Abstract
Purpose
Sleeve gastrectomy (SG) patients have substantially altered anatomy. The mechanism of rapid gastric emptying and the role of esophageal contractile function in esophago-gastric transit has not been defined. We aimed to determine the mechanisms of esophago-gastric transit and role of esophageal function following sleeve gastrectomy.
Methods
Prospective study of twenty-six asymptomatic participants post SG underwent nuclear scintigraphy and high-resolution manometry. Fourteen had semi-solid stress barium to model the emptying process. Concurrent video fluoroscopy and manometry were performed on 7 participants.
Results
Demographic data are as follows: age 45.3 ± 15.0 years, 73.1% female, excess weight loss 62.2 ± 28.1% at 8 months. Scintigraphy showed rapid gastric emptying (24.4 ± 11.4 vs. 75.80 ± 45.19 min in control, p < 0.001) with 35.24 ± 17.12% of bolus transited into small bowel on initial frame. Triggered deglutitive reflux was common (54.4% vs. 18.2%, p = 0.017). Stress barium delineated separate vertical and antral gastric compartments with cyclical emptying of 8 stages, including reflux-induced repeated esophageal peristalsis. During manometry, ramping effects were noted, with sequential swallows producing sustained isobaric pressurizations in proximal stomach (33.6 ± 29.5 mmHg). Video fluoroscopy showed individual esophageal peristalsis generating pressurizations at 5.0 ± 1.4 cm below lower esophageal sphincter (LES), at amplitude of 31.6 ± 13.1 mmHg, associated with intragastric transit. Pressurizations were sustained for 17.3 ± 8.2 s, similar to the prolonged LES contraction (18.5 ± 9.0 s, p = 0.355).
Conclusions
Repeated esophageal peristaltic contractions induced isobaric pressurization of proximal stomach, thus providing the drive to pressurize and empty the vertical compartment of the gastric sleeve. Transit following SG appeared to be esophageal-mediated and followed a distinct cycle with strong associations with reflux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sleeve gastrectomy (SG) is the most common bariatric surgical procedure performed worldwide. It has rapidly gained popularity with use outstripping the establishment of comprehensive physiological understanding of the procedure. The mechanics of gastric transit and normal physiological processes have not been comprehensively delineated. Significant controversy exists relating to key aspects of the gastric sleeve including its impact on gastroesophageal reflux disease (GERD), potential for longer term dilatation and weight regain and its perioperative complications [1].
Substantial physiological changes have been observed following the gastric sleeve including reduced gastric emptying half-time [2]. The mechanism and significance of this have not been defined. Variable effects of the sleeve on esophageal function have been proposed in studies conducted pre and post the procedure, although a definitive role of esophageal function in the mediating mechanisms of the gastric sleeve has not been established.
GERD remains a highly controversial issue following SG and likely linked to esophageal function and the intrinsically altered physiology post procedure [3, 4]. GERD appears a common and significant problem, reported as 39% in some series [5] with 48% of patients requiring proton pump inhibitors after surgery [6]. This is a significant conundrum due to the already increased propensity of GERD in patients with obesity [7, 8].
Staple line leak is one of the most significant perioperative complications [9, 10]. Leaks tend to have a prolonged and difficult course, resulting in substantial morbidity and economic cost [11]. Ninety percent of leaks occur close to angle of His, indicating a common etiology and causative mechanism [12,13,14]. The initiation and perpetuation of sleeve leaks are significantly attributed to the narrow tube demonstrating a high resting intraluminal pressure [13, 15]. Similar pathological basis has been inferred to perpetuate reflux following sleeve gastrectomy [16]. Limited objective evidence supports this premise and an improved physiological understanding may well translate to improved preventative and treatment strategies.
We felt that a more precisely defined mechanical physiology of the gastric sleeve would add significant insights into a range of key clinical issues. Our central aim was to better define the key physiological processes associated with the gastric sleeve relating to the mechanisms of transit, intraluminal pressurization, and the role of esophageal contractile function.
