Abstract
Dynamic changes in growth characteristics and endogenous hormone contents in the leaves and roots of Sophora davidii seedlings under low-phosphorus stress were studied to provide a reference for further study of the internal regulatory mechanism of the response strategy of this species to low-phosphorus stress. Normal phosphorus (0.5 mmol KH2PO4, NP) and low-phosphorus (0.005 mmol KH2PO4, LP) levels and different treatment times were applied to study growth characteristics, phosphorus utilization and hormone contents in the leaves and roots of potted Sophora davidii by tissue culture and sand culture. The results first showed that compared with NP, LP significantly decreased the plant height, leaf length, leaf width, leaf area, leaf perimeter and root–shoot ratio by 20.10%, 21.08%, 22.73%, 51.33%, 24.94% and 18.92%, respectively. LP decreased the total root length and root dry weight, and increased the root surface area, average root diameter, root tip number, root volume and dry weight of the aerial part on day 9, but these effects were not significant. Second, compared with NP, LP significantly decreased P contents in the aerial part and roots and P uptake efficiency in the aerial part and roots on day 9 by 23.33%, 53.89%, 14.04% and 58.06%, respectively. LP significantly increased the P utilization efficiency and leaf acid phosphatase (ACP) activity on day 9 by 82.79% and 84.38%, respectively. LP increased root ACP activity on day 9, but the effect was not significant. Third, compared with NP, LP significantly decreased abscisic acid (ABA), cytokinin (CTK) and strigolactone (SL) contents in leaves by 21.52%, 36.65% and 45.86%, respectively, and significantly increased gibberellin (GA) contents in roots by 28.92% on day 9. LP decreased GA contents in leaves and CTK contents in roots and increased indole-3-acetic acid (IAA) contents in leaves and roots and ABA and SL contents in roots on day 9, but these effects were not significant. Correlation analysis indicated that endogenous hormone contents in Sophora davidii leaves and roots under different treatment conditions had certain correlations with growth characteristics. In conclusion, Sophora davidii can improve its P utilization efficiency by changing growth characteristics and endogenous hormones to enhance its adaptive response to low-phosphorus stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus is one of the essential nutrient elements for plant growth and development and is not only a component of many important compounds in plants (such as ATP, phospholipids and nucleic acids), but also serves as the main component and energy provider of energy carriers (Kisko et al. 2015; Vitousek et al. 2010). However, phosphorus deficiency is one of the main factors restricting agricultural production. Phosphorus is abundant in soil but easily forms insoluble compounds with Fe3+, Al3+, Ca2+ and Mg2+ (Strock et al. 2018). A large amount of phosphorus is stored in soil in a bound state, resulting in a low relative availability of phosphorus to plants (Johnston et al. 2014). Plants have adopted a series of adaptation strategies in the long evolutionary process to increase the likelihood of completing their life cycle from seed germination to flowering and fruiting under low-phosphorus conditions (Giehl et al. 2014; Shen et al. 2013). In the process of adapting to low phosphorus, some plants can enhance the interception and absorption of this nutrient through a change in root morphology; some plants can transform organic phosphorus into inorganic phosphorus through the enhancement of phosphatase activity for recycling purposes; some plants can secrete a large amount of low-molecular-weight organic acids from their roots under low-phosphorus stress to improve the rhizosphere ecological environment and increase the amount of available soil phosphorus to meet their needs. The adaptive strategies of different plants to changes in root morphology under low-phosphorus stress are different, and endogenous hormones play an important role in the process (Xiong et al. 2018). A lack of phosphorus affects the synthesis, transport, metabolism and effects of hormones in plants (Waadt et al. 2015). However, changes in endogenous hormone balance will induce changes in plant morphological characteristics, biomass distribution and physiological and biochemical characteristics to adapt to a low-phosphorus environment (van de Wiel et al. 2016; Nadira et al. 2016).
All phytohormones, including indole-3-acetic acid (IAA), cytokinin (CTK), gibberellin (GA), abscisic acid (ABA) and strigolactone (SL), have been implicated in the phosphorus deficiency response (Zhang et al. 2019a, b; Wang et al. 2020). Among them, IAA is a key hormone that regulates the growth and development of plant roots (Jia et al. 2017a, b), and it plays a positive regulatory role during the initial development of lateral roots and the formation of the lateral root primordium (Du and Scheres 2018). Yamagishi et al. (2011) confirmed that some genes involved in IAA synthesis and signal transduction were largely expressed in the formation of the fasciculus root of white lupine. CTK plays a negative regulatory role in low-phosphorus stress signaling pathways. Schaefer et al. (2015) transformed the cytokinase gene into Arabidopsis thaliana and tobacco for over expression analysis and found that CTK synthesis was inhibited, which promoted the development of primary and lateral roots. GA can regulate root morphogenesis and anthocyanin synthesis in Arabidopsis thaliana under phosphorus starvation (Jiang et al. 2007), and it can lead to decreased phosphorus absorption, acid phosphatase (ACP) activity and phosphorus content in stems (Devaiah et al. 2009). Radin et al. (1982) found that ABA accumulates in cotton leaves under low-phosphorus stress. In addition, under low-phosphorus conditions, the SL content of Arabidopsis obviously increases, which inhibits bud development and stem branching, and the stem branch SL content is low or not sensitive to the inhibition of the mutant. In addition to regulating the formation of the root system, SL can also be applied for stem growth and formation of the spatial structure, which indicates that SL not only regulates the morphogenesis of the root system but also regulates the growth and development of stems and the formation of the spatial structure (Czarnecki et al. 2013). Because of the variety of plant hormones that are involved in many metabolic processes, plant growth and development are usually regulated by the interaction of multiple hormones.
