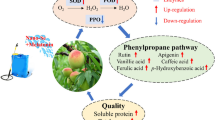
Abstract
Selenium (Se) is an essential trace element for both animals and plants. Se treatment can increase fruit Se concentration and shelf life. However, the mechanism underlying Se-delayed fruit ripening is still unclear. ‘Nanhong’ pear (Pyrus ussuriensis) is a typical climacteric fruit with high ethylene production and a rapid drop in firmness during the ripening process. In this research, two groups of Se (A and B treatments) were used to treat ‘Nanhong’ pear fruit. The results showed that these treatments could greatly increase the Se content but decreased the titratable acid content. Treatment A significantly decreased ethylene production, and the key genes controlling ethylene production, PuACSs and PuERF2, were inhibited by Se treatment. Our findings suggest that PuERF2 may play an important role in Se-mediated ethylene reduction. In addition, treatment A significantly decreased the stone cell content, and one lignin biosynthesis gene, PuC4H, was downregulated by treatment A, indicating that PuC4H may be the key gene responsible for stone cell reduction under Se treatment. Our findings provide a new medium to extend pear shelf life and improve fruit quality, which is beneficial to the development of the pear industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element for both animals and plants; however, Se deficiency in the diet is now a global problem (Schiavon et al. 2020). Around one billion people worldwide may be Se deficiency (Trippe and Pilon-Smits 2021), including those in many areas in China (Dinh et al. 2018). Se deficiency in the human body may be related to some serious medical complications, such as hypothyroidism (Arthur et al. 1992), cataracts, cardiomyopathy, and even cancer (Natasha et al. 2018; Newman et al. 2019). Diets are the major Se source for humans, but the Se contents in staple food are very low and are unable to meet the requirement for health (Zhou et al. 2020). Therefore, biofortification of Se-enriched food is very important for increasing the Se concentration in food and will be helpful for human health (White and Broadley 2009). Foliar spraying and soil application are both effective means to increase the Se content in food, and the latter is much more effective (Deng et al. 2019).
Low and moderate Se concentrations in plants can promote growth and ameliorate the adverse effects of environmental stresses (Hamilton 2004; Zhu et al. 2016), such as drought (Hasanuzzaman and Fujita 2011), water (Wang 2011), and salt (Hasanuzzaman et al. 2011). Se has been used to alleviate Cd-inhibited plant growth in cucumber (Sun et al. 2020). Se is generally effective in decreasing ethylene production and phenylalanine ammonia lyase (PAL) activity in lettuce and chicory (Malorgio et al. 2009). Furthermore, the effects of Se on fruit physiology has been raising concern. For example, Se makes tomato fruit more resistant to postharvest decay and increases the concentration of non-enzymatic antioxidants (Zhu et al. 2016; Schiavon et al. 2013). Se can decrease the rate of ethylene biosynthesis in tomato, leading to a delay in fruit ripening (Pezzarossa et al. 2014). Foliar Se treatment can decrease ethylene production by repressing the expression of ACS and ACO in tomato fruit (Zhu et al. 2017). Moreover, Se treatment increases the Se concentration in fruit and prolong the shelf life by delaying the reduction of flesh firmness and fruit ripening in peach and pear (Pezzarossa et al. 2012). In apple, foliar application of Se could retard flesh firmness reduction and fruit ripening by lowering ethylene biosynthesis rate (Babalar et al 2019). However, the mechanism of how Se delays fruit ripening is still unclear.
‘Nanhong’ pear (Pyrus ussuriensis) is a red bud sport variety of ‘Nanguo’ pear. The fruit of ‘Nanhong’ pear is typical climacteric fruit, and ethylene is the main factor controlling the ripening process (Yuan et al. 2020). During ripening, the fruits produce high amounts of ethylene, and firmness drops rapidly. ‘Nanhong’ pear fruits quality declined, including a rapid increase in the stone cell content and a decrease in the aroma, which seriously influence the shelf life and economic benefits of ‘Nanhong’ pear (Liu 2019). Thus, finding a way to improve fruit quality is very important for ‘Nanhong’ pear. As an important trace element, Se is increasingly used for vegetables, fruits, and food crops to produce selenium-rich foods (Nawaz et al. 2017). However, little is known about whether Se treatment can increase the Se content and fruit quality of ‘Nanhong’ pear.