Methods
Patient Selection
Ethics approvals were obtained from The Alfred Hospital Human Research and Ethics Committee (HREC) no. 380/16 and The Avenue Hospital HREC no. 236. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Asymptomatic patients following SG were recruited in this prospective study.
Inclusion Criteria
Age above 18 and below 65 years, primary SG, greater than 4 months postoperative, significant weight loss (> 30 kg or > 20% total body weight loss [17]), or were demonstrating ongoing weight loss, asymptomatic without significant reflux, or other gastrointestinal symptoms (dysphagia, abdominal pain, bloating, nausea/vomiting) or perioperative complications were the inclusion criteria. An anatomical unremarkable gastric sleeve was confirmed with a liquid contrast barium swallow.
Exclusion Criteria
Current pregnancy or breast-feeding, previous esophago-gastric or bariatric surgery, known esophago-gastric motility disorder, neurological or metabolic condition such as uncontrolled hypothyroidism, or scleroderma that significantly affect esophago-gastric motility (patients with uncomplicated diabetes mellitus were accepted into the study) were the exclusion criteria.
Controls (matched for age, gender, preoperative weight, and BMI; ratio 2 cohort: 1 control) were used for comparative purposes for nuclear scintigraphy only [18].
Surgical Technique
All procedures were performed laparoscopically as previously described [19]. The stomach was mobilized and sleeved using tri-staplers (ECHELON FLEX™ GST system), commenced 4 cm from the pylorus, over 36 French bougie. The staple line was imbricated using a running suture. Contrast swallow was performed day 1 postoperative. All patients underwent modified diet protocol of gradual transition from liquid to semi-solid diet with addition of a proton pump inhibitor for 6 weeks postoperative. Normal diet was instituted after 6 weeks with cessation of proton pump inhibitor if clinically not required.
Data Collection and Research Protocol
Baseline and postoperative weight and BMI, and the use of proton pump inhibitor (PPI) were recorded. Previously validated questionnaires on reflux, dysphagia, and SF-36 quality of life were used to evaluate postoperative outcomes [20,21,22]. Overall satisfaction with the surgery was graded using a Likert scale: 0 (not satisfied) to 10 (very satisfied).
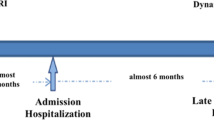
All patients underwent nuclear scintigraphy and high-resolution manometry. Willing participants were subsequently consented for stress barium and concurrent high-resolution manometry with video fluoroscopy to better delineate and demonstrate key concepts identified in the initial nuclear scintigraphy and high-resolution manometry studies (Fig. 1).
Nuclear Scintigraphy
Scintigraphy was performed using a Siemens Symbia™ Evo Excel Gamma Camera. Images were processed on a General Electric Xeleris™ Functional Imaging Workstation Version 4.
Following an overnight fast, two erect semi-solid esophageal transits were performed, each with a swallow of 1 spoonful of prepared radiolabeled semi-solid porridge: 30 g of oatmeal, 100 ml of full cream milk, 1 teaspoon of sugar, and 30 MBq of Tc-99m Calcium Phytate (Austin Health, Melbourne, Australia). Images were acquired at 1 s per frame for 60 s in the posterior projection. Following this, the patient was instructed to consume as much of the meal as tolerable in 15 min. With the patient in supine position, gastric emptying images were acquired in the left anterior oblique 30° projection at 5 s per frame for 90 min. Two liquid esophageal transits were then performed in supine position, each with a single swallow of 10 MBq of Tc-99m Calcium Phytate in 10 ml of water administered by a syringe. Images were acquired at 1 s per frame for 60 s in posterior projection.
The semi-solid and liquid transit images were processed as follows:
-
1)
A region of interest (ROI) was drawn around the esophagus and radioactivity counts were reformatted into a time-activity curve (TAC).
-
2)
Each swallow was graded by a nuclear medicine physician qualitatively as normal or delayed. A normal transit was defined as complete clearance across the gastroesophageal junction within 15 s in at least one of the swallows.
