Abstract
Isopoda, a widely distributed crustacean order, exhibits the unusual biphasic moulting in which the posterior part of the exoskeleton constantly sheds before the anterior region. This paper presents a literature review on isopods, emphasizing the association of the biphasic mode of moulting with the adaptation of isopods to different habitats and lifestyles. Owing to the biphasic pattern of moulting, the two halves of the body take shifts to carry out essential functions such as oxygen consumption, resorption of cuticular calcium, evading the risk of water loss, and compartmentalising the processes of moulting and mating. Biphasic moulting is also advantageous for the parasitic isopods to cling to their host, regulate their feeding habitat and taxis, resist water flow, withstand strong forces in their microhabitat and synchronize mating. Histology and enzyme-linked immunosorbent assay (ELISA) experiments conducted in few isopods demonstrated the differential responses of anterior and posterior body parts to neurohormones such as ecdysteroids. Taken together, the conserved phenomenon of biphasic moulting in isopods should offer several advantages for adapting to diverse environments even though there is no direct evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isopods are crustaceans belonging to the class Malacostraca of phylum Arthropoda. Isopoda is possibly the most morphologically diverse order of all the Crustacea (Hickman et al. 2006). Isopods comprising over 10,300 species and 11 suborders have a cosmopolitan distribution worldwide (Wilson 2008). Habitat-wise, isopods are marine, freshwater, or terrestrial (Kussakin 1979; Kensley and Schotte 1989; Brusca et al. 2007; Hornung 2011). They have also inhabited deep-sea trenches, groundwaters, and deserts (Bruce 2004; Hua et al. 2018). Considering the mode of living, while many of the isopods are free-living (Oniscidae), some are scavengers (Haploniscidae), grazers (Asellidae), and temporary parasites (Gnathidae) to obligatory parasites (Cymothoidae) (Wetzer 2001).
Habitat and lifestyle-based phenotypic differences in isopods
Isopods from different habitats and lifestyles follow a distinct pattern in their morphology, growth rates, reproductive strategies, digestive physiologies, and life spans (Hessler et al. 1979). The schematic representation shows the significant changes in their morphology and specific structural features (Fig. 1). The majority of isopods possess dorso-ventrally compressed body with a vaulted dorsum (Cymothoida and Sphaeromatidae), some are elongated or worm-like (Anthuroidea), or flattened (Serolidae and Sphaeromatidae), and some others possess spines and nodules (Valvifera and Sphaeromatidae) (Brandt and Poore 2003; Wilson 2008). Differences in morphology and physiology are apparent between isopods of terrestrial and aquatic inhabitants (Schmidt 2008). The terrestrial oniscideans are generally oval, broader at the fourth pereonite, and slightly vaulted towards the distal end (Brandt and Poore 2003). The other key differences include (1) smaller size, (2) water-resistant cuticle, (3) diverse surface morphologies, (4) pleopodal lungs, (5) water-conducting system, and (6) closed brood pouch (Edney 1954; Bursell 1955; Schmalfuss 1978; Hoese 1981, 1984; Holdich 1984; Cloudsley-Thompson 1988; Schmidt and Wägele 2001; Horiguchi et al. 2007). Studies on moulting demonstrated that the terrestrial isopods possess sclerotized outer cuticles, unlike those in marine and freshwater environments (Csonka et al. 2018). The sclerotized tegument assists as the primary protective barrier from their environment (Hornung 2011).
A certain level of inter-population morphological divergence is also evident in aquatic isopods. For instance, the sub-order Asellota which includes freshwater and marine isopods (especially deep sea), exhibits remarkable morphological diversity (Brandt and Poore 2003; Raupach et al. 2009). Though most freshwater forms are with a flattened body, some are thin and vermiform with legs arising close to the dorsal surface (Wilson 2008). According to Brandt and Poore 2003, marine isopods are the morphologically most diverse among the crustaceans. Shallow-water marine species may be cryptically coloured or patterned (Guarino et al. 1993). The deep-sea isopods have highly ornamented and bizarre shapes to facilitate burrowing, and their antennae are prominent (Hessler and Strömberg 1989; Bruce 2004). For instance, Haploniscidae are pill-bug-like, Ischnomesidae are elongated, Mesosignidae and Dendrotionidae possess spines on the body, Nannoniscidae are slender, and Eurycopidae are fat (Hessler et al. 1979). The deep-sea isopods are scavengers with a modified morphology (Wilson and Fenwick 1999). They possess large size ingesting organs and ambit to cover wider areas for scavenging (Hessler and Strömberg 1989). Deepwater isopods do not follow any specific pattern in colour. Their eyes are rudimentary compared to freshwater, marine, and terrestrial habitats (Hessler and Thistle 1975). For effective nutrient uptake, extensive modifications in their feeding morphology, including the size of the ingesting organs and ambit (the amount of space covered during the activity of an individual), are required. Furthermore, these isopods need efficient metabolic approaches to cope with the temperature fluctuation in the deep- sea environment.
Characteristic differences were also noticed in the structural features of isopods located in different habitats. Among the abdominal appendages, pleopods serve as oxygen uptake organs, mainly those belonging to the mesic and xeric habitats. In such isopod lineages, pleopods support the gas exchange function and aid the propulsive movement of the animals (Alexander 1988; Wägele 1992). In addition, these terrestrial inhabitants do not require the water micro-environment for any biological activities in any of their developmental stages (Broly et al. 2013). Instead, they can take up moisture from the substratum through the uropods and transfer it to their capillary water system, which acts as an interface to transport the absorbed moisture from uropods to the pleopods (Warburg 1968). All these different traits of pleopod are supposed to have evolved during terrestrial adaptation (Hoese 1982). In contrast, the capillary water system is absent in aquatic isopods, though some transitional species (e.g., Ligia) show the signs of developing a capillary water system (Barnes 1932). Similarly, the rare forms of isopods adapted to xeric conditions (Armadillo, Venezillo, and Hemilepistus) did not possess the capillary water system, as they are efficient in water vapour absorption (Warburg 1968; Harris et al. 2020). Significant differences were also found in the brood pouches. In terrestrial isopods, the brood pouch, apart from protecting the eggs against desiccation and microbes, also ensures an aquatic milieu with sufficient fluid and oxygen (Mrak et al. 2012). In unfavourable thermal conditions, the females can even remove their brood pouch (Linsenmair 1989).
Reports on the morphological adaptations for parasitic life are also available in isopods. The attachment site possibly influences the major changes in the body shape and feeding mode of the host. The obligate parasites of the sub-order Cymothoidea have a long, slender body tapering towards both ends with an efficient contour that offers resistance to water flow, and they can withstand strong forces in their micro-habitats (Fig. 2). They possess a very heavily thickened and calcified cuticle for protection and sharply curved hooks (dactyli) on all pereopods allowing them to attach to the host (Nagler et al. 2017; Kottarathil et al. 2019). Body segments become increasingly smooth, and the number of setae is less than that of the free-living species. Pereopod morphology changes, and decreasing numbers of setae occur as the level of parasitism increases (Smit et al. 2014). The mouthparts become a distinct buccal cone with strongly recurved and robust hooked setae or abrading serrated scales (Poore and Bruce 2012). Unlike free-living isopods, the eyes are more petite in parasitic forms, and the body colour varying pale to red-pink (Aegidae) or white to pale (Cymothoidae) (Poore and Bruce 2012). Generally, the parasitic isopods do not swim, crawl, or leave their final host (Poore and Bruce 2012). The aforementioned changes in the body form of parasitic isopods from their free-living counterparts might be due to the evolution of the former from the latter. Further, the parasitic cymothoids were supposed to have invaded from marine to freshwater habitats as reflected from the increased body size (Poulin 1995).