The acidic soil in southern China has a strong fixation effect on phosphorus, and therefore, the soil has a low available phosphorus content. Selecting plants that can adapt to these conditions is important. Sophora davidii is a perennial shrub of the genus Sophora Leguminosae. Due to its advantages of drought tolerance, strong adaptability, rich nutrition and medicinal value, Sophora davidii has become an important plant resource for the feed industry, ecological reconstruction and soil restoration in recent years (Lin et al. 2019). At present, research on Sophora davidii is focused on drought stress (Wang et al. 2016; Wu et al. 2018), and low-phosphorus stress is rarely reported.
Therefore, in this research, Sophora davidii was selected as the research object, three low-phosphorus stress processing times (3, 6 and 9 days) were established and dynamic changes in hormone levels were analyzed in leaves and roots of Sophora davidii on days 3, 6 and 9. By combining the morphological changes and distribution of dry matter and nutrient allocation in different stages, this study reveals the internal regulatory mechanism of the response strategy of Sophora davidii to low-phosphorus stress and provides a theoretical basis for its planting in southern China.
Materials and methods
Sand culture experiment
Sophora davidii seeds obtained from the Grassland Science Laboratory of The College of Animal Science of Guizhou University of China were soaked in 3% NaClO for 20 min, rinsed with sterilized distilled water 3–4 times, soaked in distilled water for 6 h and incubated at room temperature between two layers of moistened filter paper. Seven days later, seedlings of the same size were selected and transferred to plastic buckets with a diameter of 20.5 cm and a height of 20.3 cm for sand culture, with 20 seedlings per pot. A half-strength nutrient solution was used when transplanting seedlings and was changed to a full-strength nutrient solution after 15 days. The full-strength nutrient solution consisted of the following nutrients: 1 mmol/L KNO3, 2 mmol/L Ca(NO3)2, 1.5 mmol/L CaCl2, 0.5 mmol/L MgSO4, 1.8 mmol/L KCl, 1 µmol/L H3BO3, 1 µmol/L MnSO4, 0.5 µmol/L CuSO4, 1 µmol/L ZnSO4, 0.1 µmol/L (NH4)6Mo7O24, 50 µmol/L Na2-EDTA, and 0.05 µmol/L FeSO4 (Ren 2018). After cultivation for 2 weeks, low-phosphorus treatment was applied, and the nutrient solution was changed every 2 days. The culture conditions were 14/10 h (day/dark) at 28 °C/22 °C. Each treatment was repeated three times. Normal phosphorus (0.5 mmol) and low-phosphorus (0.005 mmol) treatment nutrient solution is prepared with KH2PO4 as the phosphorus source (Ren 2018; Jia et al. 2017a, b). To compensate for the lack of K element, 0.495 mmol of KCl was added to the low-phosphorus nutrient solution. The remainder of the nutrient solution had the same composition. Finally, the pH of the nutrient solution was adjusted to 6.0 ± 0.1 with hydrochloric acid or sodium hydroxide. On days 3, 6 and 9 after low-P treatment, the plants were collected, washed with tap water and rinsed three times with distilled water. The plants were then blotted dry with tissue paper, and the growth characteristics and related physiological and biochemical indexes of Sophora davidii were measured.
Determination of plant growth characteristics
Root and leaf samples were captured with an Epson Perfection V800 photo scanner. The root parameters, including total root length, root surface area, root volume, average root diameter and root tip number, were analyzed using WinRHIZO software (Regent Instructions, Canada Inc). The leaf parameters, including leaf width, leaf length, leaf surface area and leaf perimeter, were analyzed using WinFOLIA software (Regent Instructions, Canada Inc). Plant height was measured with a ruler. The fresh seedlings were divided into aerial parts and root systems, and then both shoots and roots were dried at 105 ℃ for 1 h and then at 80 ℃ for 72 h followed by weighing. The root–shoot ratio was calculated using the formula: root–shoot ratio = dry weight of the roots (mg)/dry weight of the aerial part (mg).
Acid phosphatase activity and P content determination
For ACP activity determination, after the excised roots were placed in a solution containing 0.5 mL of H2O, 0.4 mL of Na–Ac buffer (0.2 mol/L, pH 5.2) and 0.1 mL of NPP substrate (0.15 mol/L) for 10 min at room temperature, the reaction was terminated by the addition of NaOH. The absorption of the reaction solution was determined at 405 nm.
For P content determination, after the dried aerial part and roots of Sophora davidii were digested with H2SO4–H2O2, the molybdenum blue method (Chapman and Pratt (1962) was used, and the final determination was performed with a microplate reader. P uptake efficiency (Pu) and P utilization efficiency (PUE) were calculated using the following formulas:
Extraction and analysis of endogenous plant hormones
Extraction and analysis of endogenous plant hormones were determined according to the method reported by Mueller and Munne-Bosch (2011) with some modifications. Approximately 0.5 g of fresh leaf and root tissue was ground in liquid nitrogen, and then 1 mL of 80% methanol was added. The sample was homogenized using a vortex for 10 s every 15 min within 2 h and then centrifuged at 9600 g for 15 min. The supernatant was collected.