-
3)
Presence of reflux into the esophagus in any swallow was considered abnormal. Mild reflux was classified as reflux into the distal third of the esophagus, moderate as reflux into the middle third, and severe as reflux into the upper third.
Gastric emptying images were processed as follows:
-
1)
Three ROIs were drawn over the esophagus, stomach, and small bowel.
-
2)
Overall gastric emptying half-time was calculated.
-
3)
TAC: counts in the esophagus, stomach, and small bowel of the first and the last frames were expressed as proportions of each ROI over total ROI. Emptying of the esophagus and stomach were measured as counts of each frame compared to counts of the first frame.
Stress Barium
Stress barium technique was adapted from a previously established technique [23]. An initial liquid barium swallow was performed after an overnight fast: 2 swallows of 5 ml liquid barium (imaged in anterior view) then 2 swallows of 5 ml liquid barium (imaged in lateral view).
To distend the stomach, patients were required to consume 2 swallows of 1 spoonful of barium-soaked porridge (30 g of oatmeal, 100 ml of full cream milk, 1 teaspoon of cane sugar and liquid barium) followed immediately by undiluted liquid barium (up to 80 ml) until they felt excessively full or displayed symptoms of dysphagia, discomfort, or nausea. Continuous fluoroscopic screening was taken in anterior view. Delayed images up to 5 min were taken to demonstrate passage of bolus into the small bowel.
Qualitative analyses of gastroesophageal bolus transit and anatomical appearance were performed, including anatomical appearance, transit, reflux events, repeat peristaltic contractions, transpyloric flow, and presence of gastric contraction.
High-Resolution Esophageal Manometry
Esophageal manometry was performed in supine position after an overnight fast. A 16-channel silicone manometry catheter attached to a water-perfused system (Mui Scientific, Ontario, Canada) was inserted trans-nasally, with the distal sensor positioned 2 cm below the inferior border of the lower esophageal sphincter (LES). Data were recorded in real time using TRACE!1.2 (written by G. Hebbard using LabVIEW, National Instruments, Austin, TX).
All subjects underwent a standardized protocol [24].
-
1)
Supine basal recording for 60 s.
-
2)
5 deep breaths.
-
3)
10 wet swallows of 5 ml of water each.
-
4)
Volume stress test.
Basal end-expiratory intragastric pressure was used as a reference. Esophageal motility was analyzed from 10 swallows of 5 ml of water as previously described [25]. LES basal pressure was defined as the median peak end-expiratory pressure over 5 consecutive respiratory cycles, following a minimum 15-s period of no peristaltic activity. LES relaxation was recorded as the median nadir pressure at the initiation of swallowing. Crural diaphragm was identified at the axial level of maximal inspiratory pressure. Axial separation of LES and diaphragm was measured from the lower border of crural diaphragm and upper border of LES in inspiration.
A water stress test to induce isobaric intraluminal pressure in the proximal stomach was performed with five consecutive swallows of 10 ml of water. Each swallow was administered once the peristalsis contraction of the previous swallow had reached the lower esophageal sphincter.
Concurrent Video Fluoroscopy and Manometry
Fluoroscopy was performed using the stress barium protocol, with an in situ manometry catheter advanced to position the distal sensor 5 cm beyond the lower esophageal sphincter for simultaneous manometric measurement.
Manometric measurements recorded were basal and peak pressure of LES and proximal intragastric, isobaric distal esophageal pressure, and LES contractile pressure. The length of the high-pressure zone was assessed as the area of contiguous end-expiratory pressure greater than 5 mmHg beyond the distal esophagus during the 10 swallows. Secondary peristaltic contractions were defined as peristalsis occurring in the absence of a conscious swallow.
Data Presentation and Statistical Analysis
Continuous values were reported as mean and standard deviation unless otherwise stated. Median and interquartile range (IQR) were used to represent non-parametric continuous data. For comparative parametric data, Students t tests or analysis of variance (ANOVA) was used. The Mann-Whitney U test and Wilcoxon rank test were used for non-parametric data. Binary data was represented in whole numbers and percentage, and analyzed using Fisher’s exact test. A two-sided p value of 0.05 was considered statistically significant.