Here, we used Se to treat ‘Nanhong’ pear and found that the Se content increased in the Se-treated fruits. In addition, both the ethylene production and the stone cell content decreased. Our results showed that Se treatment could improve the quality of ‘Nanhong’ pear fruit, which provides new insights for improving fruit quality.
Materials and methods
Plant materials and treatments
‘Nanhong’ pear (Pyrus ussuriensis Maxim.) was grafted on ‘Shanli’ (Pyrus ussuriensis Maxim.) and grown at the experimental farm of Shenyang Agricultural University (Shenyang, China). The experimental farm had a sandy loam soil and the pH was 7.2. The soil contains 1.98% of organic matters, 0.085% of total nitrogen, 3 and 56 mg/kg of available phosphorus and potassium, respectively. The trees were planted at 2.0 m × 4.0 m spacing. We designed two treatments, and distilled water was used as a control. Treatment A was a 7000-fold dilution of a mixture of 0.1% nano-selenium fertilizer (Hengshui Gemei Micronutrient Co., Ltd., Hengshui, China), 40% ethephon (an ethylene precursor compound; Sigma, http://www.sigmaaldrich.com/), and a 1000-fold dilution of 1% sodium selenite (10102-18-8, Nanjing Chemical Reagent Co., Ltd., Nanjing, China). Treatment B was a 5000-fold dilution of a mixture of 0.1% nano-selenium fertilizer, 40% ethephon, and a 1000-fold dilution of 1% sodium selenite. The treatments were used to spray the trees, including leaves and fruits, at 60, 75, and 95 days after flowering. Three trees were selected as one biological replicate and every treatment included three biological replicates. After harvest (139 days after flowering), the fruits were transported to the laboratory and stored at room temperature for 15 days; sampling was conducted every 5 days. At each sampling time, fruits were used to measure ethylene production and other indexes, and then the fruits were sliced, frozen in liquid nitrogen, and stored at - 80 °C for later use.
Measurement of fruit firmness
The fruit firmness measurement was carried out according to Yuan et al. (2017) using a portable pressure tester (FT-327, Facchini, Italy) fitted with an 11-mm-diameter probe. Each fruit was cut into four thin discs (approximately 2.5 cm in diameter) from opposite sides. The probe was pressed into the cut surface of the fruit to a depth of 8–9 mm. Five fruits were used per sample.
Measurement of soluble solids and the sugar content
For the measurement of soluble solids, the fruit flesh was ground into a powder and filtered through a cell strainer (Cat. no. CSS010040, Jet Biofil, https://www.jetbiofil.com), and then the soluble solids remaining on the filter were measured by a sugar meter (PAL-1, Atago, Tokyo, Japan). Measurement of the sugar content was carried out using an HPLC 1260 Infinity Series (Agilent Technologies, Santa Clara, CA, USA) according to Li et al. (2020b). First, 1 g of each sample was placed in a 50 ml centrifuge tube to which 10 ml 80% (v/v) ethanol was added. The sample was incubated at 80 °C for 30 min and then centrifuged at 12,000 rpm for 5 min. The supernatant was transferred into a new tube, and the above steps were repeated twice to ensure complete extraction. The supernatant was dried by distillation, and then 1 ml distilled water was used to dissolve the sugar. The solution was passed through a 0.45 mm membrane, and then the soluble sugar content was measured by HPLC (Agilent 1260). The specific methods were as follows: 3 μm, 7.8 × 300 mm Carbomix Ca-NP column (Sepax Technologies, Inc., Newark, DE, USA); column temperature of 80 °C; refractive index detector temperature of 35 °C; and an injection volume of 10 ml. The standards for sugar content measurement were sucrose (CAS:57-50-1), D-(+)-Glucose (14431-43-7), D-(-)-Fructose and D-Sorbitol (CAS:50-70-5), respectively. Nine fruits were collected at each sampling time and were randomly divided into three groups as three biological replicates.
