytoplasm or in chloroplast and thus directly affects photosynthetic metabolism (Ben-Rouina et al. 2006; Bose et al. 2017). Similarly, salt stress cause imbalance in generation and consumption of reducing equivalents (NADPH and ATPs) in chloroplast during chloroplastic electron transport and in Calvin Cycle, respectively (Ogbaga et al. 2018). This results in production of reactive oxygen species (ROS) at acceptor end of PSI thereby causing membrane damage (Foyer and Shigeoka 2011; Foyer et al. 2017). In order to protect plant cellular machinery, antioxidative defense system becomes activated to scavenge ROS. Some studies suggested that enhancing non-enzymatic antioxidants or up-regulation of antioxidants enzymes may protect the plants from salt-induced-oxidative damage or improved salt tolerance in different crop species such as in Triticum aestivum (Arfan et al. 2007; Athar et al. 2008), Torreya grandis (Li et al. 2014), and Vigna radiata (Khan et al. 2014). Exogenous application of different antioxidants compounds can enhance growth of plants under salt stress by increasing antioxidant enzymatic activities, accumulation of osmolytes and improving plant photosynthetic capacity (Ashraf et al. 2010; Plaut et al. 2013).
Salicylic acid is an important plant growth regulator that modulates plant growth and development under normal or abiotic stress conditions by affecting stomatal conductance, activities of antioxidant enzymes, and nutrient uptake (Ashraf et al. 2010; Noreen et al. 2013). In addition, SA is one of the important signaling molecules which trigger variety of biochemical pathways responsible to counteract abiotic stresses including salt stress. In contrast, some reports are also available in which no positive association was found between activities of antioxidant enzymes and degree of salt tolerance (Munns and Tester 2008). For example, mutants of Arabidopsis thaliana lacking functional chloroplastic and cytosolic antioxidant enzymes were more salt tolerant than wild type plants which showed greater antioxidant potential (Miller et al. 2007). It is suggested that salt tolerance of a crop species may depends on interactive effect of stomatal conduction for CO2 fixation and photosynthetic electron transport to avoid photoinhibition which leads to alter in activities of antioxidants. This aspect is generally ignored in most of the studies, particularly when assessing SA-induced salt tolerance in plants.
Radish (Raphanus sativus L.), belongs to Brassicaceae family, is moderately salt sensitive or moderately salt tolerant root vegetable (Sonneveld 2000). The present study was aimed to assess as to whether SA could efficiently alleviate the detrimental effects of salt stress on Raphanus sativus L. and up to what extent exogenously applied SA has a role in redox balance, photosynthetic electron transport, and ion transport in mediating salt tolerance.
Materials and Methods
Plant Material and Growth Conditions
A pot experiment involving five cultivars of Raphanus sativus L. [Red Neck Purple (RNP), Early Long White (ELW), Minnu Radish (MR), 40 Day Radish (40-D), and Gong Swang Radish (GSR)] was conducted at the Institute of Molecular Biology and Biotechnology, Bahauddin Zakariya University, Multan, under the following controlled conditions: Day/Night temperature: 22/18 ± 2 °C, photoperiod: 16 h and humidity: 55–60%. Briefly, plastic pots were filled with sand and four to five seeds/pot were sown and watered with Hoagland nutrient solution. Three days after germination, two same sized plants were kept in each pot after thinning. At 5th day, SA 0 mM (mock spray with distilled water only), 2 and 5 mM (SA was dissolved initially in 70% ethanol and the concentrations of 2 and 5 mM were made up with distilled water along with 0.01% Tween-20) was applied exogenously by foliar spray. Salt treatment was started post 48 h of SA spray. Two NaCl treatments 100 and 200 mM were given for 10 days. Treatment was started from 50 mM and increased gradually to reach the desired concentration. After 24 h of last salt treatment, samples were collected for further analysis. Plants were carefully uprooted from pots and were separated into roots and shoots. Fresh weight of roots and leaves were evaluated on digital scale while length of roots was recorded by using measuring scale. For dry weight, samples were oven dried at 65 °C for 15 days.
Photosynthetic Components
PSII Photochemistry
The maximal efficiency of PSII photochemistry was monitored by fast chlorophyll a fluorescence kinetic analysis using Flour-Pen-FP-100 (PSI, CZ). The plants were placed in dark for 30 min at the room temperature and the light intensity of 3000 µmol m−2 s−1 was implicated on the leaf area of 5 mm. The initial fluorescence was observed at O-step (50 µs) then on intermediate steps as K, J, I (300 µs, 2 ms, 20 ms) and the maximum fluorescence was observed at P-step at 300 ms respectively. Parameters that were calculated: Fv/Fm = (Fm − Fo)/Fm, PIABS = (RC/ABS) [φPo/(1 − φPo)] [ψEo/(1 − ψEo)], ABS/RC = Mo (1/VJ) (1/φPo), DIo/RC = (ABS/RC) − (TRo/RC), ETo/RC = Mo (1/VJ) ψo, and TR/RC = Mo (1/VJ; Cessna et al. 2010).