Data was compiled using a customized Microsoft Access 2010 (Microsoft Corporation, Redmont, WA, USA) connected to SQL server. Statistical analysis was performed using SPSS version 26 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 8.3.0 (GraphPad Software, San Diego, CA USA).
Results
Patient Details
The demographic and postoperative clinical outcomes of twenty-six patients are summarized in Table 1. Median follow-up duration from surgery was 8.0 (IQR 13.0) months with excess body weight loss of 62.2 ± 28.1%, high reported satisfaction, and few adverse symptoms.
Nuclear Scintigraphy Esophageal Transit and Esophago-gastric Clearance
Esophageal Bolus Clearance
Semi-solid and liquid transits in SG patients were comparable to the controls. Nineteen SG patients (72.7%) demonstrated no hold-up of liquid and 21 (81.8%) demonstrated no hold up of semi-solid materials, compared to control at 81.8% (p = 0.228) and 45.5% (p = 0.183) respectively. Reflux of semi-solid material were similar; 81.8% of SG patients did not demonstrate reflux compared to 90.9% of controls (p = 0.999). However, reflux during liquid swallows, characterized by immediate triggered deglutitive reflux (Fig. 2), was more common post SG. Fourteen (54.4%) demonstrated moderate reflux compared to two (18.2%) in the control groups (p = 0.017).
Schematic of triggered deglutitive reflux on esophageal swallow. The red arrows represent the trajectory of the food bolus. No reflux—the majority of the food bolus was seen moving from the proximal esophagus to the distal esophagus within 60 s after ingestion. Triggered deglutitive reflux—the majority of the radioactive food bolus moved to the distal esophagus, however refluxed up to the mid esophagus
Accelerated Gastric Transit
Rapid gastric emptying was observed post SG, with emptying half-time of 24.4 ± 11.4 min compared to 75.80 ± 45.19 min (p < 0.001) (Fig. 3a).
Nuclear scintigraphy—gastric emptying. a Gastric emptying half-time. b Proportion of counts in the small bowel in the first 2 minutes. c Schematic of proportional emptying in controls and sleeve gastrectomy. d Emptying of the esophagus and stomach in sleeve gastrectomy—the proportional emptying of the esophagus mirrored the pattern of emptying of the esophagus. e Emptying of the esophagus and stomach in obese controls—the esophagus emptied independently of the emptying of the stomach. (min = minutes)
On the initial acquisition frame, most of the radioactivity counts expectedly accumulated in the stomach (SG cohort 62.6 ± 18.1% vs. controls 74.3 ± 18.5%, p = 0.08). Notably, a significant proportion of counts were found in the small bowel in post SG patients compared to controls on the initial frames (35.24 ± 17.12% vs. 18.66 ± 13.42%, p = 0.007) (Fig. 3b). Expectantly, most of the counts were observed in the small bowel at the conclusion of the scan in post SG patients, significantly more than the controls (94.8 ± 3.8% vs. 65.1 ± 25.9%, p < 0.001). Little remained in the esophagus (0.5 ± 0.3% vs. 2.2 ± 1.6%, p < 0.001) and stomach (4.7 ± 3.6% vs. 32.7 ± 24.6%, p = 0.08) at 90 min.
Graduated Co-dependent Esophageal Clearance
Further analysis of esophagus and stomach emptying revealed a co-dependent emptying pattern post SG (Fig. 3c) compared to controls (Fig. 3d). The esophagus and stomach appeared to empty proportionally rather than as two distinct entities as was observed in the control patients with an intact stomach.
Stress Barium
Stress barium was performed on fourteen willing SG patients. The initial liquid barium studies were unremarkable and demonstrated rapid flow within a narrow gastric tube into the duodenum, without substantial hold up, stricture, or active reflux.