Isopods also vary in their feeding habits and taxis. The primitive Phreatoicideans feed on decaying leaves; other freshwater-inhabiting asellotes are either detritivores or omnivores with adjusted feeding morphology (Wilson and Fenwick 1999). Isopods belonging to Sphaeromatidae are omnivorous, and those of Cirolanidae are carnivorous and have been observed with piercing and suctorial mouthparts (Wilson 2008). The maxillule of Lanocira has the form of a large hook, eminently suited mouthparts to grasp small polychaetes (Poore and Bruce 2012). Oniscideans and Asellidae eat decaying leaves combined with bacterial endo-symbiont (Zimmer 2002; Zimmer and Bartholmé 2003). Certain terrestrial isopods aggregate in dark, moist places, possibly due to kinetic and tactic responses (Edney 1954). In some cases, isopods can conglobate or curl up their bodies to form a ball against various physical stimuli (Warburg 1968).
Habitat and lifestyle-based genetic differences in isopods
Though most species might have undergone considerable genetic changes, the biphasic moulting phenomenon is common to all isopods. The common biological changes (such as variation in the moulting duration, seasonality, feeding mode, etc.) observed in isopods of different habitats and living modes prove this. To further test this, the present study investigated the genetic variation of isopods belonging to different habitats and modes of living. For this purpose, 16 s rDNA sequences of 6–10 individuals representing different habitats from marine, freshwater, terrestrial, and deep-sea, and marine parasitic/free-living forms were retrieved from the NCBI. Phylogenetic relationships inferred from these isopod species showed a clear genetic difference between the populations inhabiting the deep-sea, marine/freshwater, and terrestrial habitats (Fig. 3). Also, it noticed clear genetic segregation of the two different forms of marine population, leading to parasitic and free-living life (Fig. 4). In order to assess the substitution saturation of nucleotides, bioinformatics tools of DAMBE (Xia 2018) have been applied. For the test of saturation, we followed the methodology by Xia et al. (2003) and Xia and Lemey 2009. The assessment result yielded Iss = 0.488, which is significantly less than Iss.c (= 0.787) (Fig. 3), and Iss = 0.4331, which is significantly less than Iss.c (= 0.6459) (Fig. 4), presuming a symmetrical topology indicating the sequence saturation. Based on previous records, intra-habitat genetic diversity is evident among the deep-sea isopods, wherein the population was grouped into different genetic haplotypes (Barnard 1920; Raupach et al. 2009). According to Porres et al. (2018), the isopod population belonging to a single habitat showed geographical variations. Even though habitat-reliant genetic diversity is the prominent one in isopods when compared to the geographical differences.
Phylogenetic tree of isopods from different habitats. The evolutionary history of the isopods was inferred by using the Maximum Likelihood method and Kimura 2-parameter model (Kimura 1980). This analysis involved 33 16 rDNA sequences collected from NCBI Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018; Sayers et al. 2020). 16S rDNA from Penaeus monodon was taken as outgroup
Phylogenetic tree of parasitic and free-living forms of isopods. The phylogenetic tree was inferred by using the Maximum Likelihood method and Kimura 2-parameter model (Kimura 1980). The analysis involved 15 16 rDNA sequences collected from NCBI Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018; Sayers et al. 2020). 16S rDNA from Penaeus monodon was taken as outgroup
Discovery of biphasic moulting in isopods
Unlike monophasic moulting followed by most malacostracan crustaceans (Decapoda, Stomatopoda, Amphipoda, Cumacea, Mysidacea, etc.), moulting in isopods is biphasic in which the posterior part of the exoskeleton is constantly shed before the anterior part. Biphasic moulting in the isopod was first discovered accidentally by Schobl in 1880 while studying the reproduction of Porcellio scaber Latreille, 1804. Following this, many researchers also noticed this phenomenon in many isopod genera such as Trichoniscus, Haplophthalmus, Porcellio, Oniscus, Armadillidium and Asellus and Ligia (Weber 1881; Friedrich 1883; Schonichen 1898; Verhoeff 1901; Pierce 1907; Zuelzer 1907; Tait 1911; Hankó 1912; Allee 1913). By the twentieth century, commendable basic level information on biphasic moult was generated in the terrestrial group, Oniscoidea, intertidal species (Ligia oceanica (Linnaeus, 1767) and Ligia exotica Roux, 1828), and marine genera (Sphaeroma, Limnoria, Cirolana, Idotea, and Asellus) (Tait 1917; Numanoi 1934). According to Tait (1917), Herold (1913) was the first to provide a general description of the biphasic moult. In 1914, Aubin (Aubin 1914) explained the moult cycle in the terrestrial isopod, Porcellio. Tait (1917) and Nicholls (1931) observed the moult-related colour changes and calcium carbonate storage in Ligia.
Characterization of biphasic moulting
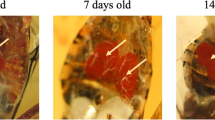
As biphasic moulting occurs in two phases, a simple microscopic observation of the isopod specimens would clearly indicate their moulting status. The digital images depicting the external morphology of the marine parasitic isopod Norileca indica (H. Milne Edwards, 1840) while undergoing biphasic moult are given for reference (Fig. 5). However, the proper stage-wise characterization of the moult cycle is essential for determining the growth rate in many crustaceans (Drach and Tchernigovtzeff 1967; Luxmoore 1982). In isopods, most of the moult cycle-related studies were focused on terrestrial forms (Montesanto and Cividini 2018). In P. scaber, Armadillidium vulgare (Latreille, 1804), Armadillo officinalis Duméril, 1816 moult related differences in shape and colour of sternites are common (Steel 1980; Suzuki et al. 1996; Zidar et al. 1998; Hagedorn and Ziegler 2002; Neues et al. 2011; Montesanto and Cividini 2018). Many researchers have documented the moult related ultra-structural changes in the integument of Oniscus asellus Linnaeus, 1758, P. scaber, Titanethes albus (C. Koch, 1841), Ligia italica Fabricius, 1798 (Price and Holdich 1980b; Štrus and Compere 1996; Ziegler 1997; Seidl and Ziegler 2012; Vittori et al. 2012; Žnidaršič et al. 2012; Vittori and Štrus 2014). In L. italica and Ligia pallasii Brandt, 1833, biphasic moult identification was made using X-ray diffraction and CT scanning (Štrus and Compere 1996; Štrus et al. 2019). In marine parasitic isopods, such as N. indica (H. Milne Edwards, 1840), Mothocya renardi (Bleeker, 1857), through light microscopic study, the moult stage-related characteristic changes in the epidermis and subsequent formation of juvenile appendages were described (Sahadevan et al. 2020; Panakkool-Thamban and Kappalli 2020). Stevenson (1961) demonstrated the biphasic moult-associated tanning first time in the terrestrial isopod A. vulgare based on the changes in the level of polyphenol oxidase secreted from the tegumental glands. In order to determine the exact moult stage at which the polyphenol oxidase was secreted, it was necessary to decipher the moult-related changes happening in the two halves of the species. For this, Stevenson (1961) followed the methods described by Drach (1939) and Charniaux-Legrand (1952) with considerable modifications. Accordingly, the moult cycle was classified into different stages such as A (postmoult stage), B (stage soon after the postmoult where the calcification of the cuticle is about to begin), C (the stage of progressive hardening), and D (preparative stage for moulting). D was further divided into D1 when the new claw is formed; D2, the new claw becomes amber-coloured; D3, no visible change and D4, when the cuticle is about to shed. In Asellus aquaticus Linnaeus, 1758, the aesthetasc sense organs on the antennules were used to identify the moult stages (Heimann 1984). According to the recent reports based on the light microscopic study of the appendages in the marine parasitic isopods, N. indica and M. renardi, the precise detection of moult stages is possible (Sahadevan et al. 2020; Panakkool-Thamban and Kappalli 2020). In N. indica, the maxillule and the exopodite (of the uropod) have been identified as the appropriate appendages showing precise moult-related changes from the anterior and the posterior parts, respectively, which enables the detection of moult stages of both body parts simultaneously (Sahadevan et al. 2020). From the studies described above, it is understood that there is no universal procedure to characterise the biphasic moult stages in isopods. Hence this aspect invites more attention since precise moult stage identification is crucial to carry out advanced studies on moulting at physiological, endocrinological, and molecular levels.