Chlorophyll and Carotenoid Contents Estimation
Chlorophyll and carotenoids contents were measured using the protocol of Arnon (1949). Briefly, 0.2 g fresh leaves were grinded in 10 ml of 80% pure acetone. The homogenate was filtered and centrifuged for 15 min at 14,000 rpm, 4 °C. The optical densities of Chlorophyll a, b, and carotenoids were measured using spectrophotometer at 645 nm, 663 nm, and 480 nm respectively.
W (weight of fresh leaves in g), V (volume of the extract).
Anthocyanin Content Determination
Anthocyanin contents were determined following the protocol of Wagner (1979). Briefly, 0.2 g of fresh leaves were homogenized in pestle mortar with 3 ml solution of 1% methanol:HCl (99:1 v/v). The homogenate was covered with aluminum foil and was placed in dark at 4 °C for 24 h. It was then centrifuged at 3000 rpm for 6 min. The concentration of anthocyanin in the supernatant was quantified by optical density at 550 nm. Anthocyanin concentration was evaluated using molar extinction coefficient 33,000 cm−2 mol−6.
Proline Estimation
The accumulation of compatible osmolyte proline was determined by using protocol described by Bates et al. (1973). 0.1 g fresh leaf samples were homogenized in 3% sulfosalicylic acid (5 ml) and were filtered using filter paper (Whatman’s No. 2). Equal volume of glacial acetic acid and acid ninhydrin were added in 2 ml of filtrate. The reaction mixture was placed in water at 100 °C for 1 h and then was placed in an ice box to cool down. An addition of toluene (2 ml) was made in the mixture and stirred for 15–20 s. The chromophore layer which contains toluene was isolated and the absorbance was measured at 520 nm taking toluene as blank. The concentration of proline was assessed using proline standards (10 mg/100 ml). The following formula was used for estimation of proline:
µmoles per g tissue = [(µg proline/ml × ml toluene)/115.5)] × 5/g sample.
Lipid Peroxidation Estimation
Salinity induced oxidative damage to membrane, also known as lipid peroxidation, was assessed by Cakmak and Horst (1991) protocol with slight modifications. 0.2 g fresh leaves were homogenized in pestle mortar with 0.1% (w/v) trichloroacetic acid (TCA) solution (1 ml) and then centrifuged for 15 min at 2000×g. 0.5% thiobarbituric acid (TBA) was prepared in 20% TCA solution. 1 ml of TBA was mixed in 0.5 ml of supernatant. This reaction mixture was heated in shaking water bath at 95 °C for 50 min and then stopped by cooling the tubes in ice box and was centrifuged for 10 min at 12,000×g. Optical density of supernatant was measured at 532 and 600 nm. Lipid peroxidation was evaluated using 156 mmol−1 cm−1 (molar extinction coefficient) with the help of following formula:
ROS Estimation
Reactive oxygen species (ROS) in plant tissues are produced under normal oxygen metabolism. However, dramatic increase in ROS level is observed under stress conditions which can be measured by assessing the amount of hydrogen peroxide (H2O2) using protocol of Velikova et al. (2000). 0.2 g fresh leaves were grinded in 0.1% (w/v) TCA solution (2 ml) using pre-chilled pestle mortar and this mixture was centrifuged for 15 min at 9000 rpm. Then, potassium iodide (KI; 0.2 ml) and potassium phosphate buffer of pH 7.0 (0.1 ml) were added in 0.1 ml of supernatant. This mixture was vortexed for 1 min and its absorbance was taken at 390 nm. The concentration of H2O2 was interpreted using a standard curve by known concentration of H2O2.
Enzymatic Antioxidants Estimation
Enzymatic antioxidants were done by using protocol of Ananieva et al. (2004). 0.2 g fresh leaves were grinded in pestle mortar using 50 mM pre-chilled potassium phosphate buffer of pH 7.8 (2 ml). The homogenate was centrifuged at 4 °C and 15,000×g for 20 min. The resulting supernatant was further used for assays. The activity of SOD was assessed using the protocol of Giannopolitis and Ries (1977). Reaction mixture contains: 4 ml distilled water, 2.5 ml of 50 mM potassium phosphate buffer pH 7.8, 1 ml of 13 mM L-methionine, 1 ml of 0.1% (v/v) triton-X-100, 0.5 ml of 1.3 mM riboflavin, 0.5 ml of 50 mM NBT, and 0.5 ml of enzyme extract. The mixture was placed under florescent light of 30 W for 15 min and reaction was stopped by turning off the lights for 10 min. The optical density of this irradiated mixture was determined at 560 nm taking reaction mixture without enzyme extract as a blank. The activity of SOD was assessed by using the standard curve made by known concentrations of NBT. The activity of catalase enzyme was evaluated using the protocol of Chance and Maehly (1955) with few modifications. Reaction mixture of CAT contained: 450 µl of 5.9 mM H2O2, 1 ml of 50 mM potassium phosphate buffer (pH 7.0), and 50 µl of enzyme extract (added at the time of measuring OD). H2O2 decomposition changes the OD value of mixture and measured at 240 nm after every 20 s up to 1 min. The catalase activity was assessed by using molar extinction coefficient of H2O2 which is 36 M−1 cm−1 expressed in µmol min−1. 1 unit activity of catalase is defined as the change in the absorbance of 0.01 units min−1. The activity of peroxidase enzyme was assessed by oxidation of guaiacol defined as the change in absorbance of 0.01 units per min using protocol of Chance and Maehly (1955). Reaction mixture contained: 200 µl of 20 mM guaiacol, 250 µl of 40 mM H2O2, 1 ml of 50 mM potassium phosphate, and 50 µl of enzyme extract. The absorbance of reaction mixture was taken at 470 nm after every 20 s up to 2 min. The activity of POD was evaluated by using 26.6 mM−1 cm−1 (molar extinction coefficient) expressed in µmol/min.