A consistent, cyclical, filling, and emptying pattern was observed. This consisted of a sequence of eight specific events (Fig. 4a, b). Two separate components of the sleeve were seen clearly in 13 patients. No peristaltic contractions were observed in the vertical component of the sleeve. In all of the patients, the vertical component appeared to fill, distend, and then demonstrate the opening of the incisura. In 4 patients, incisural opening was observed in concert with a suspected repeated peristalsis.
The cyclical pattern of gastric sleeve emptying and intragastric pressure. Components of the anatomical appearance (a) demonstrated at differing time points, with corresponding proximal gastric intraluminal pressures (b). In the cycle, the vertical compartment is seen to fill (1), with a rapid increase in pressure where the incisura is seen to open (2). At point (3), there is a closed LES and a bi-compartmental appearance to the sleeve, with movement around the incisura noted. At point (4), there is a macro-reflux event noted with a reduction in intraluminal pressure. Subsequently, a repeat peristaltic contraction (5) with an increase in intraluminal pressure noted. A continuing peristaltic contraction and pan-compartmentalization (6) was noted thereafter, followed by antral contraction (7). Trans-pyloric flow then occurred with reflux across the incisura into the vertical compartment (8)
After filling of the vertical compartment of the sleeve, 8 patients demonstrated a complete cyclical pattern and 5 demonstrated a partial cyclical pattern (with omission of some elements of the cycle) and 1 patient demonstrated unimpeded flow, without evidence of the cyclical pattern. This was presumed due to a larger diameter sleeve.
With opening of the incisura, delivery was achieved into the antrum, often secondary to lateral movement of the sleeve. Conversion of the two compartments into one occurred in 13 patients. Subsequently, in 78.6% of the cases, the antrum appeared to contract as a reflex (due to distension) and immediately deliver content to the duodenum. Reflux of semi-solid and liquid was clearly observed (n = 4) or suspected (based on a small volume of liquid contrast reflux) (n = 7) from the vertical component of the sleeve into the esophagus.
Notably, patients did not complain of discomfort or reflux upon distension of the vertical component of the sleeve.
Figure 4 demonstrates the eight components of the sleeve emptying cycle:
-
(1)
Vertical compartment filling
-
(2)
Incisural opening allows flow into the distal compartment
-
(3)
A bi-compartmental appearance to the stomach with separate vertical proximal and antrum components
-
(4)
Reflux events
-
(5)
Repeated peristalsis
-
(6)
Pan-compartmentalization
-
(7)
Antral contractions in response to distention
-
(8)
Transpyloric flow
Esophageal Motility
Stationary Manometry
Esophageal motor function was generally unremarkable with a low normal LES basal tone (median 12.6 mmHg, IQR 35.7 mmHg) and LES relaxation (median 61.3%, IQR 14.1%). Fifty percent demonstrated axial separation of LES and diaphragm, with a median separation of 3.0 cm (IQR 1.1 cm). Thirteen had mildly impaired esophageal peristalsis, and none had any other motility disorder.
Volume Stress Test
Twenty out of 26 SG patients demonstrated a ramping effect with subsequent consecutive liquid swallows during stationary manometry. This was noted as a sustained (> 2 s) post-deglutitive isobaric pressurization in the proximal stomach (> 10 mmHg). Isobaric elevation in proximal intragastric pressure was noted with a mean peak pressure of 33.6 ± 29.5 mmHg. Mean difference in proximal intragastric pressure from nadir swallow pressure (ΔP) was 29.5 ± 29.4 mmHg (p < 0.001). An example of ramping is shown in Fig. 5a.
Ramping effects (stationary manometry—volume-stress test). a An example of proximal intragastric pressure sequential increase with ramping with consecutive swallows (arrows demonstrating proximal intragastric pressure). b Coordinated pattern of esophageal response to ramping with generation of an isobaric pressure in the proximal stomach. c Uncoordinated pattern of response to repeated swallows where repeated swallows demonstrating uncoordinated lower esophageal contractions were noted. d Uncoordinated secondary or reflex contraction (red arrow) likely due to a reflux event
Two physiological patterns of esophageal response and intragastric pressurization were noted.