Biphasic moulting pattern is uniform in all isopods irrespective of their habitats and lifestyles
All isopods evolved exhibit biphasic moulting irrespective of their habitats, lifestyle, and different forms (Wilson 2009). An exception to this was reported in Antarctic isopod Glyptonotus in which moulting was monophasic (George 1972). The sub-order Phreatoicidea is considered as the most primitive isopod group originating in the marine environment (Brusca and Wilson 1991); they successfully colonized freshwater, and terrestrial ecosystems; some are the inhabitants of the groundwaters and others of the abyssal benthic region while some live in the desert (Wilson and Johnson 1999; Poore and Bruce 2012). To invade entirely different habitats, the isopod has undergone several morphological and physiological changes (Wetzer 2001; Wilson and Edgecombe 2003; Broly et al. 2013). Despite the morphological and physiological changes, no other evidence shows a change in the mode/pattern of moulting in any of the isopods beginning from the ancient Phreatoicidea to the present Cymothoidea. The fossil of the cirolanid isopod, Cirolana garassinoi Feldmann, 2009 lived during the late Cretaceous period showed the presence of three pairs of dermoliths (sites of mineral/calcium storage) at the anterior body part and a thin exocuticle which indicate that the isopod was at the premoult stage (Feldmann 2009). From this evidence, it is presumed that these marine isopods were already equipped with the calcium-storing mechanism to invade the terrestrial habitat in the late Cretaceous period itself.
Duration of biphasic moult cycle/ moulting is different among the isopods
Although biphasic moulting is common to all the isopods, its duration varies among the species. Most of the studies reported the shedding of the anterior exoskeleton within 17–40 h after shedding the posterior part. For instance, in the sand beach isopod, Excirolana chiltoni Richardson, 1905, the reported duration of biphasic moulting is 25 h (Klapow 1972). In the terrestrial isopod A. officinalis, the duration of the premoult phase is 12 days, and the biphasic ecdysis is 1.5 days (Montesanto and Cividini 2018). In the common woodlouse (O. asellus), the duration of the biphasic moulting is 1.8 days, and in the case of P. scaber the period is 17–24 h (George and Sheard 1954; Price and Holdich 1980b). Marcus (1990) reported that for the freshwater isopod A. aquaticus, the anterior ecdysis occurred only after 24 h upon the posterior ecdysis.
In the sub-terranean isopod T. albus, the duration of its biphasic moult ranges from one day to several days, and the anterior ecdysis follows the posterior ecdysis after 3–5 days. The duration of the premoult is also extended to approximately seven weeks (Vittori et al. 2012). Despite the changes in the duration of a moult cycle, certain isopods also display a seasonal-dependent variation in the number of moult cycles. In the marine cymothoid N. indica, there is a considerable decrease in moulting events during the monsoon season compared to the summer and post-monsoon/winter season (Sahadevan et al. 2020).
Behavioural changes related to biphasic moulting in isopods
Table 1 listed the behavioural changes associated with biphasic moulting in isopods reported from different habitats.
Physical/ motivational behaviours
During the moulting process, the isopods can move to reduce the rate of predation (Price and Holdich 1980a, 1980b). The exo-receptors of one-half of the body are always functional together with the activity of the tegumental glands (Gorvett 1956; Price and Holdich 1980a). Biphasic moulting is unavoidable in many parasitic isopods as they utilize this approach to cling to their host. Pereopods emerging from anterior and posterior regions help cling to the host when either region is moulting (Kottarathil et al. 2019). On the other hand, biphasic moulting is not a requisite in the manca stage as the calcification is relatively meager as their exoskeleton appears very soft (Mrak et al. 2014).
Isopods exert certain motivational behaviour like pushing their body upright by stretching their moulted half upward, possibly protecting their newly moulted region, preventing it from touching the substratum as it may cause some damage to the animal (Vittori et al. 2012). In some cases, the mating was seen co-occurring with the posterior moulting (Shuster 1989). In females, the oviduct opens outward through the base of the 5th or 6th pereopod at the posterior part of the body. Though not common, in some terrestrial isopods, as soon as the posterior exuvium sheds, the animal feeds on the exuviae to cope with the loss of calcium from the body (Steel 1993). This behaviour, however, is not seen in marine forms (Alikhan 1972). Sparrevik (1999) reported cannibalism in Saduria entomon (Linnaeus, 1758), i.e., the non-moulting individuals feed on those undergoing moulting if they are size-wise smaller than the predators.
Physiological behaviours
Terrestrial isopods face the risk of transpiration, especially during the moulting event. For the rapid water loss recovery, they take up either water or water vapour (Hoese 1981; Wright and Machin 1990, 1993). Biphasic moulting might be helpful to conserve the water content as the cuticle will always be present on one-half the body, thereby reducing the rate of transpiration. In Idotea balthica Pallas, 1772, two peaks of oxygen consumption, one during the posterior ecdysis and another during the anterior ecdysis, were reported (Bulnheim 1974). The studies on A. vulgare reported the minimum haemolymph pressures and oxygen consumption rates during the moulting period, especially during the posterior ecdysis (Alikhan 1983). Through biphasic moulting, the anterior half of the isopod takes the shift to absorb the required amount of oxygen while the posterior part is undergoing moulting. In another report by Chiang and Steel (1986), the activity of the sinus gland in O. asellus increases at the postmoult stage in both anterior and posterior regions. Whiteley and El Haj (1997) also noted a difference in the rate of muscle protein synthesis between the two halves in Idotea rescata Stimpson, 1857. Unlike other crustaceans, isopods can feed during the period of biphasic moulting. For instance, Porcellio laevis Latreille, 1804 continues feeding when it undergoes posterior moulting. However, during anterior moulting, it depends on the stored hepatopancreatic lipids (Alikhan 1972) and epithelial cell glycogen reserves for the energy requirements (Štrus and Compere 1996).