Standard compounds, i.e., indole-3-acetic acid (CAS: 87-51-4; IAA), cytokinin (CAS: 13114-27-7; CTK), gibberellin (CAS: 77-06-5; GA), abscisic acid (CAS: 14375-45-2; ABA) and strigolactone (CAS: 76974-79-3; SL), were purchased from Sigma (China), and the purity was ≥ 99%. These standards were dissolved in 80% methanol, and every standard was run five times using LC–MS–MS (Agilent Technologies 6460 Triple Quad LC/MS, Agilent Technologies, Santa Clara, CA) under the same conditions as the extracted samples. XBridgeTM C18 was the chromatographic column (2.5 μm, 2.1 mm × 50 mm Column, Waters, USA), mobile phase A was the aqueous solution, and mobile phase B was methanol. The column temperature was 30 ℃, and the flow rate was 0.2 mL·min−1. Mass spectrometry consisted of an electrospray negative ion source (ESI-source) to detect IAA, CTK, GA, ABA and SL in multi-reaction monitoring mode and compare the hormone peak area with the standard curve to calculate the concentration of each hormone. Three replicates were performed for each treatment.
Statistical analysis
All the data are presented as the mean values of each treatment. Two-way analysis of variance (ANOVA) was carried out between Sophora davidii under the control and treatments, followed by a least significant difference (LSD) multiple range test (p < 0.05), using SigmaPlot 14.0 statistical software.
Results
Impact of low-phosphorus stress on plant height and leaf shape of Sophora davidii
Plant height and leaf shape gradually increased from days 3 to 9 (Figs. 1, 2). Compared with NP, LP decreased the plant height and leaf shape at the three sampling times, and LP significantly decreased the plant height, leaf length, leaf width, leaf area and leaf perimeter on day 9 by 20.10%, 21.08%, 22.73%, 51.33% and 24.94%, respectively.
Plant height and leaf shape measured at the three sampling times under the two P levels (mean ± SD). a Plant height. b Leaf length. c Leaf width. d Leaf surface. e Leaf perimeter. LP and NP represent the low and normal phosphorus treatments, respectively. Different lowercase letters indicate significant differences (p < 0.05) among the sampling periods within each index
Impact of low-phosphorus stress on root characteristic of Sophora davidii
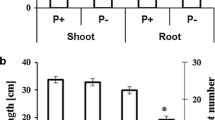
Root characteristic gradually increased from days 3 to 9 (Fig. 1). Compared with NP, LP decreased the total root length on days 3, 6 and 9, but this effect was not significant (Fig. 3a). Compared with NP, LP increased the root surface area, average root diameter and root volume on days 3, 6 and 9, but only the effect on the average root diameter was significant on day 3, which increased by 80% (Fig. 3b, c, e). Compared with NP, LP decreased the root tip number on days 3 and 6 and increased the root tip number on day 9, but these effects were not significant effect (Fig. 3d).
Root morphology at the three sampling times under the two P levels (mean ± SD). a Total root length. b Root surface area. c Average root diameter. d Root tip number. e Root volume. LP and NP represent the low and normal phosphorus treatments, respectively. Different lowercase letters indicate significant differences (p < 0.05) among the sampling periods within each index
Impact of low-phosphorus stress on dry weight and root–shoot ratio of Sophora davidii
Compared with NP, LP increased the dry weight of the aerial part and decreased the dry weight of the roots on day 9, but these effects were not significant. LP significantly decreased the root–shoot ratio by 18.92% on day 9 (Table 1).
Impact of low-phosphorus stress on P content, P uptake efficiency and P utilization efficiency of Sophora davidii
Compared with NP, LP significantly decreased the P content of the aerial part and roots on day 9 by 23.33% and 53.89%, respectively; a similar trend was observed for the P uptake efficiency of the aerial parts and roots, which decreased by 14.04% and 58.06%, respectively, but the opposite trend was observed for P utilization efficiency. Compared with NP, LP significantly increased the P utilization efficiency by 82.79% on day 9 (Table 2).
Impact of low-phosphorus stress on acid phosphatase activity of Sophora davidii
The leaf ACP activity under NP and LP conditions peaked on days 3 and 9, respectively. Compared with NP, LP increased leaf ACP activity on days 3, 6 and 9 but had a significant effect only on day 9, increasing the activity by 84.38% (Fig. 4a). Root ACP activity under NP and LP conditions peaked on days 6 and 9, respectively. Compared with NP, LP significantly decreased root ACP activity on days 3 and 6 by 41.11% and 34.74%, respectively, and increased the activity on day 9, but the effect was not significant (Fig. 4b).
Impact of low-phosphorus stress on endogenous hormone concentrations in leaves of Sophora davidii
The IAA content in leaves under NP and LP conditions peaked on days 9 and 3, respectively. Compared with NP, LP significantly increased the IAA content in leaves by 25.13% on day 3 and by 156.84% on day 6 (Fig. 5a). The ABA content in leaves under NP and LP conditions peaked on day 9. Compared with NP, LP significantly decreased the ABA content in leaves by 64.37%, 50.02% and 21.52% on days 3, 6 and 9, respectively (Fig. 5b). The GA content in leaves under NP and LP conditions peaked on days 9 and 6, respectively, but the effect was not significant (Fig. 5c). The CTK content in leaves under NP and LP conditions peaked on days 9 and 6, respectively. Compared with NP, LP significantly decreased the CTK content in leaves by 36.65% on the 9th day (Fig. 5d). The SL content in leaves under NP and LP conditions peaked on days 9 and 3, respectively. Compared with NP, LP significantly decreased the SL content in leaves by 45.86% on day 9 (Fig. 5e).