Statistical Analysis
All the data were statistically analyzed to determine significant variation between different factors using Statistical Software, COSTATv.6.3 (Cohort Software, Berkley, California). Means comparison was done by least significant difference (LSD) test. The statistical analysis was performed on raw measurement values, not on the fold change values.
Results
Physiological Parameters
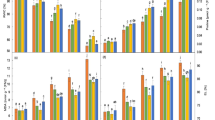
Salt stress significantly decreased the fresh and dry weights of leaves and roots of all five radish cultivars and exogenous treatment of SA significantly improved the biomass of leaves and roots under saline and non-saline conditions (Table 1, Figs. 1a–d, 2, Supplementary Table 1). In addition, application of 2 mM SA was more effective in improving these growth attributes of all radish cultivars under salt stress, particularly at 200 mM NaCl. This is also confirmed from the graphs of fold change for fresh and dry weights of leaves and roots (Fig. 1a–d). The fold change values for 2 or 5 mM SA and 100 or 200 mM NaCl are calculated as: [(2 or 5 mM SA)/(Control)] and [(100 or 200 mM NaCl)/(Control)] respectively, whereas all the fold change values for 2 or 5 mM SA at 100 mM NaCl are calculated as: [(2 or 5 mM SA at 100 mM NaCl)]/(100 mM NaCl) and for 2 or 5 mM SA at 200 mM NaCl are calculated as: [(2 or 5 mM SA at 200 mM NaCl)]/(200 mM NaCl). The fold change values for control (0 mM NaCl, 0 mM SA) is taken as 1.00. The response of each cultivar was significantly different to SA application under salt stress. From Fig. 1a–d, it is apparent that 2 mM SA application particularly at highest salt treatment (200 mM NaCl) had maximum increasing effect on fresh and dry weights of leaves of cv. GSR (2.80-fold) and cv. MR (2.89-fold), respectively. Similarly, the exogenous application of 2 mM SA at 200 mM NaCl increased fresh and dry weights of roots of cv. MR (5.12-fold) and cv. ELW (3.67-fold), respectively. Salt stress also reduced root lengths of all five radish cultivars and SA application particularly 2 mM significantly increased the length of roots (Table 1, Fig. 1e, Supplementary Table 1). However, no varietal difference was observed among cultivars. Overall, 2 mM SA was found more effective to protect from the damaging effect of higher salt concentration (200 mM) for plant biomass attributes and prominent cultivar differences were observed expect for root length.
Effect of Salicylic acid on biomass and proline content of five radish cultivars grown under varying concentrations of salt stress. a Fold change for fresh weight of leaves, b fold change for fresh weight of roots, c fold change for dry weight of leaves, d fold change for dry weight of roots, e fold change for length of roots, f fold change for proline. Fold change (FC) values for 2 or 5 mM SA and 100 or 200 mM NaCl are calculated as: [(2 or 5 mM SA)/(control)] and [(100 or 200 mM NaCl)/(control)] respectively, whereas FC values for 2 or 5 mM SA at 100 mM NaCl are calculated as: [(2 or 5 mM SA at 100 mM NaCl)]/(100 mM NaCl) and for 2 or 5 mM SA at 200 mM NaCl are calculated as: [(2 or 5 mM SA at 200 mM NaCl)]/(200 mM NaCl). FC value for control (0 mM NaCl, 0 mM SA) is taken as 1.00
Representative plants of different radish cultivars grown under varying concentrations of salt stress and salicylic acid. a 0 mM SA, b 100 mM NaCl, c 200 mM NaCl, d 2 mM SA, e 2 mM SA plus 100 mM NaCl, f 2 mM SA plus 200 mM NaCl, g 5 mM SA, h 5 mM SA plus 100 mM NaCl, i 5 mM SA plus 200 mM NaCl. For each treatment, the representative plants belong to different cultivars and are placed randomly for this figure panel
Biochemical Parameters
Chlorophyll a, chlorophyll b, total chlorophyll and carotenoids contents were significantly decreased due to salt stress in all cultivars. Foliar application of SA, particularly 2 mM, significantly enhanced these photosynthetic pigments in the leaves of all five radish cultivars under saline and non-saline conditions (Table 1, Fig. 3a–c, e, Supplementary Table 2). From Fig. 3a–c and e, it is apparent that the improving effect of 2 mM SA at 200 mM NaCl on chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents was maximum in cv. ELW (2.26-fold), cv. RNP (2.07-fold), cv. RNP (2.17-fold), and cv. ELW (3.08-fold), respectively. However, effect of SA application in terms of carotenoids/chlorophyll ratio was non-significant under non-saline conditions (Table 1, Fig. 3f, Supplementary Table 2). In contrast, anthocyanin pigments increased with salt stress while SA treatment decreased anthocyanin pigments under non-saline conditions; however, a significant increase was observed in anthocyanin pigments in all radish cultivars, particularly with 5 mM SA application at 100 mM NaCl (Table 1, Fig. 5c, Supplementary Table 2). The response of each cultivar was similar to SA application under salt stress.