According to Vernet and Charmatier-Daures (1994), biphasic moulting favours calcium saving through recycling; when the posterior half is at premoult, the integumental calcium from this part is withdrawn and store at the anterior sternites. When the posterior half completes moulting, the calcium is re-absorbed from the anterior half and re-calcify the posterior part. This physiological behaviour is much essential for terrestrial isopods (Montesanto and Cividini 2018). In Ligia, the calcium present in the endo-cuticle is cycled between the anterior and posterior parts (Numanoi 1942). Studies in T. albus showed the signs of sternal calcium deposits in the apical plasma membrane of their epidermal cells during cuticle formation (Vittori et al. 2012). Similar reports are also available in other terrestrial isopods (Price and Holdich 1980a; Ziegler 1997; Štrus and Blejec 2001). In the haemolymph of P. scaber, the amount of Ca2+ is significantly increased by 13%, 19%, and 18% during premoult, intermoult, and postmoult, respectively, which might be due to the resorption of cuticular calcium (Ziegler and Scholz 1997). But in some isopods, either gastroliths or hepatopancreas act as the storehouse or supplier of calcium (Numanoi 1942). In freshwater isopods, biphasic moulting may be more beneficial to meet the calcium demand as this shows wide fluctuation in freshwater (Greenavvay 1985). Biphasic moulting may also favour reproductive function: in Natatolana borealis Lilljeborg, 1851, N. indica, and M. renardi, the oostegites are formed during biphasic partial-parturial ecdysis (Johansen 1996; Kottarathil and Kappalli 2019; Panakkool-Thamban and Kappalli 2020). Females can rejuvenate their genitalia after the biphasic moult without interfering their reproduction (Suzuki 2002).
Biochemical and molecular factors defining the mechanism of biphasic moulting – Future perspectives
Studies on the neuronal and hormonal control of biphasic moulting are limited, and most of the available reports are based on a terrestrial isopod O. asellus. Matsumoto (1959) suggested the homologous nature of beta cells identified from this species with the X organ of decapod crustaceans. Carefoot (1993) demonstrated the connection of beta cell with the sinus glands. Chiang and Steel (1984, 1985) histologically demonstrated the presence of neuro- secretory terminals in this species (O. asellus). They (Chiang and Steel 1989) also found a decreased level of action potential from the sinus gland while the animal was at the late premoult stage with maximum moulting hormone, ecdysteroids. This supports the fact that the moult-inhibiting hormone secreted from the sinus gland is inversely correlated with the ecdysteroids (Martin et al. 1979; Lachaise et al. 1993). Despite this information, no satisfactory explanation is available on the question of how it controls the biphasic moulting.
According to the researchers, the resorption of calcium from the old cuticle is under the control of the ecdysteroids (McWhinnie et al. 1972; Kleinholz and Keller 1979). But the role of this hormone in the calcification of the postmoulted part is not clear. However, the role of ecdysteroids in triggering the premoulting and ecdysis events was experimentally proven by measuring its titre with respect to moult cycle stages. Reports are available for free-living terrestrial isopods A. vulgare (Suzuki et al. 1996) and O. asellus (Steel and Vafopoulou 1998), and also for a parasitic isopod N. indica (Sahadevan et al. 2020). Steel and Vafopoulou (1998) in their experiments in O. asellus showed that when the ecdysteroids level was maximum, there occurs cuticle deposition in the posterior part indicating the differential response of anterior and posterior body parts to the ecdysteroids released at one time. They speculate that even though both the anterior and posterior epidermal cells receive the signal for simultaneous secretion of the cuticle, the response might be consecutive. According to Steel (1977), this calcium translocation is controlled by the brain hormones stored in the sinus gland for timely release as they could record the increased electrical activity of this gland after each partial ecdysis.
In a marine parasitic isopod (N. indica), the level of ecdysteroids is maximum when the posterior half is at the late premoult stage. The titre, however, showed a dramatic decrease when the anterior part attains late premoult, indicating that ecdysteroid receptor activity was initiated at the posterior half first, then at the anterior half (Sahadevan et al. 2020). This time difference in the hormone receptivity of the two halves might be one of the reasons for biphasic moulting. This also indicates that the nervous system does have only the initial control over ecdysteroids production when the posterior part undergoes premoult agreeing with the observation of Steel and Vafopoulou (1998). Since the isopods possess the open haemocoel, released ecdysteroids may be accessed equally by anterior and posterior body parts. To demonstrate this hypothesis, more research in this line is required. Studies also showed that cellular uptake of ecdysteroids involves the activity of Na+/K+-ATPase (Spindler and Spindler-Barth 1989). Presumably, the animal controls the expression of the Na+/K+-ATPase differently in the two halves. Since as a steroid hormone, ecdysteroids need no pump to enter the target cell. So a comprehensive study related to the expression of ecdysteroid receptors during biphasic moulting is also necessary.
During the biphasic moult of N. indica the posterior half becomes wider first, followed by the anterior part indicating the differential growth of muscle (Sahadevan et al. 2020). The differential growth of the muscle in two halves of the isopod body and its hormonal control also needs attention. According to Whiteley and El Haj (1997), the rate of protein synthesis in the anterior part is higher than that of the posterior part; once the animal completes postmoult in the anterior half, the rate of protein synthesis becomes equal in both halves. They found that mRNA level of actin and myosin remained the same over the biphasic moult, which leads to the conclusion that the change in expression might have occurred only at the translation level, not in the transcription rate. More clearly, though mRNA for the muscle protein is synthesised in equal amounts, its translation into protein happened at two different times when the two halves moulted. The question remains: why is the protein synthesis rate higher in the anterior part compared to the posterior? A molecular-level study about the translation mechanism is also needed for answering these questions. Another important finding was that in I. rescata, the rate of protein synthesis increased with a rise in temperature; i.e., transcription and translation are directly proportional to temperature (Whiteley and El Haj 1997). This may be the reason behind the seasonal moulting in some isopods. Cymothoid-like N. indica shows a higher moulting rate in the summer than in the monsoon season (Kottarathil et al. 2019).
Conclusions
Reviewing the biphasic moulting in isopods enabled us to reach the following conclusions. 1) Isopods belonging to different habitats and lifestyles varied both phenotypically as well as genetically. 2) Despite the minor moult-related changes in the physical and physiological behaviours, the general pattern of biphasic moulting is conserved in all isopods, either in aquatic (including the parasitic) or terrestrial inhabitants. 3) The conserved biphasic moulting phenomenon has varying functional significance to favour the successful adaptation of isopods inhabiting a wide range of habitats and lifestyles. The application of advanced molecular studies would help address the exact mechanism of biphasic moulting and its control in isopods.
Data availability
Not applicable.
Code availability
Not applicable.