Measurement of ethylene production
One or two fruits were enclosed in an airtight container (0.86 l, 24 °C) equipped with septa, and 1 ml gas was sampled using a syringe. The ethylene production was measured with a gas chromatograph (Agilent 7890A, Santa Clara, USA) according to Tan et al. (2013). Five fruits per sample were measured.
Measurement of the stone cell content
The stone cell contents were measured using the hydrochloric acid treatment method according to Lee et al. (2006) with slight modification as follows: 10 g of each sample was mixed and diluted with distilled water. Solutions were allowed to settle for 30 min, after which the supernatant was decanted; the above steps were repeated twice. The sediment was suspended in 0.5 M/L HCl for 30 min, decanted and washed twice using distilled water. Finally, the sediment was dried in an oven at 65 °C, and the stone cell content was measured. Stone cell content (%) = weight of stone cells (g DW)/weight of pulp (g FW) × 100 (Yan et al. 2014). Nine fruits were collected at each sampling time and were randomly divided into three groups as three biological replicates.
Measurement of the titratable acid content
An acid-base titration was used to measure the titratable acid content, with phenolphthalein as an indicator. Nine fruits were collected at each sampling time and were randomly divided into three groups as three biological replicates.
Measurement of Se content
The Se content measurement was performed according to Zao and Burau (1977), with minor modification. The sample was soaked with nitric and perchloric acid (7:1) overnight, after boiling at 100 °C for 12 h, digested at 150 °C until the digests were clear and transparent. Then the digests were diluted with distilled water to 25 ml. Finally, the digests were analyzed by ICP-MS (inductively coupled plasma mass spectrometry). Nine fruits were collected at each sample point, and were divided into three groups randomly as three biological replicates.
Quantitative RT-PCR
Total RNAs of each sample point were extracted according to Li et al. (2017), with little modification. 1 μg of total RNA was used to synthesize first-strand cDNA using a PrimeScript First-Strand cDNA Synthesis Kit (Takara, Japan).
Quantitative RT-PCR (qRT-PCR) was performed as described by Bu et al. (2020), and the pear Actin gene was used as the internal control (Yuan et al. 2017). The primers for each gene were designed by the online website Primer3 (http://primer3.ut.ee/) and were listed in supplemental Table 1. Three replications were conducted.
Results
The Se content increases in treated pear
In order to clarify whether the application of exogenous Se could increase the Se content in ‘Nanhong’ pear, we treated ‘Nanhong’ pear during fruit development. After harvested, the fruits were stored at room temperature for 15 days (Fig. 1A). We then measured the Se content in treated fruits, finding that both treatments A and B greatly increased the Se content in these fruits (Fig. 1B). These results indicated that our treatments were effective and suitable for the following research.
Exogenous Se treatment increased Se content in Nanhong pear fruits. Nanhong pear fruits of Se treatment were harvested at 139 DAH (days after harvest), and stored for 15 days at room temperature (A). Se content of treated fruits was investigated using ICP-MS (B). **Significant differences (p < 0.01, Student’s t test). Error bars indicated the standard deviation (SD) of 10 fruits. Bar, 10 mm
Effect of Se on fruit storage quality
Storage quality is one of the most important economic traits of fruits; a good storage quality has a decisive influence on the fruit quality and market value. In order to detect the effect of Se on fruit storage quality, we measured the ethylene production and hardness of the fruits under Se treatment. The results showed that both treatments retained fruit hardness during storage, especially on 10 and 15 DAH (Fig. 2A). The ethylene production was inhibited by these two treatments, especially treatment A (Fig. 2B).