-
a)
Coordinated (Fig. 5b): there was a peristaltic event followed by completion of the prolonged LES contraction and increased intragastric pressure (IIGP).
-
b)
Uncoordinated: there was a contraction of esophagus followed by a reduction in pressure (Fig. 5c), occasionally with ineffective uncoordinated pan-esophageal contractions (esophageal decompensation) (Fig. 5d).
Concurrent Video Fluoroscopy and Manometry
Concurrent video fluoroscopy and intraluminal pressure measurement were performed on seven of the SG cohort. Table 2 and Fig. 4b summarize the intraluminal pressures recorded and correlation with cyclical pattern of emptying on stress barium. Isobaric intragastric pressure during swallows with the manometry catheter advanced (mean 5.0 ± 1.4 cm) into the stomach was 21.6 ± 11.3 mmHg.
Similar to the findings of stationary manometry, individual esophageal peristaltic contractions were noted to generate isobaric pressurizations of the stomach (mean peak proximal intragastric pressure 31.6 ± 13.1 mmHg). The ΔP achieved was 27.5 ± 13.3 mmHg (p = 0.002). These intragastric pressurizations were sustained for a mean duration of 17.3 ± 8.2 s, almost identical to the duration of the prolonged LES contraction (18.5 ± 9.0 s, p = 0.355).
Secondary peristaltic contractions repeat swallows and an oscillatory pattern was observed in all seven patients. Five patients demonstrated at least one escape event where bolus escape with relaxation of LES followed by synchronous contractions. Four patients demonstrated abnormal peristalsis as a response to reflux events.
Overall, this confirmed the role of esophageal peristaltic contractions in producing periods of high isobaric intragastric pressure due to filling and distension of the vertical compartment of the sleeve with prolonged contraction of the lower esophageal sphincter. A pattern of repeated reflux events was stimulating repeated esophageal contractions resulting in re-pressurization of the vertical gastric compartment.
Discussion
We have conducted a series of sequentially linked studies evaluating the mechanisms of esophageal and gastric transit following sleeve gastrectomy. A substantially altered paradigm of mechanical physiology has been established. This principally consists of esophageal-driven gastric clearance via anatomically and functionally separate vertical and antral gastric compartments. A gastric clearance cycle consisting of 8 distinct components resulting in accelerated transit was observed rather than an increased rate of regulated emptying. Triggered reflux events provoking repeated esophageal peristalsis were intrinsic to the emptying process.
Our initial nuclear scintigraphy studies confirmed previous work, demonstrating that gastric emptying half-time was reduced. We extended that observation by performing a focused analysis in addition to a simple analysis of time activity curves. This led to the key observation that a large proportion of the meal had transited into the small bowel (accelerated transit), prior to the commencement of the scan.
This finding suggested that the gastric clearance was immediate and potentially represented a distinct transit process rather than an increase in the rate of regulated emptying. Further interrogation of the scan led us to identify that a high proportion of deglutitive reflux occurred immediately on swallowing.
To delineate the mechanism of accelerated transit, we modeled the initial consumption of a meal using stress barium and screening fluoroscopy. This permitted concurrent observation of transit and anatomical change in higher resolution. Stress barium demonstrated rapid transit of a bolus to the duodenum via a dynamic emptying cycle of eight separate components. The vertical compartment filled, reflux developed, followed by further peristalsis, then opening of the incisura, and ultimately antral contraction leading to transpyloric flow.
The filling of the vertical component appeared directly associated with esophageal peristaltic contractions and repeated contractions of variable nature were also suspected. This suggested an esophageal mechanism or piston effect as a principal driver of sleeve emptying.
Standard esophageal manometry showed that overall motility was unremarkable and did not suggest these components were critical to emptying of the sleeve. A key finding was a ramping effect with the observation of peristaltic-induced isobaric pressurization in the proximal stomach following consumption of a small volume of liquid. This was highly indicative of a low compliance system that developed high intraluminal pressures (at a low volume threshold and from a low basal pressure), as a response to esophageal peristalsis.