Effect of Salicylic acid on photosynthetic pigments of five radish cultivars grown under varying concentrations of salt stress. a Fold change for Chl a, b fold change for Chl b, c fold change for total Chl, d Fold change for Chl a/b, e fold change for carotenoids, f fold change for carotenoids/total chl. Fold change (FC) values for 2 or 5 mM SA and 100 or 200 mM NaCl are calculated as: [(2 or 5 mM SA)/(Control)] and [(100 or 200 mM NaCl)/(Control)] respectively, whereas FC values for 2 or 5 mM SA at 100 mM NaCl are calculated as: [(2 or 5 mM SA at 100 mM NaCl)]/(100 mM NaCl) and for 2 or 5 mM SA at 200 mM NaCl are calculated as: [(2 or 5 mM SA at 200 mM NaCl)]/(200 mM NaCl). FC value for control (0 mM NaCl, 0 mM SA) is taken as 1.00
Salt-induced reduction in maximum quantum yield of photosystem II (Fv/Fm) and performance index (PIABS) of different radish cultivars was alleviated by exogenously applied SA (2 & 5 mM SA) under saline and non-saline conditions (Table 1, Fig. 4a, b, Supplementary Table 3). The more pronounced effect was observed by 2 mM SA at highest salt stress (200 mM NaCl). Foliar spray of 2 mM SA at 200 mM NaCl had maximum increasing effect on quantum yield of PSII and PIABS of cv. ELW (1.14-fold) and cv. GSR (4.57-fold), respectively (Fig. 4a, b). The light absorption flux per reaction center (ABS/RC), trapping energy flux per reaction center (TRo/RC) and energy flux for heat dissipation per reaction center (DIo/RC) increased significantly in all radish cultivars with increasing NaCl concentration, whereas energy flux for electron transport further than QA (ETo/RC) decreased with salt stress. However, exogenous foliar spray of SA specifically 2 mM SA significantly decreased the ABS/RC, TRo/RC and DIo/RC, while it increased ETo/RC in salt stress plants of radish cultivars (Table 1, Fig. 4c–f, Supplementary Table 3). The response of radish cultivars to SA treatment under saline condition was significantly different. For example, exogenous application of 2 Mm SA at 200 mM NaCl caused a greater decrease in ABS/RC, TRo/RC and DIo/RC of cv. 40-D (0.65-fold), cv. 40-D (0.73-fold), and cv. ELW (0.40-fold), respectively and the maximum increase in ETo/RC was found in cv. GSR (1.74-fold; Fig. 4c–f).
Effect of Salicylic acid on photochemistry of PSII of five radish cultivars grown under varying concentrations of salt stress. a Fold change for Fv/Fm, b fold change for Pi_Abs, c fold change for ABS/RC, d fold change for TRo/RC, e fold change for ETo/RC, f fold change for DIo/RC. Fold change (FC) values for 2 or 5 mM SA and 100 or 200 mM NaCl are calculated as: [(2 or 5 mM SA)/(Control)] and [(100 or 200 mM NaCl)/(Control)] respectively, whereas FC values for 2 or 5 mM SA at 100 mM NaCl are calculated as: [(2 or 5 mM SA at 100 mM NaCl)]/(100 mM NaCl) and for 2 or 5 mM SA at 200 mM NaCl are calculated as: [(2 or 5 mM SA at 200 mM NaCl)]/(200 mM NaCl). FC value for control (0 mM NaCl, 0 mM SA) is taken as 1.00
The results of present work showed that salt stress, SA, and salt stress plus SA treatments caused significant leaf proline accumulation in the leaves of different radish cultivars. However, higher accumulation was observed in plants treated with 5 mM SA. Exogenous application of 2 & 5 mM SA caused maximum accumulation of proline in salt stressed plants of cv. GSR whereas the reverse was true for cv. MR (Table 1, Fig. 1f, Supplementary Table 4).
Salt stress increased the accumulation of H2O2 and MDA contents in leaves of radish cultivars that was decreased by exogenous treatment of SA (2 & 5 mM) in salt stressed and non-salt stressed radish cultivars under investigation (Table 1, Fig. 5a, b, Supplementary Table 4). The foliar spray of 2 mM SA had greater reduction of leaf MDA contents at both salt concentrations (100 & 200 mM NaCl) in a cultivar dependent manner. For example, 100 mM NaCl, cv. RNP (0.37-fold) and cv. 40-D (0.37-fold), had maximum reduction while at 200 mM NaCl cv. MR (0.35-fold) had maximum decrease in MDA content as compared to other cultivars. For leaf H2O2, the response of each cultivar was significantly different to SA treatment under non-saline condition; however, in saline conditions the interactive term (Salt * Var * SA) was not significant.