Endogenous hormone contents in the leaves of Sophora davidii seedlings at different sampling times (mean ± SD). a The indole-3-acetic acid content in leaves. b The abscisic acid content in leaves. c The gibberellin content in leaves. d The cytokinin content in leaves. e The strigolactone content in leaves. LP and NP represent the low and normal phosphorus treatments, respectively. Different lowercase letters indicate significant differences (p < 0.05) among the sampling periods within each index
Impact of low-phosphorus stress on endogenous hormone concentrations in roots of Sophora davidii
The IAA content in roots under NP and LP conditions peaked on days 9 and 6, respectively. Compared with NP, LP significantly increased the IAA content in roots by 18.31% on day 6 (Fig. 6a). The ABA content in roots under NP and LP conditions peaked on days 3 and 6, respectively. Compared with NP, LP significantly increased the ABA content in roots by 55.25% on day 3 and by 590.90% on day 6 (Fig. 6b). The GA content in roots under NP and LP conditions peaked on days 3 and 9, respectively. Compared with NP, LP significantly decreased the GA content in roots by 21.13% on day 3 and significantly increased the GA content by 28.92% on day 9 (Fig. 6c). The CTK content in the roots under NP and LP conditions peaked on days 9 and 3, respectively. Compared with NP, LP significantly increased the CTK content in roots by 71.73% on day 3 (Fig. 6d). The SL content in roots under NP and LP conditions peaked on days 3 and 9, respectively. Compared with NP, LP significantly decreased the SL content in roots by 31.63% on day 3 (Fig. 6e).
Endogenous hormone contents in the roots of Sophora davidii seedlings at different sampling times (mean ± SD). a The indole-3-acetic acid content in roots. b The abscisic acid content in roots. c The gibberellin content in roots. d The cytokinin content in roots. e The strigolactone content in roots. LP and NP represent the low and normal phosphorus treatments, respectively. Different lowercase letters indicate significant differences (p < 0.05) among the sampling periods within each index
Correlational analyses
The content of endogenous hormones in leaves and roots was correlated with the growth characteristics of Sophora davidii under different treatment conditions. Under NP conditions, the IAA content of leaves was significantly negatively correlated with the dry weight of the aerial part and the P uptake efficiency of roots. The GA content of leaves was significantly negatively correlated with the root volume and P utilization efficiency. The CTK content of leaves was significantly positively correlated with the P uptake efficiency of roots, and the ABA content of roots was significantly negatively correlated with the root–shoot ratio and average root diameter.
Under LP conditions, the GA content of leaves was significantly negatively correlated with the root tip number. The CTK content of leaves was significantly negatively correlated with the average root diameter and P utilization efficiency. The SL content of leaves was significantly positively correlated with leaf length, which was significantly negatively correlated with leaf ACP activity and P accumulation in the roots. The ABA content of roots was significantly negatively correlated with root tip number. The IAA content of roots was significantly positively correlated with the P content of the aerial part. The GA content of roots was significantly positively correlated with the P utilization efficiency. The CTK content of roots was significantly positively correlated with the plant height and leaf perimeter, which was significantly negatively correlated with the dry weight of roots. The SL content of roots was significantly positively correlated with the dry weight of the aerial part and the P content of roots, which was significantly negatively correlated with the total root length and root surface area (Fig. 7).
Correlations between endogenous hormones and growth characteristics in Sophora davidii under NP (a) and LP (b) conditions. Red and blue are positive and negative R values, respectively. *Indicates a significant correlation at the 0.05 level (bilateral), **Indicates a significant correlation at the 0.01 level (bilateral)
Discussion
Plants are very sensitive to phosphorus deficiency in the early stages of growth. When the supply of phosphorus is low, the synthesis of carbohydrates will be affected, cell division will be hindered and the plant will grow slowly (Liu et al. 2015). In this study, the growth of Sophora davidii was significantly affected by the LP treatment, and leaf growth was the most obvious response of plants under stress. In this study, plant height and leaf shape gradually increased from days 3 to 9. Compared with NP, LP decreased the plant height and leaf shape in the three sampling periods, and LP significantly decreased the plant height, leaf length, leaf width, leaf area and leaf perimeter on day 9. This finding is consistent with the research results for Chinese fir (Yu et al. 2017) and alfalfa (Jia et al. 2017a, b).
Under the combined influence of biotic stress and abiotic stress, the root system of a plant also exhibits strong morphological plasticity, and the root morphology will change significantly to cope with phosphorus stress (Cao et al. 2014; Tyburski et al. 2010; Bayuelo-Jimenez et al. 2012). In this study, compared with NP, LP decreased the total root length and increased the root surface area, average root diameter, root volume and root tip number on day 9. Results presented by Zou et al. (2015), who conducted research on phosphorus starvation in Chinese fir seedlings, were similar to our findings. This might be because on the basis of a certain number of roots, Sophora davidii can increase the root diameter, root surface area, root volume and root tip number by reducing the root length, expanding the spatial extent of root absorption and increasing the contact area between roots and phosphorus, promoting the absorption and utilization of more phosphorus to match the plant needs.
The biomass allocation model is believed to assist in phosphorus acquisition by improving root foraging and absorption capacity (Richardson et al. 2011). The results showed that compared with NP, LP increased the dry weight of the aerial part and decreased the dry weight of the roots on day 9, but these effects were not significant. LP significantly decreased the root–shoot ratio on day 9. This result shows that Sophora davidii can still maintain a high biomass by improving the absorption efficiency of roots under a low-phosphorus concentration. By contrast, a low-phosphorus supply leads to a higher root biomass distribution and a higher root–shoot ratio in wheat and alfalfa (Van Lam and Stangoulis 2019; Pang et al. 2015). This phenomenon may be due to the greater effect of low-phosphorus stress on the root dry weight of Sophora davidii than stem and leaves, resulting in an absolute reduction of the root biomass.