References
Alexander DE (1988) Kinematics of swimming in two species of Idotea (Isopoda: Valvifera). J Exp Biol 138(1):37–49. https://doi.org/10.1242/jeb.138.1.37
Alikhan MA (1972) Changes in the hepatopancreas metabolic reserves of Porcellio laevis Latreille during starvation and the moult-cycle. Am Midl Nat 87(2):503–514. https://doi.org/10.2307/2423579
Alikhan MA (1983) Oxygen consumption and haemolymph pressure measurements in Armadillidium vulgare (Latreille) (Armadillidiidae: Isopoda) during ecdysis. J Crust Biol 3(1):25–33. https://doi.org/10.2307/1547851
Allee WC (1913) Further studies on physiological states and rheotaxis in isopoda. J Exp Zool 15(3):257–295. https://doi.org/10.1002/jez.1400150302
Aubin PA (1914) Some notes on the terrestrial Isopoda. J Eton Biol 9:15–20
Barnard KH (1920) Contributions to the crustacean fauna of South Africa. 6. Further additions to the list of marine Isopoda. Ann S Afr Mus 17:319–438
Barnes TC (1932) Salt requirements and space orientation of the littoral isopod Ligia in Bermuda. Biol Bull 63(3):496–504. https://doi.org/10.2307/1537351
Brandt A, Poore GCB (2003) Higher classification of the Flabelliferan and related isopoda based on a reappraisal of relationships. Invertebr Syst 17(6):893–923. https://doi.org/10.1071/IS02032
Broly P, Deville P, Maillet S (2013) The origin of terrestrial isopods (Crustacea: Isopoda: Oniscidea). Evol Ecol 27(3):461–476. https://doi.org/10.1007/s10682-012-9625-8
Bruce NL (2004) New species of the Cirolana 'parva-group' (Crustacea: Isopoda: Cirolanidae) from coastal habitats around New Zealand. Species Divers 9(1):47–66. https://doi.org/10.12782/specdiv.9.47
Brusca RC, Wilson GDF (1991) Phylogenetic analysis of the isopoda with some classificatory recommendations. Memoirs of the Queensland Museum 31:143–204
Brusca RC, Coelho VR, Taiti S (2007) Isopoda. In: Carlton JT (ed) The light and smith manual: intertidal invertebrates from central California to Oregon. UC Press, Berkeley, pp 503–542
Bulnheim HP (1974) Respiratory metabolism of Idotea balthica (Crustacea, Isopoda) in relation to environmental variables, acclimation processes and moulting. Helgoland Wiss Meer 26(3–4):464–480. https://doi.org/10.1007/BF01627627
Bursell E (1955) The transpiration of terrestrial isopods. J Exp Biol 32(2):238–255. https://doi.org/10.1242/jeb.32.2.238
Carefoot TH (1993) Physiology of terrestrial isopods. Comp Biol Physiol A Physiol 106(3):413–429. https://doi.org/10.1016/0300-9629(93)90235-V
Charniaux-Legrand H (1952) Le cycle d’intermue des amphipodes et ses particularités chez les formes terrestres (Talitridae). Arch Zool Exp Gen 88:1297–1298
Chiang RG, Steel CH (1984) Neuroendocrinology of growth and moulting in terrestrial isopods. Symp Zool Soc Lond 53:109–125
Chiang RG, Steel CGH (1985) Ultrastructure and distribution of identified neurosecretory terminals in the sinus gland of the terrestrial isopod Oniscus asellus. Tissue Cell 17(3):405–415. https://doi.org/10.1016/0040-8166(85)90058-8
Chiang RG, Steel CGH (1986) Electrical activity of the sinus gland of the terrestrial isopod, Oniscus asellus: characteristics of identified potentials recorded extracellularly from neurosecretory terminals. Brain Res J 377(1):83–95. https://doi.org/10.1016/0006-8993(86)91193-5
Chiang RG, Steel CH (1989) Neurobiology of the brain-sinus gland neurosecretory system of the terrestrial isopod, Oniscus asellus L, 1758. Monit Zool Ital 4:333–334
Cloudsley-Thompson JL (1988) Adaptations to extreme environments. In: Evolution and adaptation of terrestrial arthropods, Springer, Berlin, Heidelbergpp 80–98. https://doi.org/10.1007/978-3-642-61360-9_6
Csonka D, Halasy K, Buczkó K, Hornung E (2018) Morphological traits - desiccation resistance - habitat characteristics: a possible key for distribution in woodlice (Isopoda, Oniscidea). ZooKeys 801:481–499. https://doi.org/10.3897/zookeys.801.23088
Drach P (1939) Mue et cycle d’intermue chez les crustacés décapodes. Ann Inst Oceanogr Monaco 19:103–391
Drach P, Tchernigovtzeff C (1967) Method for determining intermoulting stages and its general application to Crustacea. Vie et Milieu Serie A – Biologie Marine 18:595
Edney EB (1954) Woodlice and the land habitat. Biol Rev 292:185–219. https://doi.org/10.1111/j1469-185X1954tb00595x
Feldmann RM (2009) A new cirolanid isopod (Crustacea) from the cretaceous of Lebanon: dermoliths document the pre-moult condition. J Crust Biol 29(3):373–378. https://doi.org/10.1651/08-30961
Friedrich H (1883) Die Geschlechtsverhaltnisse der Onisciden. Ztschr Naturw 56:468–469
George RY (1972) Biphasic moulting in isopod crustacea and the finding of an unusual mode of moulting in the Antarctic genus Glyptonotus. J Nat Hist 6(6):651–656. https://doi.org/10.1080/00222937200770591
George RW, Sheard K (1954) Ecdysis in the isopod Porcellio scaber (Latreille). Aust J Zool 2(1):75–85. https://doi.org/10.1071/ZO9540075
Gorvett H (1956) Tegumental glands and terrestrial life in woodlice. Proc Zool Soc Lond 126(2):291–314. https://doi.org/10.1111/j.1096-3642.1956.tb00439.x
Greenavvay P (1985) Calcium balance and moulting in the crustacea. Biol Rev 6:425–454
Guarino SM, Gambardella C, Ianniruberto M, De Nicola M (1993) Colour polymorphism in Idotea baltica from the bay of Naples and its ecological significance. J Marine Biol Assoc UK 73(4):785–794. https://doi.org/10.1017/S002531540003472X
Hagedorn M, Ziegler A (2002) Analysis of Ca2+ uptake into the smooth endoplasmic reticulum of permeabilised sternal epithelial cells during the moulting cycle of the terrestrial isopod Porcellio scaber. J Exp Biol 205(13):1935–1942. https://doi.org/10.1242/jeb.205.13.1935
Hankó B (1912) Über den Einfluß einiger Lösungen auf die Häutung, Regeneration und das Wachstum von Asellus aquaticus. Archiv für Entwicklungsmechanik der Organismen 34(3):477–488. 34(3):477–488. https://doi.org/10.1007/BF02287907
Harris ZC, Charles JT, Jonathan CW (2020) The physiology of Venezillo arizonicus: water balance and the cuticular water barrier. J Insect Physiol 120:103991. https://doi.org/10.1016/j.jinsphys.2019.103991
Heimann P (1984) Fine structure and moulting of aesthetasc sense organs on the antennules of the isopod, Asellus aquaticus (Crustacea). Cell Tissue Res 235(1):117–128. https://doi.org/10.1007/BF00213731