Effect of Se on fruit sensory quality
In order to clarify the influence of Se treatment on the stone cell content, we measured the stone cell content of the pears treated with Se during fruit storage. Because the fruits on 15 DAH were too soft, we only present the results for 0, 5, and 10 DAH. On 0 and 5 DAH, both treatments decreased the stone cell content. On 10 DAH, treatment A decreased the stone cell content, whereas B had the opposite effect (Fig. 3A).
The influencing of Se treatment on Nanhong pear fruits sensory quality during storage. The stone cell content (A), soluble acid content (B) and titratable acid content (C) were measured. Solidity-acid ratio was the ratio of soluble solid to acid (D). DAH (days after harvest). **Significant differences (p < 0.01, Student’s t-test). Error bars indicate the standard deviation (SD) of three biological replicates
We then analyzed the soluble solid content and found that the soluble solid content continued to increase as the storage time prolonged, with no significant difference between the control and treatments (Fig. 3B). Soluble solid includes sugar, titratable acid (TA), and other components, so we measured the titratable acid content. Both treatments A and B greatly decreased the titratable acid content (Fig. 3C), leading to an increase in the solidity-acid ratio (Fig. 3D). We believed that treatment A was much more effective than treatment B, so we chose treatment A for the following research.
We measured the soluble sugar content in the treated and control fruits. Glucose, fructose, and sorbitol increased in response to Se treatment, but no significant difference was observed for sucrose (Fig. 4).
Sugar content of Se treatment of Nanhong pear fruits during storage. Sucrose (A), glucose (B), fructose (C) and sorbitol (D) content were measured using high-performance liquid chromatography (HPLC) of Nanhong fruits. Commercial harvest day was 134 DAH (days after harvest). **Significant differences (p < 0.01, Student’s t test). Error bars indicate the standard deviation (SD) of three biological replicates
Effect of Se on expression patterns of ethylene biosynthesis and signal transduction genes
Treatment A inhibited ethylene production (Fig. 2B), so we analyzed the expressions of PuACS1 and PuACS4, which are key enzymes in pear ethylene biosynthesis (Yuan et al. 2020), and we found that the expressions of PuACS1 and PuACS4 were inhibited by Se treatment (Fig. 5A and B), showing a similar tendency as that observed for ethylene (Fig. 2B).
Se treatment suppressed the expression of ethylene biosynthesis and signal transduction genes. The expression of ethylene biosynthesis genes PuACS1 (A) and PuACS4 (B) were investigated in Nanhong pear fruits. Ethylene signal transduction gene PuERF2 was investigated in Nanhong pear fruits (C). DAH (days after harvest). **Significant differences (p < 0.01, Student’s t test). Error bars indicate the standard deviation (SD) of three biological replicates
PuERF2 was proved to play an important role in pear ethylene signal transduction (Yue et al. 2019), and our result showed that the expression of PuERF2 was greatly inhibited by Se treatment (Fig. 5C).
Effect of Se on the expression patterns of lignin-related genes
We examined the expressions of the COMT (caffeic acid 3-O-methyltransferase), C4H (cinnamate‑4‑hydroxylase), and CAD (cinnamyl alcohol dehydrogenase) genes, which are important for lignin biosynthesis. The expression of PuCOMT decreased during fruit storage, but it was not inhibited by Se treatment (Fig. 6A). The expression pattern of PuCAD was not regular (Fig. 6B). PuC4H was greatly inhibited by Se treatment, especially on 10 and 15 DAH (Fig. 6C), indicating that PuC4H may be the key gene responsible for stone cell reduction in Se treatment.
The influence of Se treatment on lignin synthesis genes of Nanhong pear fruits. On-tree Nanhong fruits were treated with Se treatment at on 60, 75 and 95 days after flower, respectively, and harvested at 139 DAH (days after harvest). The expression of PuCOMT (A), PuCAD (B) and PuC4H (C) were investigated by qRT-PCR in untreated and A treatment fruits. **Significant differences (p < 0.01, Student’s t test). Error bars indicate the standard deviation (SD) of three biological replicates
Discussion
Se is indispensable for life, and a moderate Se concentration is beneficial to both animals and plants (Zhu et al. 2016, 2017). However, hyper-accumulated levels of Se are toxic to the creatures mentioned above. For adults, the Se dietary allowance is 55–75 μg/day (National Academy of Sciences 2000), whereas a toxic critical concentration is 400 μg/day (Combs 2001). Through Se treatment, the Se concentration in ‘Nanhong’ pear fruit was approximately 40–60 μg/kg (Fig. 1B), and the average weight of ‘Nanhong’ pear fruit used in this research was about 100 g, indicating approximately 4–6 μg Se in each treated fruit. Therefore, our method used here to produce Se-biofortified pear fruit is available and safe for humans.