Low-phosphorus stress not only causes adaptive changes in leaf and root morphology, but also causes a series of physiological changes in roots and even the whole plant. Phosphorus uptake efficiency and phosphorus utilization efficiency are two key characteristics of phosphorus efficiency. Some studies have shown that compared with normal phosphorus, low phosphorus significantly reduces the phosphorus content and phosphorus absorption rate of the aerial part and the root system (Tang et al. 2020). This study showed that compared to NP, LP significantly decreased the P content and P uptake efficiency of the aerial part and roots on day 9, thus demonstrating that the phosphorus concentration was closely related to the phosphorus absorption of Sophora davidii, which is consistent with a previous study (Deng et al. 2020). In this study, compared with NP, LP significantly increased the P utilization efficiency on day 9, which indicated that Sophora davidii increased its internal phosphorus utilization efficiency when phosphorus absorption was low to maintain phosphorus metabolism in the root system. ACP is an important hydrolase in the process of plant growth, which can convert organic phosphorus in soil into more easily absorbed inorganic phosphorus (Wang et al. 2018a, b). Under low-phosphorus stress, the ACP activity of plant roots was significantly enhanced, increasing the organic phosphate content in the plant root environment, accelerating the mineralization and decomposition of organic phosphorus and increasing the biological availability of organic phosphorus in the root ecosystem to improve plant phosphorus absorption and utilization efficiency (Harvey et al. 2009). Previous results have shown that the ACP activity of plants increases under phosphorus deficiency (Sun et al. 2018). Our study provided the same conclusion. In this study, compared with NP, LP increased the leaf and root ACP activity on day 9, indicating that the increase in ACP activity was the adaptive response of plants to low-phosphorus stress.
Low-phosphorus stress affects the normal growth of plants, but in the long-term evolutionary process, the plants themselves also produce a set of countermeasures. Vysotskaya et al. (2016) have shown that plant hormones are involved in these regulatory processes. IAA is the most important member of the auxin family and can promote cell elongation and maintain apical dominance, and it also plays an important role in plant growth and development (Normanly 2010). Phosphorus deficiency can increase the IAA content, thereby inducing root growth, and a high accumulation of IAA could promote remodeling of the root structure induced by low phosphorus (as shown in Arabidopsis thaliana, Lupinus albus, etc.) (Miura et al. 2011; Wang et al. 2015). In this study, compared with NP, LP increased the IAA content in the leaves and roots on days 3, 6 and 9, and the IAA content of roots was positively correlated with the P content of the aerial part. Low-phosphorus stress has been suggested to cause changes in enzymes such as YUCCA and CYP79/CYP79B3, which may increase the IAA content in the root system, thus improving the phosphorus absorption efficiency of Sophora davidii (Tang et al. 2013; Meng et al. 2013; Liu et al. 2013; Yamamoto et al. 2007).
CTK, a natural cytokinin, can promote the cell division of plant roots and shoots and plays an important role under low-phosphorus stress (Marquez-Lopez et al. 2019). In this study, compared with NP, LP decreased the CTK content in the leaves and roots of Sophora davidii on day 9, which is consistent with previous studies on corn (Shen et al. 2012) and European rape (Shi et al. 2012). Under LP stress, the CTK content in leaves was negatively correlated with the average root diameter and phosphorus utilization efficiency; the CTK content in roots was positively correlated with plant height and leaf length and negatively correlated with root dry weight. The results showed that P utilization efficiency could be improved by reducing the CTK content in the root system, thus changing the morphology of the above- and below-ground components.
SL is a hormone that has recently been found to regulate the development of different parts of plants. SL regulates axillary bud growth in branches and the root structure and the length and density of root hairs in the root system (Al-Babili and Bouwmeester 2015). In this study, compared with NP, LP significantly decreased the SL content in leaves by 45.86% on day 9, whereas the change in SL content in roots was opposite to that in leaves. LP increased the SL content in roots, which is consistent with previous studies (Kapulnik and Koltai 2016; Sun et al. 2016). The SL content was negatively correlated with the leaf length and leaf ACP activity; these effects were extremely significant. In addition, the SL content was positively correlated with the P content in roots and was negatively correlated with the total root length and root surface area. The accumulation of SL in leaves has been suggested to lead to the transport of SL from leaves to roots, improving the efficiency of root phosphorus uptake and further consolidating the role of SL as a regulator of the steady-state system of plants under PI deficiency (Gho et al. 2018; Liu et al. 2018).
GA, the main form of gibberellin, can promote cell growth and the development of leaves and roots. In this study, compared with NP, LP significantly increased the GA content in roots and decreased the GA content in leaves on day 9, and under LP conditions, the GA content in leaves was negatively correlated with the number of root tips, while the GA content in roots was positively correlated with the P utilization efficiency, which is consistent with previous studies on barley and cotton. The interaction between GA and low-phosphorus stress was confirmed (Devaiah et al. 2009). This phenomenon may be the self-defense reaction of Sophora davidii under stress, and the accumulation of GA in leaves may lead to the transport of GA from leaves to roots under phosphorus deficiency; that is, by increasing the content of GA in roots, the effects of phosphorus deficiency on plant growth and development can be alleviated, the morphological structure of roots can be changed and the efficiency of phosphorus utilization can be increased to enhance the adaptive response of plants to low-phosphorus stress (Jiang et al. 2007).