Herold W (1913) Beiträge zur Anatomie und Physiologie einiger Landisopoden. Zool Jahrb Anat Jena 35:455–526
Hessler RR, Strömberg JO (1989) Behavior of janiroidean isopods (Asellota), with special reference to deep-sea genera. Sarsia 74(3):145–159. https://doi.org/10.1080/00364827.1989.10413424
Hessler RR, Thistle D (1975) On the place of origin of deep-sea isopods. Mar Biol 32(2):155–165. https://doi.org/10.1007/BF00388508
Hessler RR, Wilson GD, Thistle D (1979) The deep-sea isopods: a biogeographic and phylogenetic overview. Sarsia 64(1–2):67–75. https://doi.org/10.1080/00364827.1979.10411365
Hickman CP, Roberts LS, Larson A (2006) Integrated principles of zoology. McGraw-Hill, New York
Hoese B (1981) Morphologie und Funktion des Wasserleitungssystems der terrestrischen Isopoden (Crustacea, Isopoda, Oniscoidea). Zoomorphology 98(2):135–167. https://doi.org/10.1007/BF00310433
Hoese B (1982) Morphologie und Evolution der Lungen bei den terrestrischen Isopoden (Crustacea, Isopoda, Oniscoidea). Zool Jahrb Abt Anat Ontog 107:396–422
Hoese B (1984) The marsupium in terrestrial isopods. Symp Zool Soc Lond 53:65–76
Holdich DM (1984) The cuticular surface of woodlice: a search for receptors. Symp Zool Soc Lond 53:9–48
Horiguchi H, Hironaka M, Meyer-Rochow VB, Hariyama T (2007) Water uptake via two pairs of specialized legs in Ligia exotica (Crustacea, Isopoda). Biol Bull 213(2):196–203. https://doi.org/10.2307/25066635
Hornung E (2011) Evolutionary adaptation of oniscidean isopods to terrestrial life: structure, physiology and behavior. Terr Arthropod Rev 4(2):95–130. https://doi.org/10.1163/187498311X576262
Hua CJ, Li WX, Zhang D, Zou H, Li M, Jakovlić I, Wu SG, Wang GT (2018) Basal position of two new complete mitochondrial genomes of parasitic Cymothoida (Crustacea: Isopoda) challenges the monophyly of the suborder and phylogeny of the entire order. Parasit Vectors 11(1):1–15. https://doi.org/10.1186/s13071-018-3162-4
Johansen PO (1996) Reproduction and sexual maturation of the scavenging Deepwater isopod Natatolana borealis (Lilljeborg) from Western Norway. Sarsia 81(4):297–306. https://doi.org/10.1080/00364827.1996.10413627
Kensley B, Schotte M (1989) Guide to the marine isopod crustaceans of the Caribbean. Smithsonian Institution Press, Washington, D.C.
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. https://doi.org/10.1007/BF01731581
Klapow LA (1972) Fortnightly moulting and reproductive cycles in the sand-beach isopod, Excirolana chiltoni. Biol Bull 143(3):568–591. https://doi.org/10.2307/1540184
Kleinholz LH, Keller R (1979) Endocrine regulation in Crustacea. In: Barrington EJW (ed) Hormones and evolution. Academic Press, New York, pp 159–214. https://doi.org/10.1007/978-3-642-68866-9_5
Kottarathil HA, Kappalli S (2019) Characterization of a protandrous hermaphroditic reproductive system in transitional and female phases of Norileca indica—morphological, histological and ultrastructural approach. Crustaceana 92(6):641–664. https://doi.org/10.1163/15685403-00003896
Kottarathil HA, Sahadevan AV, Kattamballi R, Kappalli S (2019) Norileca indica (Crustacea: Isopoda, Cymothoidae) infects Rastrelliger kanagurta along the Malabar coast of India—seasonal variation in the prevalence and aspects of host-parasite interactions. Zool Stud 58:e35. https://doi.org/10.6620/ZS.2019.58-35
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kussakin OG (1979) Marine and brackish isopods (Isopoda) of cold and temperate waters of the northern hemisphere, 1 suborder Flabellifera. Opredeliteli po faune SSSR, izdavaemye Zoologicheskim institutom Akademii Nauk SSSR 122:1–470
Lachaise F, Le Roux A, Hubert M, Lafont R (1993) The molting gland of crustaceans: localization, activity, and endocrine control (a review). J Crust Biol 13(2):198–234. https://doi.org/10.1163/193724093X00020
Linsenmair KE (1989) Sex-specific reproductive patterns in some terrestrial isopods. In: Rasa AE, Vogel C, Voland E (eds) The sociobiology of sexual and reproductive strategies, Chapman & Hall, London, pp 19–47
Luxmoore RA (1982) The reproductive biology of some serolid isopods from the Antarctic. Polar Biol 1(1):3–11. https://doi.org/10.1007/BF00568750
Marcus JH (1990) Moulting as an indicator of growth in Asellus aquaticus (L, 1758) and A. meridianus Racovitza, 1919 (Isopoda). Crustaceana 58(2):136–143. https://doi.org/10.1163/156854090X00048
Martin G, Mocquard JP, Besse G (1979) Controle neurohumoral de la mue chez les femelles de l’oniscoide Porcellio dilatatus Brandt. Bull Soc Sci Nat Tunisie 14:47–57
Matsumoto T (1959) Upper cretaceous ammonites of California Part II. Mem Fac Sci Kyushu Univ Ser D Geol 1:172
McWhinnie MA, Kirchenberg RJ, Urbanski RJ, Schwarz JE (1972) Crustecdysone mediated changes in crayfish. Am Zool 12(2):357–372. https://doi.org/10.1093/icb/122357
Montesanto G, Cividini S (2018) The moult cycle of the terrestrial isopod Armadillo officinalis Duméril, 1816 (Crustacea: Isopoda: Oniscidea). Acta Zool 99(3):263–273. Montesanto G, Cividini S (2018) The moult cycle of the terrestrial isopod Armadillo officinalis Duméril, 1816 (Crustacea: Isopoda: Oniscidea). Acta Zool 99(3):263–273. https://doi.org/10.1111/azo.12210
Mrak P, Žnidaršič N, Tušek-Žnidarič M, Klepal W, Gruber D, Štrus J (2012) Egg envelopes and cuticle renewal in Porcellio embryos and marsupial mancas. ZooKeys 55(176):55–72. https://doi.org/10.3897/zookeys1762418
Mrak P, Žnidaršič N, Žagar K, Čeh M, Štrus J (2014) Exoskeletal cuticle differentiation during intramarsupial development of Porcellio scaber (Crustacea: Isopoda). Arthropod Struct Dev 43(5):423–439. https://doi.org/10.1016/j.asd.2014.07.002
Nagler C, Hyžný M, Haug JT (2017) 168 million years old' marine lice' and the evolution of parasitism within isopods. BMC Evol Biol 17(1):1–14. https://doi.org/10.1186/s12862-017-0915-1
Neues F, Hild S, Epple M, Marti O, Ziegler A (2011) Amorphous and crystalline calcium carbonate distribution in the tergite cuticle of moulting Porcellio scaber (Isopoda, Crustacea). J Struct Biol 175(1):10–20. https://doi.org/10.1016/j.jsb.2011.03.019