Effect of Salicylic acid on lipid peroxidation, ROS, anthocyanin and enzymatic antioxidants of five radish cultivars grown under varying concentrations of salt stress. a Fold change for MDA, b fold change for H2O2, c fold change for anthocyanin, d fold change for CAT, e fold change for POD, f fold change for SOD. Fold change (FC) values for 2 or 5 mM SA and 100 or 200 mM NaCl are calculated as: [(2 or 5 mM SA)/(control)] and [(100 or 200 mM NaCl)/(control)] respectively, whereas FC values for 2 or 5 mM SA at 100 mM NaCl are calculated as: [(2 or 5 mM SA at 100 mM NaCl)]/(100 mM NaCl) and for 2 or 5 mM SA at 200 mM NaCl are calculated as: [(2 or 5 mM SA at 200 mM NaCl)]/(200 mM NaCl). FC value for control (0 mM NaCl, 0 mM SA) is taken as 1.00
Antioxidant Enzymes
The activities of SOD and CAT remained unchanged due to salt stress while the application of SA increased their activities significantly under saline and non-saline conditions (Table 1, Fig. 5d, f, Supplementary Table 4). All the radish cultivars show significantly different behavior due to SA application under salt stress. The treatment of 2 mM SA at 100 mM NaCl showed maximum activities of SOD and CAT in the leaves of cv. GSR (1.61-fold) and cv. RNP (1.48-fold), respectively while at 200 mM NaCl cv. GSR (1.23-fold) and cv. MR (1.14-fold) showed greater SOD and CAT activities, respectively (Fig. 5d, f). In different radish varieties, POD activity significantly increased upon salinity treatment as well as due to exogenous SA application under saline and non-saline conditions (Table 1, Fig. 5e, Supplementary Table 4). The increasing effect of 2 mM SA at 100 & 200 mM NaCl on activities of POD was maximal in cv. RNP (1.40-fold) and cv. MR (1.26-fold), respectively (Fig. 5e).
Discussion
Plant growth regulators and signaling molecules like salicylic acid have the potential to overcome the deleterious effects of abiotic stresses on plants. In the present study, exogenous application of SA had ameliorative effect on all five radish cultivars. However, lower concentration of SA application was more effective as compared to higher concentration of SA (5 mM SA). The effectiveness of low concentration of SA was also reported in Brassica juncea (Fariduddin et al. 2003) and Triticum aestivum (Hayat et al. 2005). In addition, growth promoting effect of SA depends on type of cultivar and degree of salt stress. For example, at 100 mM NaCl salt stress, SA application caused maximal increase in leaf dry weight in cultivar RNP, whereas the least growth improvement was found in radish cultivar ELW. However, at 200 mM NaCl salt stress, SA application caused growth improvement in cv MR followed by cv. GSR. Similar genotypic differences in growth due to SA application under saline conditions has already been observed in different crop species such as in Triticum aestivum (Arfan et al. 2007), Helianthus annuuas L. (Noreen and Ashraf 2008), Zea mays L. (Khodary 2004; Gunes et al. 2007). SA-induced increase in biomass (Table 1, Fig. 1a–e, Supplementary Table 1) of salt affected plants might have been due to the enhanced photosynthetic capacity, and/or reduction oxidative stress (Khan 2003; Fariduddin et al. 2003; Szepesi et al. 2005).
In salt stressed plants of radish cultivars, the chlorophyll (a, b & total chlorophyll; Table 1, Fig. 3a–c, Supplementary Table 2) and carotenoids contents (Table 1, Fig. 3e, Supplementary Table 2) decreased with increasing salinity level. This decrease in chlorophyll might be due to reduction in the biosynthesis of chlorophyll. For example, while working with Penniseturm typhoides Reddy and Vora (1986), showed that salt stress destabilized protein complexes involved in Chl biosynthesis. In addition, salt stress increased the activity of chlorophyllase enzyme. In another study, it has been reported that salt stress caused degradation of the chlorophyll a/b binding proteins in light harvesting complex II (Levitt 1980).
Photosynthesis is one of the premier physiological processes that govern the plant growth and productivity. Plant photosynthetic capacity can be measured through CO2 fixation rate or functional activity of photosystem II (PSII). It has been documented that reduction in photosynthetic capacity due to salt stress is due to structural and functional damages to photosystems and electron carriers. However, structural damages to photosystems occur at high salt stress or for a longer period of stress such as exposure of plants to 150–200 mM NaCl salt stress for more than three weeks or 300–400 mM NaCl for 10–15 days (Kalaji et al. 2016). In the present study, the salt stress significantly decreased the quantum yield of PSII and performance index (PI) in all cultivars radish while the application of SA increased quantum yield of PSII and PI in all radish cultivars under saline conditions (Table 1, Fig. 4a, b, Supplementary Table 3). A positive correlation between PI and active reaction center density, quantum yield of electron transport and primary photochemistry was recorded. Therefore, exogenous application of SA improved the photosynthetic activity of salt stressed plants of radish cultivars by stabilizing PSII and electron transport. Previous studies suggested that SA treatment can protect the function of PSII by increasing ΦPSII under environmental stresses (Shi et al. 2006; Khalid et al. 2015). It is well established that changes in quantum yield of PSII are associated either with inhibition in electron transport from donor side (damage to oxygen evolving complex OEC) or reduction in pool of oxidize QA− (Kalaji et al. 2016). In the present study, salt stress increased the ABS/RC and TRo/RC along with reduction ETo/RC in all radish cultivars which indicated that effective antenna size become increased due to inactivation of reaction centers (Table 1, Fig. 4c–e, Supplementary Table 3). Moreover, this result in over-reduction of QA− and efficiency of electron transfer from QA− to QB also