ABA plays an important role in plant growth, especially under stress. ABA regulates the unloading of photosynthetic products and the opening and closing of stomata, and when plants are exposed to environmental stresses such as drought, salinity and low phosphorus, the ABA content increases rapidly, inducing the initiation and expression of plant stress resistance systems (Wang et al. 2018a, b; Zhang et al. 2019a, b). Studies have found that ABA can promote the closure of stomata to reduce the loss of water vapor and also reduce stress damage by activating stress response genes encoding LEA proteases to jointly improve plant stress resistance (Bray 2010). In this study, compared with NP, LP significantly decreased the ABA content in leaves on days 3, 6 and 9, and the ABA content was negatively correlated with the root tip number. Radin et al (1982) found that more ABA could accumulate in cotton leaves when phosphorus was deficient compared with when phosphorus was sufficient, in opposition to our research results. In this study, the ABA content in roots increased to varying degrees compared with normal P supply, which is consistent with previous research results (Postma and Lynch 2011). This finding may have been observed because under phosphorus deficiency, the polar transport of ABA from the shoot to the root increases its concentration in the roots, thereby reducing the leaf ABA concentration. ABA triggers the expression of stress-responsive genes encoding LEA proteases in root cells and increases the phosphorus content and improves the phosphorus utilization efficiency of the root system, thus enhancing the ability of Sophora davidii to resist stress (Sharp and LeNoble 2002).
In this study, the morphological characteristics, phosphorus utilization and changes in endogenous hormones of Sophora davidii under low-phosphorus stress showed that the effect of low-phosphorus stress was greater on the Sophora davidii root system than on leaves. Therefore, the root system was speculated to be the first to sense the stress signal and to process and transmit this signal because the root system is not only an important synthesis and transformation site of plant hormones, but also an important synthesis and transformation site of plant hormones. The roots are the main organs for plants to absorb nutrients and can detect phosphorus deficiency earlier than the aboveground parts. After receiving the stress signal from the roots, the whole plant responds rapidly to the nutrient stress, and corresponding adjustment measures are used to adapt to the stress. Concurrently, a plant is not the result of a single hormone in the process of its growth and development but a comprehensive result of the coordinated regulation of various hormones. Under environmental stress, plants can alleviate growth inhibition by changing the content of various hormones to maintain normal growth and development.
Conclusion
Sophora davidii can quickly induce a series of anti-stress responses under low-phosphorus stress by changing the morphological characteristics of the aerial plant parts, reducing the length of the main root, increasing the root surface area, average root diameter, number of root tips and root volume, changing the allocation of biomass, reducing P accumulation, improving P utilization efficiency and ACP activity and changing the content of various hormones, thus reducing the negative effects caused by low-phosphorus stress and maintaining high phosphorus efficiency.
Author contribution statement
XZ: experiment design, experiment performance, experimental data collection, data analysis, manuscript writing and revision. ZZL: seeds provision, manuscript writing, resources provision and funding acquisition. LJH and XFS: experiment performance, experimental data collection and data analysis. PCW: manuscript writing and revision. All the authors read and approved the final manuscript.
References
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. In: Merchant SS (ed.) Ann Rev Plant Biol 66(1): 161–186. https://doi.org/10.1146/annurev-arplant-043014-114759.
Bayuelo-Jimenez JS, Ochoa I, Perez-Decelis VA, de Loudes M-A, Cardenas-Navarro R (2012) Phosphorus-efficiency in maize germplasm at seedling stage from the P’urhepecha plateau. Rev Fitotec Mex 35(3):199–208
Bray EA (2010) Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environm 25(2):153–161. https://doi.org/10.1046/j.1365-3040.2002.00746.x
Cao P, Ren Y, Zhang K, Teng W, Zhao X, Dong Z, Liu X, Qin H, Li Z, Wang D, Tong Y (2014) Further genetic analysis of a major quantitative trait locus controlling root length and related traits in common wheat. Mol Breed 33(4):975–985. https://doi.org/10.1007/s11032-013-0013-z
Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants and waters. Soil Sci 93(1):68
Czarnecki O, Yang J, Weston DJ, Tuskan GA, Chen J (2013) A dual role of strigolactones in phosphate acquisition and utilization in plants. Int J Mol Sci 14(4):7681–7701. https://doi.org/10.3390/ijms14047681
Deng Y, Men C, Qiao S, Wang W, Yang J (2020) Tolerance to low phosphorus in rice varieties is conferred by regulation of root growth. Crop J 8(4): 534–547. https://doi.org/10.1016/j.cj.2020.01.002
Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2(1):43–58. https://doi.org/10.1093/mp/ssn081
Du Y, Scheres B (2018) Lateral root formation and the multiple roles of auxin. J Experim Botany 69:155–167. https://doi.org/10.1093/jxb/erx223
Gho Y, An G, Park H, Jung K (2018) A systemic view of phosphate starvation-responsive genes in rice roots to enhance phosphate use efficiency in rice. Plant Biotechnol Rep 12(4):249–264. https://doi.org/10.1007/s11816-018-0490-y