Se treatment decreased the biosynthetic rate of ethylene in red tomato fruit (Pezzarossa et al. 2014) and increased the fruit firmness in peach and pear (Pezzarossa et al. 2012). This study showed that Se treatment inhibited ethylene production during the pear storage period (Fig. 2B), which was consistent with the results mentioned above. Based on previous studies, ACS (ACC synthase) is the rate-limiting enzyme in ethylene biosynthesis (Kende 1993). PuACS1 and PuACS4 are the main ACS genes controlling ethylene production in ‘Nanguo’ pear, which is similar to ‘Nanhong’ pear (Yuan et al. 2020). Here, for the first time, we showed that Se could inhibit the expression of PuACS1 and PuACS4 in pear (Fig. 5A and B), leading to ethylene reduction. This is consistent with a previous study in which ACS2 and ACS4 of tomato fruit were inhibited by Se treatment (Zhu et al. 2017). As ERF (ethylene response factor) is a key transcription factor in the ethylene signaling pathway (Stepanova and Alonso 2009), it could regulate the transcription of ethylene-responsive genes, including ACS genes. The expression of PuERF2 was induced by ethephon treatment and was considered to play an important role in ‘Nanguo’ pear fruit ripening (Yue et al. 2019). In addition, Se treatment also inhibited the expression of PuERF2 (Fig. 5C), with a similar expression pattern to PuACS1 and PuACS4 (Fig. 5). Therefore, we speculated that PuERF2 could control ethylene production by regulating the expression of PuACS2 and PuACS4, and Se could effectively inhibit the expression of PuERF2, finally leading to a decrease in ethylene. However, further research may be needed to determine how Se treatment regulates PuERF2 expression.
The stone cell content is very important for pear fruit and can influence the pear fruit quality, such as flesh hardness and chewiness (Zhang et al. 2017). Therefore, it is very important to lower the stone cell content in pear. CaCl2 treatment (0.50%) reduced the stone cell distribution density in pear (Tao et al. 2009). In addition, ethephon reduced the stone cell content in ‘Korla fragrant pear’ (Chen et al. 2020). In the present study, we found that Se treatment reduced the stone cell content in pear fruit (Fig. 3A), but the fruit firmness was promoted by Se treatment (Fig. 2A). We speculate that pear fruit softening was mainly due to the degradation of polysaccharides, such as pectin and hemicellulose (Fischer and Bennett 1991). Lignin is the main component of stone cells in pears (Zhang et al. 2020). Lignin biosynthesis is controlled by enzymes that participate in lignin monomer transport and polymerization (Li et al. 2020a), and COMT, CAD and C4H are key enzyme of lignin biosynthesis. Here, the expression of PuCOMT declined during fruit storage in the untreated fruit (Fig. 6A), showing a same tendency with stone cell content (Fig. 3A). But under Se treatment, the expression pattern of PuCOMT was not in consistent with stone cell content, which means that the inhibition of lignin biosynthesis by Se treatment is independent of PuCOMT expression. Overexpression of PbC4H1 and PbC4H3 increased the lignin content of xylem cells in Arabidopsis (Li et al. 2020a). When C4H expression is inhibited, the total lignin content is significantly reduced (Sykes et al. 2015). The expression level of GbC4H was correlated with the lignin content in different tissues (Cheng et al. 2018). The expression of C4H can be regulated by certain abiotic stresses and hormonal treatments. The C4H gene in kenaf was induced by ABA treatment (Kim et al. 2013), similar to the result for GbC4H (Cheng et al. 2018). Low temperature can also induce the expression of C4H genes, such as Rhododendron catawbiense RcC3H and Hibiscus cannabinus HcC4H (Wei et al. 2006; Janská et al. 2011). Here for the first time, we showed that Se treatment inhibited the expression of PuC4H in pear (Fig. 6C). These results indicate that in addition to abiotic stresses and hormonal treatments, Se can also influence the expression of C4H. Therefore, we speculate that Se may reduce the stone cell content by inhibiting the expression of PuC4H.