reduced due to salt stress. Thus, dissipation of absorbed energy as heat (DIo/RC) might have increased by active reaction centers which results in overall lowering in PIABS. However, the adverse effect of salt stress on donor end of PSII was greater than that on acceptor end of PSII as has earlier been observed in wheat plants (Mehta et al. 2010). These results can be explained in view of the argument of Zhang and Xing (2008) who reported that salt-induced osmotic stress affected antenna size heterogeneity with no change in acceptor end of PSII, while salt-induced ionic stress affected both donor and acceptor ends of PSII. Improvement in quantum yield of PSII and PIABS due to SA application might have been due to increase in density of active reactive centers per antenna chlorophylls thereby lowering in ABS/RC, TR/RC. These results are in parallel with those of Ghassemi-Golezani and Lotfi (2015) who found that exogenous application of SA enhanced the quantum yield of PSII and electron transfer efficiency in mung bean plants under salt stress. From these reports and results from the present study, it can be inferred that salt stress not only reduced the photosynthetic pigments in antenna, but it also disturb the conformation of antenna pigment assemblies that resulted in efficiency of PSII. This is further supported by the fact that salt stress increased the MDA in all radish cultivars and exogenous application of SA lowered the MDA contents along with improved the chlorophyll contents and carotenoids in salt stressed plants of radish cultivars. Exogenous application of SA substantially enhanced the photosynthetic rate in wheat (Arfan et al. 2007) and cotton (Noreen et al. 2013), which may be associated with higher CO2 assimilation rate, pigment level or activities of antioxidants. In contrast, application of high concentration of SA reduced photosynthetic rate by lowering in photosynthetic pigments, amount and activity of rubisco, and affecting ultrastructure of chloroplast (Pancheva et al. 1996; Uzunova and Popova 2000; Sahu 2013) or reducing quantum efficiency of PSII by inhibiting electron transport in guard cells of tomato (Poór and Tari 2012). Thus changes in SA-induced photosynthetic capacity of radish cultivars might have been linked with osmoprotection and antioxidant capacity of plants.
Salt stress causes the generation of reactive oxygen species in chloroplast, a wide variety of osmoprotectants and antioxidants become accumulated to protect thylakoidal protein complexes. Proline is one of the potential osmoprotectants having role in osmotic adjustment, protecting PSII activity, as antioxidant, and stabilization of cell membrane (Szabados and Savouré 2010). Results of present study showed that SA treatment and salt stress increased proline accumulation; however, the increase in proline accumulation was more due to SA application (Table 1, Fig. 1f, Supplementary Table 4). Similar increase in proline by SA treatment has earlier been observed in Panax ginseng (Ali et al. 2007), Solanum lycopersicum (Mimouni et al. 2016) and Cucumis sativus (Youssef et al. 2018). A positive association between accumulation of proline and PIABS has been found which indicated that SA-induced increase in PSII efficiency can be linked to proline accumulation. However, exogenous application of 5 mM SA caused greater accumulation of proline with relative lower PIABS in salt stressed plants of different radish cultivars than with those of applied with 2 mM SA. These results suggest that contribution of proline in improving PSII efficiency of salt stressed plants radish cultivars is partial. Other factors may include non-enzymic and enzymic antioxidants (Foyer and Shigeoka 2011; Foyer et al. 2017).
Anthocyanins are group of flavonoid pigments that act as non-enzymic antioxidants (Taiz et al. 2015). In the present study, salt stress increased the anthocyanin pigments in radish cultivars while SA treatment decreased their amount (Table 1, Fig. 5c, Supplementary Table 2). Similar increase in anthocyanins due to salt stress has already observed in Pelargonium (Breś et al. 2016) and spinach (Xu and Mou 2016). However, SA application decreased anthocyanin pigments under non-saline conditions but increase in anthocyanins was observed under SA plus salt stress. Stress induced anthocyanins are thought to function as ROS scavengers (Agati et al. 2012). Szepesi et al. (2008) demonstrated that pre-treatment with SA increased anthocyanin accumulation under saline and non-saline conditions. Based on our results, it can be suggested that stress increased ROS accumulation and in order to detoxification of ROS radicals SA increased production of anthocyanins as non-enzymic antioxidants. Anthocyanin accumulation is mainly localized to upper epidermis where it shields lower mesophyll cells from light intensity or lower oxidative stress. Thus, it is assumed that SA treatment reduced the oxidative stress thereby increasing anthocyanin accumulation.
The malondialdehyde (MDA) content is considered as key indicator to show lipid peroxidation level (Shen et al. 2014). In the present study, the salt stress increased H2O2 and MDA content in all radish cultivars, while SA application reduced the concentrations of both H2O2 and MDA content, which indicated that the salt stress caused oxidative damage and SA application lowers the oxidative damage (Table 1, Fig. 5a, b, Supplementary Table 4). While working with five cultivars of turnip (Brassica rapa L.) Noreen et al. (2010) reported that decrease in photosynthetic capacity of turnip cultivars due to salinity stress is related with higher ROS production. Similarly, seedlings of Torreya grandis treated with SA had lower electrolyte leakage and MDA content (Li et al. 2014). Generally, plants activate their antioxidant enzymes (SOD, CAT and POD etc.) for protecting their tissues from lethal effects of ROS accumulated due to salt stress (Foyer et al. 2017).