In a final study, video fluoroscopy was performed with an in situ manometry catheter advanced into the vertical compartment of the sleeve. This showed that peristaltic-mediated isobaric pressurization was occurring at least 5 cm into the common vertical compartment of the sleeve in concert with filling. Importantly, these studies confirmed that repeated esophageal peristaltic contractions (interspersed with reflux events) were providing the drive to fill, distend, pressurize, and empty the vertical compartment of the sleeve.
Strengths of our study include the systematic design and use of multiple complimentary investigational modalities. Detailed analysis and sophisticated purpose-designed algorithms were used to answer questions that were posed by initial experiments. In particular, we conducted more detailed analysis of the nuclear scintigraphy scans, beyond simple measurement of gastric emptying half-time.
Stress barium was purpose-designed to provide better anatomical delineation of the mechanism of accelerated gastric transit suggested by the lower anatomical resolution nuclear scintigraphy scans. Standard esophageal manometry provided minimal mechanistic information. However, by specific evaluation of the ramping effects and utilizing video fluoroscopy to identify peristaltic contractions during the previously defined emptying cycle, we were able to extract key data and validate our findings.
While our study demonstrated consistent results from different experimental modalities, we were only able to model eating and we have not accounted for every possible situation. We also have not linked these findings to better understanding the mechanism of weight loss. We have yet to fully characterize the motility aspect of the gastric component of the sleeve itself, although we strongly suspect the vertical compartment is immotile and acts as a transit vessel. Furthermore, the finding of proximal compartment pressurization may elude to potential mechanical narrowing of the distal compartment, which were not characterized in this study.
Our future endeavors will focus on reflux and defining these syndromes, particularly in the context of the novel finding that mechanical reflux events and substrate retention in the esophagus appear central to the procedure. Validation of these findings in another group of sleeve gastrectomy patients with optimal progress, with repeated measure preoperative and postoperative, would be of value. We will also focus on linking this physiology paradigm to neurohormonal processes to better understand the mechanisms of weight loss [26].
Conclusion
Our study had identified a new physiological schema relating to emptying following gastric sleeve and defined the role of esophageal function and reflux events. It is hoped that this will form a highly useful basis for surgeons addressing key challenges associated with the gastric sleeve, link to development of improved diagnostic algorithms, and inform further investigations of the mechanism of weight loss.
References
Arman GA, Himpens J, Dhaenens J, et al. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(10):1778–86.
Vigneshwaran B, Wahal A, Aggarwal S, et al. Impact of sleeve gastrectomy on type 2 diabetes mellitus, gastric emptying time, glucagon-like peptide 1 (GLP-1), ghrelin and leptin in non-morbidly obese subjects with BMI 30-35.0 kg/m(2): a prospective study. Obes Surg. 2016;26(12):2817–23.
Tolone S, Savarino E, Yates RB. The impact of bariatric surgery on esophageal function. Ann N Y Acad Sci. 2016;1381(1):98–103.
Bou Daher H, Sharara AI. Gastroesophageal reflux disease, obesity and laparoscopic sleeve gastrectomy: the burning questions. World J Gastroenterol. 2019;25(33):4805–13.
Nocca D, Loureiro M, Skalli EM, et al. Five-year results of laparoscopic sleeve gastrectomy for the treatment of severe obesity. Surg Endosc. 2017;31(8):3251–7.
Barr AC, Frelich MJ, Bosler ME, et al. GERD and acid reduction medication use following gastric bypass and sleeve gastrectomy. Surg Endosc. 2017;31(1):410–5.
Locke III GR, Talley NJ, Fett SL, et al. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–9.
Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization—the NHANES I Epidemiologic Followup Study. Ann Epidemiol. 1999;9:424–35.
Iossa A, Abdelgawad M, Watkins BM, et al. Leaks after laparoscopic sleeve gastrectomy: overview of pathogenesis and risk factors. Langenbeck's Arch Surg. 2016;401(6):757–66.
Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257(2):231–7.
Stroh C, Kockerling F, Volker L, et al. Results of more than 11,800 sleeve gastrectomies: data analysis of the German Bariatric Surgery Registry. Ann Surg. 2016;263(5):949–55.
Cesana G, Cioffi S, Giorgi R, et al. Proximal leakage after laparoscopic sleeve gastrectomy: an analysis of preoperative and operative predictors on 1738 consecutive procedures. Obes Surg. 2018;28(3):627–35.
Yuval JB, Mintz Y, Cohen MJ, et al. The effects of bougie caliber on leaks and excess weight loss following laparoscopic sleeve gastrectomy. Is there an ideal bougie size? Obes Surg. 2013;23(10):1685–91.
Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19(12):1672–7.
Al Hajj G, Chemaly R. Fistula following laparoscopic sleeve gastrectomy: a proposed classification and algorithm for optimal management. Obes Surg. 2018;28(3):656–64.
Mion F, Tolone S, Garros A, et al. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–56.
Corcelles R, Boules M, Froylich D, et al. Total weight loss as the outcome measure of choice after Roux-en-Y gastric bypass. Obes Surg. 2016;26(8):1794–8.
Burton PR, Yap K, Brown WA, et al. Changes in satiety, supra- and infraband transit, and gastric emptying following laparoscopic adjustable gastric banding: a prospective follow-up study. Obes Surg. 2011;21(2):217–23.
Johari Y, Ooi G, Burton P, et al. Long-term matched comparison of adjustable gastric banding versus sleeve gastrectomy: weight loss, quality of life, hospital resource use and patient-reported outcome measures. Obes Surg. 2020;30(1):214–23.
Dakkak M, Bennett JR. A new dysphagia score with objective validation. J Clin Gastroenterol. 1992;14(2):354–8.
Anvari M, Allen C, Born A. Laparoscopic Nissen fundoplication is a satisfactory alternative to long-term omeprazole therapy. Br J Surg. 1995;82(7):938–42.
McHorney CA, Ware Jr JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63.
Burton PR, Brown WA, Laurie C, et al. Mechanisms of bolus clearance in patients with laparoscopic adjustable gastric bands. Obes Surg. 2010;20(9):1265–72.
Burton PR, Brown WA, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction; analysis using high resolution video manometry. Obes Surg. 2009;19(7):905–14.
Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago Classification. J Clin Gastroenterol. 2008;42:627–35.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Author information
Authors and Affiliations
Contributions
Yazmin Johari was extensively involved in design, data collection, data analysis, presentation, and write up.
Anagi Wickremasinghe, Pradipta Kiswadono, Helen Yue, Geraldine Ooi, and Cheryl Laurie were involved in the initial design, patient recruitment, data collection, and data representation.
Geoffrey Hebbard, Paul Beech, Kenneth SK Yap, Wendy Brown, and Paul Burton were extensively involved in initial concept, design, supervision, data analysis, presentation, write up, and final approval of the paper.
Corresponding author
Ethics declarations
Conflict of Interest
Wendy Brown received grants from Johnson and Johnson, grants from Medtronic, grants from GORE, personal fees from GORE, grants from Applied Medical, grants from Apollo Endosurgery, grants and personal fees from Novo Nordisc, and personal fees from Merck Sharpe and Dohme, outside the submitted work. The other authors declare that they have no conflict of interest.
Ethical Approval
Ethics approval was obtained from the Alfred Hospital Human Research and Ethics Committee (HREC) no. 380/16 and The Avenue Hospital HREC no. 236.
Informed Consent
An information statement was provided prior to commencement. Written informed consent was obtained in an opt-in manner.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Johari, Y., Wickremasinghe, A., Kiswandono, P. et al. Mechanisms of Esophageal and Gastric Transit Following Sleeve Gastrectomy. OBES SURG 31, 725–737 (2021). https://doi.org/10.1007/s11695-020-04988-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04988-1