Se application influences TA and soluble solid content (SSC). Studies showed that one and 1.5 mg/l Se could increase the TA content in ‘Starking Delicious’ apple, but 0.5 mg/l Se treatment had no significant effect on TA content (Babalar et al. 2019), which was in agreement with the results reported in peach (Wu and Tian 2009). But Se treatment did not affect the TA and SSC content in tomato fruit (Pezzarossa et al. 2014). Nevertheless, the finding that Se could decrease the pear TA content in this study is just the opposite. The discrepancy might be due to the difference in sensitivity to Se among pear, tomato and apple. Another explanation might be related to the Se concentration. The SSC was not affected in apple (Babalar et al. 2019), pear-jujube (Zhao et al. 2013) and other plants (Pezzarossa et al. 2012, 2014). Here, the SSC also showed no differences between control and treated fruits. The SSC includes sugar, TA and other components. During fruit ripening, organic acid is used as respiratory substrates and carbon skeleton for the synthesis of new compounds (Stanley 1991). Meanwhile, starch converts to soluble sugars. These may lead to TA content decrease, while SSC increase during fruit ripening.
In conclusion, Se treatment increased the Se content in pear fruit. In addition, Se decreased ethylene production and the stone cell content. Moreover, the key genes for ethylene production (PuACSs and PuERF2) and lignin biosynthesis (PuC4H) were also inhibited by Se treatment. This provides a practical method for fruit quality improvement and is beneficial for prolonging shelf life.
Author contribution statement
AW: conceived this project. CY: performed most of the experiments. JZ, JL and GW: treated the sample. HB and HY: wrote the manuscript. All the authors have read and agreed to the published version of the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Arthur JR, Nicol F, Beckett GJ (1992) The role of selenium in thyroid hormone metabolism and effects of selenium deficiency on thyroid hormone and iodine metabolism. Biol Trace Elem Res 33(1–3):37–42
Babalar M, Mohebbi S, Zamani Z, Askari MA (2019) Effect of foliar application with sodium selenate on selenium biofortification and fruit quality maintenance of ‘Starking Delicious’ apple during storage. J Sci Food Agric 99(11):5149–5156
Bu HD, Yu WQ, Yuan H, Yue PT, Wei Y, Wang AD (2020) Endogenous auxin content contributes to larger size of apple fruit. Front Plant Sci 11:592540
Chen Y, Zhang Q, Dong YZ, Tao ST, Zhang TZ, Bao JP (2020) Effects of ethephon on the rate of calyx-abscission and fruit quality of ‘Korla Fragrant Pear’ and ‘Xinli No.7.’ J Henan Agric Univ. 54(6):949–955
Cheng SY, Yan JP, Meng XX, Zhang WW, Liao YL, Ye JB, Xu F (2018) Characterization and expression patterns of a cinnamate-4-hydroxylase gene involved in lignin biosynthesis and in response to various stresses and hormonal treatments in ginkgo biloba. Acta Physiol Plant 40(1):7
Combs G (2001) Selenium in global food systems. Br J Nutr 85:517–547
Deng XF, Zhao ZQ, Han ZY, Huang LQ, Liu XW (2019) Selenium uptake and fruit quality of pear (Pyrus communis L.) treated with foliar Se application. J Plant Nutr Soil Sci 182(4):637–646
Dinh QT, Cui ZW, Huang J, Tran TT, Wang D, Yang WX, Zhou F, Wng MK, Yu DS, Liang DL (2018) Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int 112:294–309
Fischer RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Mol Biol 42(1):675–703
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326(1–3):1–31
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confer enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143(3):1758–1776
Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143(3):1704–1721
Janská A, Aprile A, Zámečník J, Cattiveli L, Ovesná J (2011) Transcriptional responses of winter barley to cold indicate nucleosome remodelling as a specific feature of crown tissues. Funct Integr Genomics 11(2):307–325
Kays SJ (1991) Post-harvest physiology and handling of perishable plant products. Van Nestrand-Reinhold, New York
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
Kim J, Choi B, Natarajan S, Bae H (2013) Expression analysis of kenaf cinnamate 4-hydroxylase (C4H) ortholog during developmental and stress responses. Plant Omics 6(1):65–72
Lee S, Choi J, Kim W, Han T, Park Y, Gemma H (2006) Effect of soil water stress on the development of stone cells in pear (Pyrus pyrifolia, cv.‘Niitaka’) flesh. Sci Hortic 110(3):247–253
Li T, Xu YX, Zhang LC, Ji YL, Tan DM, Yuan H, Wang AD (2017) The Jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29(6):1316–1334
Li GH, Liu X, Zhang Y, Muhammad A, Han WL, Li DH, Cheng X, Cai YP (2020a) Cloning and functional characterization of two cinnamate 4-hydroxylase genes from Pyrus bretschneideri. Plant Physiol Biochem 156:135–145
Li XY, Guo W, Li JC, Yue PT, Bu HD, Jiang J, Liu WT, Xu YX, Yuan H, Li T, Wang AD (2020b) Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol 182:2035–2046
Liu C (2019) Effect of calcium on fruit quality and the formation of stone cell in Nanguo pear. Shenyang Agricult Univ. 50(4):399–405
Malorgio F, Diaz K, Ferrante A, Mensuali-Sodi A, Pezzarossa B (2009) Effects of selenium addition on minimally processed leafy vegetables grown in floating system. J Sci Food Agric 89(13):2243–2251
Natasha SM, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an Inference to human health. Environ Pollut 234:915–934
National Academy of Sciences (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids: a report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes. Washington, DC.
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Hussain RA (2017) Selenium supply methods and time of application influence Spring Wheat (Triticum aestivum L.) yield under water deficit conditions. J Agric Sci 155(04):643–656
Newman R, Waterland N, Moon Y, Tou JC (2019) Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention-a review. Plant Food Hum Nutr 74:449–460
Pezzarossa B, Remorini D, Gentile ML, Massai R (2012) Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J Sci Food Agric 92:781–786
Pezzarossa B, Rosellini I, Borghesi E, Tonutti P, Malorgio F (2014) Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci Hortic 65:106–110
Schiavon M, Dall’Acqua S, Mietto A, Pilon-Smits E, Sambo P, Masi A, Malagoli M (2013) Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J Agric Food Chem 61(44):10542–10554
Schiavon M, Nardi S, Vecchia FD, Ertani A (2020) Selenium biofortification in the 21st century: status and challenges for healthy human nutrition. Plant Soil 453:245–270
Stepanova AN, Alonso JM (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12(5):548–555
Sun HY, Wang XY, Li HM, Bi JH, Yu J, Liu XJ, Zhou HX, Rong ZJ (2020) Selenium modulates cadmium-induced ultrastructural and metabolic changes in cucumber seedlings. RSC Adv 10:17892
Sykes RW, Gjersing EL, Foutz K, Rottmann WH, Kuhn SA, Foster CE, Ziebell A, Turner GB, Decker SR, Hinchee MAW, Davis MF (2015) Down-regulation of p-coumaroyl quinate/shikimate 3′- hydroxylase (C3′H) and cinnamate 4-hydroxylase (C4H) genes in the lignin biosynthetic pathway of Eucalyptus urophylla x E. grandis leads to improved sugar release. Biotechnol Biofuels 8(1):128