In the present study, the foliar spray with SA significantly increased POD, CAT and SOD activity which is inversely related with MDA and H2O2, which indicate foliar spray with SA ameliorated the salt-induced oxidative damage to PSII by modulating activities of antioxidant enzymes (Table 1, Fig. 5d–f, Supplementary Table 4). These results can be explained in view of the argument of Bose et al. (2017) that SA activates multiple pathways to scavenge ROS in chloroplast to protect photosynthetic machinery including battery of antioxidant enzymes. Signaling between SA and ROS is closely related, as ROS signaling is inhibited by SA which stimulates the activation of defensive genes (Xu and Brosche 2014).
Conclusion
Salt stress adversely affect the physiological and biochemical properties of radish plant causing growth inhibition, destruction of photosynthetic machinery, ROS accumulation, increased membrane damage, and reduced efficiency of PSII. However, SA treatment reduced the detrimental effect of salt stress by combination of osmolytes, non-enzymic and enzymic antioxidants. However, mode of action of SA in ameliorating the adverse effects of salt stress on radish cultivars depends on concentration of SA application. As observed in previous studies, that higher dose of SA become inhibitory to some photosynthetic processes is another supportive argument in this regard.
References
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Ali MB, Hahn EJ, Paek KY (2007) Methyljasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor. Molecules 12:607–621. https://doi.org/10.3390/12030607
Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161:319–328. https://doi.org/10.1078/0176-1617-01022
Arfan M, Athar HR, Ashraf M (2007) Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J Plant Physiol 164:685–694. https://doi.org/10.1016/j.jplph.2006.05.010
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1. https://doi.org/10.1104/pp.24.1.1
Ashraf M, Akram NA, Arteca RN, Foolad MR (2010) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci 29:162–190. https://doi.org/10.1080/07352689.2010.483580
Athar HR, Ashraf M (2009) Strategies for crop improvement against salinity and drought stress: an overview. In: Ashraf M, Ozturk M, Athar HR (eds) Salinity and water stress: improving crop efficiency. Springer, Netherlands, pp 1–16
Athar HR, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63:224–231. https://doi.org/10.1016/j.envexpbot.2007.10.018
Athar HR, Zafar ZU, Ashraf M (2015) Glycinebetaine improved photosynthesis in canola under salt stress: evaluation of chlorophyll fluorescence parameters as potential indicators. J Agron Crop Sci 201:428–442. https://doi.org/10.1111/jac.12120
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Ben-Rouina B, Ben-Ahmed C, Athar HR, Boukhriss M (2006) Water relations, proline accumulation and photosynthetic activity in olive tree (Olea europaea L. CV "Chemlali") in response to salt stress. Pak J Bot 38:1397–1406
Bose J, Munns R, Shabala S, Gilliham M, Pogson B, Tyerman SD (2017) Chloroplast function and ion regulation in plants growing on saline soils: lessons from halophytes. J Exp Bot 68:3129–3143. https://doi.org/10.1093/jxb/erx142
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x
Cessna S, Demmig-Adams B, Adams WW (2010) Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J Nat Resour Life Sci Educ 39:22–30. https://doi.org/10.4195/jnrlse.2009.0024u
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41:281–284. https://doi.org/10.1023/B:PHOT.0000011962.05991.6c
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Foyer CH, Ruban AV, Nixon PJ (2017) Photosynthesis solutions to enhance productivity. Philos Trans R Soc Lond B Biol Sci 372:20160374. https://doi.org/10.1098/rstb.2016.0374
Ghassemi-Golezani K, Lotfi R (2015) The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ J Plant Physiol 62:611–616. https://doi.org/10.1134/S1021443715040081
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol 164:728–736. https://doi.org/10.1016/j.jplph.2005.12.009
Hayat S, Fariduddin Q, Ali B, Ahmad A (2005) Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron Hung 53:433–437
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102. https://doi.org/10.1007/s11738-016-2113-y
Khalid A, Zafar ZU, Akram A, Hussain K, Manzoor H, Al- Qurainy F, Ashraf MA (2015) Photosynthetic capacity of canola (Brassica napus L.) plants as affected by glycinebetaine under the salt stress. J Appl Bot Food Qual 88:78–86. https://doi.org/10.5073/JABFQ.2015.088.011
Khan NA (2003) NaCl inhibited chlorophyll synthesis and associated changes in ethylene evolution and antioxidative enzyme activities in wheat. Biol Plant 47:437–440
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74. https://doi.org/10.1016/j.plaphy.2014.03.026
Khodary SEA (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int J Agric Biol 6:5–8
Levitt J (1980) Responses of plants to environmental stresses. Academic Press, New York
Li T, Hu Y, Du X, Tang H, Shen C, Wu J (2014) Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE. https://doi.org/10.1371/journal.pone.0109492