Nicholls AG (1931) Studies on Ligia oceanica part II the processes of feeding, digestion and absorption, with a description of the structure of the foregut. J Mar Biol Assoc UK 17(3):675–706. https://doi.org/10.1017/S0025315400051924
Numanoi H (1934) Calcium in the blood of Ligia exotica during non-moulting and moulting phases. J Fac Sci Univ Tokyo 3:351–358
Numanoi H (1942) Comparison of three types of calcium storing during moulting in several crustaceans. Proceedings of the Imperial Academy 18(7):415–419
Panakkool-Thamban A, Kappalli S (2020) Occurrence of life cycle dependent monophasic and biphasic moulting in a parasitic isopod. Mothocya renardi. Thalassas 36(1):115–124. https://doi.org/10.1007/s41208-019-00188-6
Pierce WD (1907) Notes on the economic importance of sowbugs. US Dept Agric Bur Entomol Bull 64:15–22
Poore GCB, Bruce NL (2012) Global diversity of marine isopods (except Asellota and crustacean symbionts). PLoS One 7(8):e43529. https://doi.org/10.1371/journalpone0043529
Porres MD, Rionda M, Madrid E, Bedano JC, Momo F, Iriarte PF (2018) Genetic variability in populations of the terrestrial isopod Armadillidium vulgare living in soils with different land uses. Pedobiologia 71:1–7. https://doi.org/10.1016/J.PEDOBI.2018.07.001
Poulin R (1995) Evolutionary influences on body size in free-living and parasitic isopods. Biol J Linn Soc 54(3):231–244. https://doi.org/10.1016/0024-4066(95)90019-5
Price JB, Holdich DM (1980a) The formation of the epicuticle and associated structures in Oniscus asellus (Crustacea, Isopoda). Zoomorphologie 94(3):321–332. https://doi.org/10.1007/BF00998208
Price JB, Holdich DM (1980b) An ultrastructural study of the integument during the moult cycle of the wood louse, Oniscus asellus (Crustacea, Isopoda). Zoomorphologie 95(3):250–263. https://doi.org/10.1007/BF00998125
Raupach MJ, Mayerv C, Malyutina M, Wägele JW (2009) Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proc R Soc B Biol Sci 2761658:799–808. https://doi.org/10.1098/rspb20081063
Sahadevan AV, Jose Priya TA, Kappalli S (2020) Biphasic moult cycle of the parasitic isopod Norileca indica (H Milne Edwards, 1840) (Isopoda: Cymothoidae): stage-wise characterisation and haemolymph ecdysteroids titre. J Crustac Biol 40:131–140. https://doi.org/10.1093/jcbiol/ruz082
Sayers EW, Beck J, Brister JR, Bolton EE, Canese K … Ostell J (2020) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 48(D1):D9–D16. https://doi.org/10.1093/nar/gkz899
Schmalfuss H (1978) Morphology and function of cuticular micro-scales and corresponding structures in terrestrial isopods (crust, Isop, Oniscoidea). Zoomorphologie 91(3):263–274. https://doi.org/10.1007/BF00999815
Schmidt C (2008) Phylogeny of the terrestrial isopoda (Oniscidea): a review. Arthropod Syst Phylogeny 66:191–226
Schmidt C, Wägele JW (2001) Morphology and evolution of respiratory structures in the pleopod exopodites of terrestrial isopoda (Crustacea, Isopoda, Oniscidea). Acta Zool 82(4):315–330. https://doi.org/10.1046/j1463-6395200100092x
Schöbl J (1880) Über die Fortpflanzung isopoder Crustaceen. Arch mikr Anat 17(1):125–140. https://doi.org/10.1007/BF02952569
Schonichen W (1898) Der darmkanal der onisciden und Aselliden. Zeit f wiss Zool 65:143–178
Seidl BHM, Ziegler A (2012) Electron microscopic and preparative methods for the analysis of isopod cuticle. Zookeys 73(176):73–85. https://doi.org/10.3897/zookeys1762294
Shuster SM (1989) Female sexual receptivity associated with moulting and differences in copulatory behavior among the three male morphs in Paracerceis sculpta (Crustacea: Isopoda). Biol Bull 1773:331–337. https://doi.org/10.2307/1541591
Smit NJ, Bruce NL, Hadfield KA (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasites Wildl 3(2):188–197. https://doi.org/10.1016/jijppaw201403004
Sparrevik E (1999) Sediment texture and cannibalism affect survival during moult in Saduria entomon (Isopoda). Mar Biol 133(3):437–441. https://doi.org/10.1007/s002270050482
Spindler KD, Spindler-Barth M (1989) Uptake of ecdysteroids. In: Koolman J (ed) Ecdysone: from chemistry to mode of action. Georg Thieme Verlag, Stuttgart, pp 245–249
Steel CGH (1977) The neurosecretory system in the aphid Megoura viciae, with reference to unusual features associated with long distance transport of neurosecretion. Gen Comp Endocrinol 31(3):307–322. https://doi.org/10.1016/0016-6480(77)90095-8
Steel CGH (1980) Mechanisms of coordination between moulting and reproduction in terrestrial isopod crustacea. The Biol Bull 159(1):206–218. https://doi.org/10.2307/1541019
Steel CGH (1993) Storage and translocation of integumentary calcium during the moult cycle of the terrestrial isopod Oniscus asellus (L). Can J Zool 71(1):4–10. https://doi.org/10.1139/z93-002
Steel CGH, Vafopoulou X (1998) Ecdysteroid titres in haemolymph and other tissues during moulting and reproduction in the terrestrial isopod, Oniscus asellus (L). Invertebr Reprod Dev 34(2–3):187–194. https://doi.org/10.1080/0792425919989652652
Stevenson JR (1961) Polyphenol oxidase in the tegumental glands in relation to the moulting cycle of the isopod crustacean Armadillidium vulgare. Biol Bull 121(3):554–560. https://doi.org/10.2307/1539454
Štrus J, Blejec A (2001) Microscopic anatomy of the integument and digestive system during the moult cycle in Ligia italica (Oniscidea). In: Kinsley B, Brusca RC (eds) Isopod systematics and evolution crustacean issues, 13th edn. Balkema, Rotterdam, pp 343–352
Štrus J, Compere P (1996) Ultrastructural analysis of the integument during the moult cycle in Ligia italica (Crustacea, Isopoda). Pflugers Arch 431(6):251–252. https://doi.org/10.1007/BF02346363
Štrus J, Tušek-Žnidarič M, Repnik U, Blejec A, Summers A (2019) Microscopy of crustacean cuticle: formation of a flexible extracellular matrix in moulting sea slaters Ligia pallasii. J Mar Biol Assoc UK9 9(4):857–865. https://doi.org/10.1017/S0025315418001017
Suzuki S (2002) Reconstruction of the female genitalia at moulting in the isopod crustacean, Armadillidium vulgare (Latreille, 1804). Crustacean Res 31:18–27. https://doi.org/10.18353/crustacea31.0_18
Suzuki S, Yamasaki Y, Fujita T, Mamiya Y, Sonobe H (1996) Ovarian and hemolymph ecdysteroids in the terrestrial isopod Armadillidium vulgare (Malacostracan Crustacea). Gen Comp Endocrinol 104(2):129–138. https://doi.org/10.1006/gcen19960155