Tan DM, Li T, Wang AD (2013) Apple 1-Aminocyclopropane-1-Carboxylic Acid Synthase Genes, MdACS1andMdACS3a, are expressed in different systems of ethylene biosynthesis. Plant Mol Biol Rep 31(1):204–209
Tao ST, Khanizadeh S, Zhang H, Zhang SL (2009) Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci 176(3):413–419
Trippe RC, Pilon-Smits E (2021) Selenium transport and metabolism in plants: phytoremediation and biofortification implications. J Hazard Mater 404:124178
Wang C (2011) Water-stress mitigation by selenium in Trifolium repens L. J Plant Nutr Soil Sci 174:276–282
Wei H, Dhanaraj AL, Arora R, Rowland LJ, Fu Y, Sun L (2006) Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: importance of moderately abundant ESTs in genomic studies. Plant Cell Environ 29:558–570
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
Wu GL, Tian JB (2009) Progress of fruit plants in selenium enriched research in China. Acta Hortic 841:599–602
Yan C, Yin M, Zhang N, Jin Q, Fang Z, Lin Y, Cai YP (2014) Stone cell distribution and lignin structure in various pear varieties. Sci Hortic 174:142–150
Yuan H, Zhang LC, Jiang ZY, Wang AD (2017) Characterization of ripening-related PuARP4 in pear ( Pyrus ussuriensis ). J Plant Growth Regul 36:766–772
Yuan H, Yue PT, Bu HD, Wang AD (2020) Genome-wide analysis of ACO and ACS genes in pear (Pyrus ussuriensis). In Vitro Cell Dev Biol-Plant 56:193–199
Yue PT, Wang YN, Bu HD, Li XY, Yuan H, Wang AD (2019) Ethylene promotes IAA reduction through PuERFs-activated PuGH3.1 during fruit ripening in pear (Pyrus ussuriensis). Postharvest Biol Technol 157:110955. https://doi.org/10.1016/j.postharvbio.2019.110955
Zasoski RJ, Burau RG (1977) A rapid nitric-perchloric acid digestion method for multi-elements tissue analysis. Commun Soil Sci Plant Anal 8:425–436
Zhang JY, Cheng X, Jin Q, Su XQ, Yan CC, Jiao XY, Li DH, Lin Y, Cai YP (2017) Comparison of the transcriptomic analysis between two Chinese white pear (Pyrus bretschneideri Rehd.) genotypes of different stone cells contents. PLoS ONE 12(10):e0187114
Zhang JY, Li JM, Xue C, Wang RZ, Zhang MY, Qi KJ, Fan J, Hu HJ, Zhang SL, Wu J (2020) The variation of stone cell content in 236 germplasms of sand pear (pyrus pyrifolia) and identification of related candidate genes. Hortic Plant J. 7(2):108–116
Zhao Y, Wu P, Wang Y, Feng H (2013) Different approaches for selenium biofortification of pear-jujube (Zizyphus jujuba cv. Lizao) and associated effects on fruit quality. J Food Agric Environ 11:529–534
Zhou XB, Yang J, Kronzucker HJ, Shi WM (2020) Selenium biofortification and interaction with other elements in plants: a review. Front Plant Sci 11:586421
Zhu Z, Chen Y, Zhang X, Li M (2016) Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci Hortic 198:304–310
Zhu Z, Chen Y, Shi G, Zhang X (2017) Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem 219:179–184
Acknowledgements
We thank the Research Square platform for publishing part of our research results on the website (https://doi.org/10.21203/rs.3.rs-626936/v1) as a preprint publication for peer communication.
Funding
This research was funded by the National Key R&D Program of China (2018YFD0201403), the National Natural Science Foundation of China (31801834) and Natural Science Foundation of Shandong Province (ZR2020QC190).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by P.K. Nagar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, C., Bu, H., Zhao, J. et al. Effect of Se application on selenium accumulation and fruit quality in pear (Pyrus ussuriensis). Acta Physiol Plant 45, 45 (2023). https://doi.org/10.1007/s11738-023-03521-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03521-y