Mehta P, Allakhverdiev SI, Jajoo A (2010) Characterization of photosystem II heterogeneity in response to high salt stress in wheat leaves (Triticum aestivum). Photosynth Res 105:249–255. https://doi.org/10.1007/s11120-010-9588-y
Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144:1777–1785
Mimouni H, Wasti S, Manaa A, Gharbi E, Chalh A, Vandoorne B, Lutts S, Ahmed HB (2016) Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. Omics 20:180–190. https://doi.org/10.1089/omi.2015.0161
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Noreen S, Ashraf M (2008) Alleviation of adverse effects of salt stress on sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak J Bot 40:1657–1663
Noreen Z, Ashraf M, Akram NA (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci 196:273–285
Noreen S, Athar HR, Ashraf M (2013) Interactive effects of watering regimes and exogenously applied osmoprotectants on earliness indices and leaf area index in cotton (Gossypium hirsutum L.) crop. Pak J Bot 45:1873–1881
Ogbaga CC, Stepien P, Athar HUR, Ashraf M (2018) Engineering Rubisco activase from thermophilic cyanobacteria into high-temperature sensitive plants. Crit Rev Biotechnol 38:559–572. https://doi.org/10.1080/07388551.2017.1378998
Pancheva TV, Popova LP, Uzunova AN (1996) Effects of salicylic acid on growth and photosynthesis in barley plants. J Plant Physiol 149:57–63. https://doi.org/10.1016/S0176-1617(96)80173-8
Plaut Z, Edelstein M, Ben-Hur M (2013) Overcoming salinity barriers to crop production using traditional methods. Crit Rev Plant Sci 32:250–291
Poór P, Tari I (2012) Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Funct Plant Biol 39:1028–1037. https://doi.org/10.1071/FP12187
Reddy MP, Vora AB (1986) Changes in pigment composition, hill reaction activity and saccharides metabolism in bajra (Pennisetum typhoides S&H) leaves under nacl salinity. Photosynthetica 20:50–55
Sahu GK (2013) Salicylic acid: role in plant physiology and stress tolerance. In: Rout GR, Das AB (eds) Molecular stress physiology of plants. Springer, Bhubaneshwar, pp 217–239
Shen C, Hu Y, Du X, Li T, Tang H, Wu J (2014) Salicylic acid induces physiological and biochemical changes in Torreya grandis cv. Merrillii seedlings under drought stress. Trees 28:961–970. https://doi.org/10.1007/s00468-014-1009-y
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135. https://doi.org/10.1007/s10725-005-5482-6
Sonneveld C (2000) Effects of salinity on substrate grown vegetables and ornamentals in greenhouse horticulture. Dissertation, Wageningen University
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Szepesi A, Csiszár J, Bajkan S, Gémes K, Horváth F, Erdei L, Deer AK, Simon ML, Tari I (2005) Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol Szeged 49:123–125
Szepesi Á, Csiszár J, Gallé Á, Gémes K, Poór P, Tari I (2008) Effects of long-term salicylic acid pre-treatment on tomato (Lycopersicon esculentum Mill. L.) salt stress tolerance: changes in glutathione-S-transferase activities and anthocyanin contents. Acta Agron Hung 58:129–138
Taiz L, Zeiger E, Moller IS, Murphy A (2015) Plant physiology and development. Sinauer Associates Inc., Massachusetts
Uzunova AN, Popova LP (2000) Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica 38:243–250. https://doi.org/10.1023/A:100722611
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wagner GJ (1979) Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol 64:88–93
Xu E, Brosche M (2014) Salicylic acid signaling inhibits apoplastic reactive oxygen species signaling. BMC Plant Biol 14:1–17. https://doi.org/10.1186/1471-2229-14-155
Xu C, Mou B (2016) Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J Am Soc Hortic Sci 141:12. https://doi.org/10.21273/JASHS.141.1.12
Youssef SM, Abd Elhady SA, Aref RM, Riad GS (2018) Salicylic acid attenuates the adverse effects of salinity on growth and yield and enhances peroxidase isozymes expression more competently than proline and glycine betaine in cucumber plants. Gesunde Pflanzen 70:75–90. https://doi.org/10.1007/s10343-017-0413-9
Zafar ZU, Manzoor H, Rasul S, Noreen S, Ali Q, Iqbal M, Javed M, Gul HS, Ahmad Z, Shahzad F, Ogbaga CC, Athar HR, Ashraf M (2017) Strategies to improve crop salt and drought tolerance: success and limitations. In: Malik CP, Wani SH, Kushwaha HB, Kaur R (eds) Quality and quantum improvement in field crops. Agribios, New Delhi, pp 265–298
Zhang L, Xing D (2008) Rapid determination of the damage to photosynthesis caused by salt and osmotic stresses using delayed fluorescence of chloroplasts. Photochem Photobiol Sci 7:352–360. https://doi.org/10.1039/b714209a
Acknowledgements
We are grateful to Ayub Agriculture Research Institute, Faisalabad, Pakistan for providing seed of radish varieties.
Funding
This work was partially funded by Directorate of Research and External Linkages, Bahauddin Zakariya University, Multan, Pakistan under Non-PhD research grant program Via grant No. DR & EL/D-43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bukhat, S., Manzoor, H., Athar, HuR. et al. Salicylic Acid Induced Photosynthetic Adaptability of Raphanus sativus to Salt Stress is Associated with Antioxidant Capacity. J Plant Growth Regul 39, 809–822 (2020). https://doi.org/10.1007/s00344-019-10024-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10024-z