Giehl RFH, Gruber BD, von Wiren N (2014) Its time to make changes: modulation of root system architecture by nutrient signals. J Experim Botany 65(3):769–778. https://doi.org/10.1093/jxb/ert421
Harvey PR, Warren RA, Wakelin S (2009) Potential to improve root access to phosphorus: the role of non-symbiotic microbial inoculants in the rhizosphere. Crop Pasture Ence 60(2):144–151. https://doi.org/10.1071/CP08084
Jia H, Zhang S, Wang L, Yang Y, Zhang H, Cui H, Shao H, Xu G (2017a) OsPht1;8, a phosphate transporter, is involved in auxin and phosphate starvation response in rice. J Experim Botany 68(18):5057–5068. https://doi.org/10.1093/jxb/erx317
Jia Z, Zhang Q, Tong Z, Li Y, Xu H, Wang X, Bi S, Cao J, He F, Wang L, Li X (2017b) Analysis of morphological and physiological responses to low Pi stress in different alfalfas. Scientia Agricultura Sinica 50(20):3898–3907. https://doi.org/10.3864/j.issn.0578-1752.2017.20.006
Jiang C, Gao X, Liao L, Harberd NP, Fu X (2007) Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol 145(4):1460–1470. https://doi.org/10.1104/pp.107.103788
Johnston AE, Poulton PR, Fixen PE, Curtin D (2014) Phosphorus: its efficient use in agriculture. Adv Agron 123:177–228. https://doi.org/10.1016/B978-0-12-420225-2.00005-4
Kapulnik Y, Koltai H (2016) Fine-tuning by strigolactones of root response to low phosphate. J Intege Plant Biol 58:203–212. https://doi.org/10.1111/jipb.12454
Kisko M, Bouain N, Rouached A, Choudhary SP, Rouached H (2015) Molecular mechanisms of phosphate and zinc signalling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environm Experim Botany 114:57–64. https://doi.org/10.1016/j.envexpbot.2014.05.013
Lin B, Liu X, Wu S, Zheng H, Huo K, Qi S, Chen C (2019) phytochemicals content, antioxidant and antibacterial activities of Sophora viciifolia. Chem Biodiv 16(7). https://doi.org/10.1002/cbdv.201900080
Liu Q, Zhou GQ, Xu F, Yan XL, Liao H, Wang JX (2013) The involvement of auxin in root architecture plasticity in Arabidopsis induced by heterogeneous phosphorus availability. Biol Plant 57(4):739–748. https://doi.org/10.1007/s10535-013-0327-z
Liu Y, Li X, Wang R, Zhang C (2015) Screen indexes for soybean tolerance to phosphorus deficiency and identification of low-P tolerant soybean varieties. J Agric Sci Technol 17: 30–41. https://doi.org/10.13304/j.nykjdb.2015.349. (Chinese)
Liu G, Pfeifer J, Francisco RDB, Emonet A, Stirnemann M, Gubeli C, Hutter O, Sasse J, Mattheyer C, Stelzer E, Walter A, Martinoia E, Borghi L (2018) Changes in the allocation of endogenous strigolactone improve plant biomass production on phosphate-poor soils. New Phytol 217(2):784–798. https://doi.org/10.1111/nph.14847
Marquez-Lopez RE, Quintana-Escobar AO, Loyola-Vargas VM (2019) Cytokinins, the Cinderella of plant growth regulators. Phytochem Rev 18:1387–1408. https://doi.org/10.1007/s11101-019-09656-6
Meng ZB, You XD, Suo D, Chen YL, Tang C, Yang JL, Zheng SJ (2013) Root-derived auxin contributes to the phosphorus-deficiency-induced cluster-root formation in white lupin (Lupinus albus). Physiol Plant 148(4):481–489. https://doi.org/10.1111/j.1399-3054.2012.01715.x
Miura K, Lee J, Gong Q, Ma S, Jin JB, Yoo CY, Miura T, Sato A, Bohnert HJ, Hasegawa PM (2011) SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiol 155(2):1000–1012. https://doi.org/10.1104/pp.110.165191
Mueller M, Munne-Bosch S (2011) Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods. https://doi.org/10.1186/1746-4811-7-37
Nadira UA, Ahmed IM, Wu F, Zhang G (2016) The regulation of root growth in response to phosphorus deficiency mediated by phytohormones in a Tibetan wild barley accession. Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2124-8
Normanly J (2010) Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harbor Perspect Biol. https://doi.org/10.1101/cshperspect.a001594
Pang J, Yang J, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2015) Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol Plant 154(4):511–525. https://doi.org/10.1111/ppl.12297
Postma JA, Lynch JP (2011) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annal Botany 107:829–841. https://doi.org/10.1093/aob/mcq199
Radin JW, Parker LL, Guinn G (1982) Water Relations of cotton plants under nitrogen deficiency: V. environmental control of abscisic acid accumulation and stomatal sensitivity to abscisic acid. Plant Physiol 70(4):1066–1070. https://doi.org/10.1104/pp.70.4.1066
Ren P (2018) Evaluation of Phosphorus Use Efficiency and Transcriptome Analysis in Barley Germplasm (Hordeum vulgare L.). Gansu Agricultural University, 125 (Chinese)
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156. https://doi.org/10.1007/s11104-011-0950-4
Schaefer M, Bruetting C, Meza-Canales ID, Grosskinsky DK, Vankova R, Baldwin IT, Meldau S (2015) The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J Experim Botany 66:4873–4884. https://doi.org/10.1093/jxb/erv214
Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53(366):33–37. https://doi.org/10.1093/jexbot/53.366.33
Shen Y, Zhang Y, Lin H, Gao S, Pan G (2012) Effect of low phosphorus stress on endogenous hormone levels of different maize genotypes in seedling stage. J Biolog Sci. https://doi.org/10.3923/jbs.2012.308.314
Shen J, Li C, Mi G, Li L, Yuan L, Jiang R, Zhang F (2013) Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J Experim Botany 64(5):1181–1192. https://doi.org/10.1093/jxb/ers342
Shi T, Zhao D, Li D, Wang N, Meng J, Xu F, Shi L (2012) Brassica napus root mutants insensitive to exogenous cytokinin show phosphorus efficiency. Plant Soil 358(1–2):57–70. https://doi.org/10.1007/s11104-012-1219-2