Tait J (1911) Types of crustacean blood coagulation. J Mar Biol Assoc 9(2):191–198. https://doi.org/10.1017/S0025315400073367
Tait J (1917) V.—Experiments and Observations on Crustacea: Part II. Moulting of Isopods. Proc R Soc Edinburgh 37:59–68. https://doi.org/10.1017/S0370164600023506
Verhoeff K (1901) Beiträge zur Kenntnis paläarktischer Myriopoden. XVI. Aufsatz: zur vergleichenden Morphologie, Systematik und Geographie der Chilopoden. Nova Acta Abhandlungen der Kaiserlichen Leopoldinisch-Carolinisch Deutschen Akademie der Naturforscher 77:369–465
Vernet G, Charmatier-Daures M (1994) Mue, autotomie et régénération. In: Grassé P (ed) Morphologie, physiologie et reproduction, 7edn. Crustace’s. Traité de Zoologie. Masson, Paris, pp 107–194
Vittori M, Štrus J (2014) The integument in troglobitic and epigean woodlice (Isopoda: Oniscidea): a comparative ultrastructural study. Zoomorphology 133(4):391–403. https://doi.org/10.1007/s00435-014-0232-9
Vittori M, Kostanjšek R, Znidaršič N, Strus J (2012) Molting and cuticle deposition in the subterranean trichoniscid Titanethes albus (Crustacea, Isopoda). ZooKeys 176:23–38. https://doi.org/10.3897/zookeys.176.2285
Wägele JW (1992) Isopoda. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates, vol 9. Wiley, New York, pp 529–611
Warburg MR (1968) Behavioral adaptations of terrestrial isopods. Am Zool 8(3):545–559. https://doi.org/10.1093/icb/83545
Weber M (1881) Anatomisches iiber Trichonisciden. Zugleich ein Beitrag zur Frage nach der Bedeutung der Chromatophoren, Pigmente und verzweigten Zellen der Hautdecke. Arch Mikr Anat 19:579–648. https://doi.org/10.1007/BF02952709
Wetzer R (2001) Hierarchical analysis of mtDNA variation and the use of mtDNA for isopod (Crustacea: Peracarida: Isopoda) systematics. Contrib Zool 701:23–39. https://doi.org/10.1163/18759866-07001002
Whiteley NM, El Haj AJ (1997) Regulation of muscle gene expression over the moult in crustacea. Comp Biochem Physiol B Biochem Mol Biol 117(3):323–331. https://doi.org/10.1016/S0305-0491(97)00130-2
Wilson GDF (2008) Gondwanan groundwater: subterranean connections of Australian phreatoicidean isopods (Crustacea) to India and New Zealand. Invertebr Syst 22(2):301–310. https://doi.org/10.1071/IS07030
Wilson GDF (2009) The phylogenetic position of the isopoda in the Peracarida (Crustacea: Malacostraca). Arthropod Syst Phylogen 67:159–198
Wilson GDF, Edgecombe GD (2003) The triassic isopod Protamphisopus wianamattensis (Chilton) and comparison with extant taxa (Crustacea, Phreatoicidea). J Paleontol 77(3):454–470. https://doi.org/10.1666/0022-3360(2003)077<0454:TTIPWC>20CO;2
Wilson GDF, Fenwick GD (1999) Taxonomy and ecology of Phreatoicus typicus Chilton, 1883 (Crustacea, Isopoda, Phreatoicidae). J R Soc N Z 29(1):41–64. https://doi.org/10.1080/0301422319999517582
Wilson GD, Johnson RT (1999) Ancient endemism among freshwater isopods (Crustacea, Phreatoicidea). In: Ponder W, Lunney D (eds) The Other 99%. The Conservation and Biodiversity of Invertebrates, Transactions of the Royal Zoological Society of New South Wales, pp 264–268. https://doi.org/10.7882/0958608512
Wright JC, Machin J (1990) Water vapour absorption in terrestrial isopods. J Exp Biol 154(1):13–30. https://doi.org/10.1242/jeb.154.1.13
Wright JC, Machin J (1993) Atmospheric water absorption and the water budget of terrestrial isopods (Crustacea, Isopoda, Oniscidea). Biol Bull 184(2):243–253. https://doi.org/10.2307/1542232
Xia X (2018) DAMBE7: new and improved tools for data analysis in molecular biology and evolution. Mol Biol Evol 35(6):1550–1552. https://doi.org/10.1093/molbev/msy073
Xia X, Lemey P (2009) Assessing substitution saturation with DAMBE. In: Lemey P, Salemi M, Vandamme AM (eds) The phylogenetic handbook: a practical approach to DNA and protein phylogeny. Cambridge University Press, Cambridge, pp 615–630
Xia X, Xie Z, Salemi M, Chen L, Wang Y (2003) An index of substitution saturation and its application. Mol Phylogenet Evol 26(1):1–7. https://doi.org/10.1016/S1055-7903(02)00326-3
Zidar P, Drobne D, Štrus J (1998) Determination of moult stages of Porcellio scaber (Isopoda) for routine use. Crustaceana 646–654. https://www.jstor.org/stable/20106039
Ziegler A (1997) Ultrastructural changes of the anterior and posterior sternal integument of the terrestrial isopod Porcellio scaber Latr (Crustacea) during the moult cycle. Tissue Cell 291:63–76. https://doi.org/10.1016/S0040-8166(97)80073-0
Ziegler A, Scholz FHE (1997) The ionic hemolymph composition of the terrestrial isopod Porcellio scaber Latr during moult. J Comp Physiol B 1678:536–542. https://doi.org/10.1007/s003600050106
Zimmer M (2002) Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biol Rev Camb Philos Soc 77(4):455–493. https://doi.org/10.1017/s1464793102005912
Zimmer M, Bartholmé S (2003) Bacterial endosymbionts in Asellus aquaticus (Isopoda) and Gammarus Pulex (Amphipoda) and their contribution to digestion. Limnol Oceanogr 48(6):2208–2213. https://doi.org/10.4319/lo20034862208
Žnidaršič N, Mrak P, Tušek-Žnidarič M, Štrus J (2012) Exoskeleton anchoring to tendon cells and muscles in moulting isopod crustaceans. ZooKeys 176:39–53. https://doi.org/10.3897/zookeys.176.2445
Zuelzer M (1907) Ueber den Einfluss der Regeneration auf die Wachstumsgeschwindigkeit von Asellus aquaticus L. Arch Entw Mech Organism 25:361–397
Funding
Authors gratefully acknowledge the Department of Science and Technology, Govt. of India (DST SERB: EMR/2016/001163 dated 28.8.2017), (DST WOS-A: SR/WOS-A/LS-78/2018 (G), 28.06.2019), (DST-RFBR: INT/RUS/RFBR/P-330, 10.01.2019), and the Kerala State Council for Science, Technology, and Environment, Government of Kerala (KSCSTE/5224/2017- SRSLS dated 28.8.2018), for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No animals used in this study require ethical clearance.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahadevan, A.V., T A, J.P. & Kappalli, S. Biphasic moulting in isopods confers advantages for their adaptation to various habitats and lifestyle. Biologia 77, 1067–1081 (2022). https://doi.org/10.1007/s11756-022-01017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01017-7