Strock CF, de la Riva LM, Lynch JP (2018) Reduction in Root Secondary Growth as a Strategy for Phosphorus Acquisition. Plant Physiol 176(1):691–703. https://doi.org/10.1104/pp.17.01583
Sun H, Bi Y, Tao J, Huang S, Hou M, Xue R, Liang Z, Gu P, Yoneyama K, Xie X, Shen Q, Xu G, Zhang Y (2016) Strigolactones are required for nitric oxide to induce root elongation in response to nitrogen and phosphate deficiencies in rice. Plant Cell Environm 39(7):1473–1484. https://doi.org/10.1111/pce.12709
Sun L, Wang L, Zheng Z, Liu D (2018) Identification and characterization of an Arabidopsis phosphate starvation-induced secreted acid phosphatase as a vegetative storage protein. Plant Sci 277:278–284. https://doi.org/10.1016/j.plantsci.2018.09.016
Tang H, Shen J, Zhang F, Rengel Z (2013) Interactive effects of phosphorus deficiency and exogenous auxin on root morphological and physiological traits in white lupin (Lupinus albus L). Sci China-Life Sci 56(4):313–323. https://doi.org/10.1007/s11427-013-4461-9
Tang H, Chen X, Gao Y, Hong L, Chen Y (2020) Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere. https://doi.org/10.1016/j.rhisph.2020.100201
Tyburski J, Dunajska K, Tretyn A (2010) A role for redox factors in shaping root architecture under phosphorus deficiency. Plant Signal Behav 5(1):64–66
van de Wiel CCM, van der Linden CG, Scholten OE (2016) Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207(1):1–22. https://doi.org/10.1007/s10681-015-1572-3
Van Lam N, Stangoulis J (2019) Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiol Plant. https://doi.org/10.1007/s11738-019-2891-0
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecolog Applic 20(1):5–15. https://doi.org/10.1890/08-0127.1
Vysotskaya LB, Trekozova AW, Kudoyarova GR (2016) Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2127-5
Waadt R, Hsu P, Schroeder JI (2015) Abscisic acid and other plant hormones: Methods to visualize distribution and signaling. BioEssays 37(12):1338–1349. https://doi.org/10.1002/bies.201500115
Wang Z, Rahman ABMM, Wang G, Ludewig U, Shen J, Neumann G (2015) Hormonal interactions during cluster-root development in phosphate-deficient white lupin (Lupinus albus L.). J Plant Physiol 177:74–82. https://doi.org/10.1016/j.jplph.2014.10.022
Wang H, Wang P, Zhao G, Sun Q, Long Z, Zhang Y (2016) Seed size and germination strategy of Sophora davidii under drought stress. Acta Ecol Sin 36:335–341. https://doi.org/10.5846/stxb201312032878
Wang YZ, Chen X, Lu CY, Huang B, Shi Y (2018a) Different mechanisms of organic and inorganic phosphorus release from Mollisols induced by low molecular weight organic acids. Canadian J Soil Sci. https://doi.org/10.1139/cjss-2017-0002
Wang L, Zhu J, Xiaoming S (2018b) Salt and drought stress and ABA responses related to bZIP genes from V. radiata and V. angularis. Gene Int J Focus Gene Clon Gene Str Funct 651:152–160. https://doi.org/10.1016/j.gene.2018.02.005
Wang Y, Li L, Wang R, Tang L, Zheng Y (2020) Change of root morphology in intercropping systems of wheat and faba bean under different phosphorus levels and its relationship with endogenous hormones. Chinese J Appl Ecol. https://doi.org/10.13287/j.1001-9332.202009.029 (Chinese)
Wu L, Wei X, Lu W, Li T, Yao Q, Zhao X (2018) Effects of seed vigor at different harvesting time and drought-heat stress on growth and development of Sophora davidii seedlings. Jiangsu Agric Sci 46(16):94–98. https://doi.org/10.15889/j.issn.1002-1302.2018.16.023 (Chinese)
Xiong R, Tang H, Xu M, Zeng C, Peng Y, He R, Yan Z, Qi Z, Cheng Y (2018) Transcriptomic analysis of banana in response to phosphorus starvation stress. AGRONOMY-BASEL. https://doi.org/10.3390/agronomy8080141
Yamagishi M, Zhou K, Osaki M, Miller SS, Vance CP (2011) Real-time RT-PCR profiling of transcription factors including 34 MYBs and signaling components in white lupin reveals their P status dependent and organ-specific expression. Plant Soil 342(1–2):481–493. https://doi.org/10.1007/s11104-010-0711-9
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143(3):1362–1371. https://doi.org/10.1104/pp.106.091561
Yu J, Li Y, Yin D, Zhou C, Ma X (2017) Response and physiological mechanism of Chinese fir to low phosphorus stress. Forest Res 30: 566–575. https://doi.org/10.13275/j.cnki.lykxyj.2017.04.005 (Chinese)
Zhang J, Jiang F, Shen Y, Zhan Q, Bai B, Chen W, Chi Y (2019a) Transcriptome analysis reveals candidate genes related to phosphorus starvation tolerance in sorghum. BMC Plant Biol. https://doi.org/10.1186/s12870-019-1914-8
Zhang S, Tang D, Helena K, Li C (2019b) Metabolic and physiological analyses reveal that Populus cathayana males adopt an energy saving strategy to cope with phosphorus deficiency. Tree Physiol 39(9):1630–1645. https://doi.org/10.1093/treephys/tpz074
Zou X, Wu P, Chen N, Wang P, Ma X (2015) Chinese fir root response to spatial and temporal heterogeneity of phosphorus availability in the soil. Canadian J Forest Res 45(4):402–410. https://doi.org/10.1139/cjfr-2014-0384
Acknowledgements
This work was funded through projects of the National Key Research and Development Programme of China (2016YFC0502607-04), the National Natural Science Foundation of China (31702173), and Science and Technology Project of Guizhou Province (QKHZC[2019]2295).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, X., Zhao, LL., Huang, LJ. et al. Response of growth characteristics and endogenous hormones of Sophora davidii to low-phosphorus stress. Acta Physiol Plant 43, 118 (2021). https://doi.org/10.1007/s11738-021-03284-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03284-4