Abstract
Background
The incidence of de novo gastroesophageal reflux disease (GERD) after LSG is substantial. However, an objective correlation with the structural gastric and EGJ changes has not been demonstrated yet. We aimed to prospectively evaluate the effects of laparoscopic sleeve gastrectomy (LSG) on the structure and function of the esophagogastric junction (EGJ) and stomach.
Methods
Investigations were performed before and after > 50% reduction in excess body weight (6–12 months after LSG). Subjects with GERD at baseline were excluded. Magnetic Resonance Imaging (MRI), high-resolution manometry (HRM), and ambulatory pH-impedance measurements were used to assess the structure and function of the EGJ and stomach before and after LSG.
Results
From 35 patients screened, 23 (66%) completed the study (age 36 ± 10 years, BMI 42 ± 5 kg/m2). Mean excess weight loss was 59 ± 18% after 7.1 ± 1.7-month follow-up. Esophageal acid exposure (2.4 (1.5–3.2) to 5.1 (2.8–7.3); p = 0.040 (normal < 4.0%)) and reflux events increased after surgery (57 ± 24 to 84 ± 38; p = 0.006 (normal < 80/day)). Esophageal motility was not altered by surgery; however, intrabdominal EGJ length and pressure were reduced (both p < 0.001); whereas the esophagogastric insertion angle (35° ± 11° to 51° ± 16°; p = 0.0004 (normal < 60°)) and esophageal opening diameter (16.9 ± 2.8 mm to 18.0 ± 3.7 mm; p = 0.029) were increased. The increase in reflux events correlated with changes in EGJ insertion angle (p = 0.010). Patients with > 80% reduction in gastric capacity (TGV) had the highest prevalence of symptomatic GERD.
Conclusion
LSG has multiple effects on the EGJ and stomach that facilitate reflux. In particular, EGJ disruption as indicated by increased (more obtuse) esophagogastric insertion angle and small gastric capacity were associated with the risk of GERD after LSG.
clinicaltrials.gov: NCT01980420
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic Sleeve Gastrectomy (LSG) has gained popularity over the last 15 years, becoming a standard procedure in the surgical treatment of morbid obesity [1, 2]. It is efficiently performed in a minimal access fashion [3] with similar morbidity and mortality rates as compared with the laparoscopic Roux-en-Y gastric bypass (RYGB) [4, 5]. Further, long-term follow-up has shown minimal differences between these two techniques in terms of durable weight loss and resolution of comorbid conditions [5,6,7].
The most frequent complication of LSG is gastroesophageal reflux disease (GERD). The Second International Consensus Summit for Sleeve Gastrectomy stated that the average incidence of de novo reflux symptoms after this form of bariatric surgery is 6.5% [8]. However, the incidence varies widely between centers with more than 1 in 5 patients complaining of new reflux symptoms after LSG in some reports [9,10,11,12,13], and underreporting by patients on acid suppressants may be an issue. In the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS), a prospective randomized trial comparing outcomes of LSG with laparoscopic RYGB with a follow-up rate of > 90% after 5 years, symptomatic reflux increased (more symptoms or increase in therapy) more often after LSG than RYGB (31.8 vs. 6.3%; p < 0.001) [5]. Moreover, other groups have described a high incidence of Barrett esophagus after this bariatric procedure [14,15,16], and cases of esophageal adenocarcinoma have been reported [17]. Thus, the risk of de novo GERD post LSG is substantial and can be linked to impaired quality of life and adverse outcomes. Notwithstanding the above, not all patients develop GERD after this form of surgery. If patient and operator dependent factors that increase the risk of de novo GERD after LSG could be identified, then patient selection and changes to surgical technique could reduce the frequency of this common complication.
Resection of the gastric fundus and body to form the gastric sleeve has obvious effects on the morphology, motility, and function of the stomach [18]. Surgeons and physiologists have proposed various factors that could increase the risk of gastroesophageal reflux after LSG. These include effacement of the esophagogastric insertion (angle of His), reduced gastric compliance with increased intraluminal pressure after the meal, and delayed gastric emptying [19,20,21]. Conversely, resection of a large proportion of acid-secreting parietal cells from the gastric corpus and reduction in central obesity must reduce gastric acid secretion and this could reduce acid reflux after bariatric surgery [22]. The pathophysiological mechanism of GERD after LSG has been investigated in anesthetized animals and in surgical preparations using invasive techniques that alter the esophagogastric junction (EGJ) structure and function [23]. In patients, to date, no one technique provides an adequate assessment. Endoscopy offers a semi-qualitative assessment of EGJ in the fasted and gas-distended stomach (Hill grading [24]), manometry provides only indirect information regarding the EGJ morphology [25, 26], and conventional radiology does not deliver adequate anatomic detail because of limited soft-tissue contrast. As a result, there is a lack of objective data to support or refute these hypotheses and the causes of GERD after LSG remain uncertain.
Magnetic Resonance Imaging (MRI) offers excellent soft-tissue contrast without exposure to ionizing radiation and can provide a comprehensive assessment of esophagogastric anatomy and function [27]. Three-dimensional (3D) models of the distal esophagus and proximal stomach are reconstructed from stacks of 2D image slices from which objective measurements can be made including the insertion angle of the esophagus into the stomach and gastric morphology [28], both of which are abnormal in GERD patients [29]. In addition, dynamic volume change of the stomach and gastric contents can be measured during ingestion of a standardized test meal and subsequent gastric emptying [30].
This study applied validated MRI and high-resolution manometry (HRM) to test the hypothesis that disruption of factors involved in reflux protection, in particular the esophagogastric insertion angle (“angle of His”), are altered in patients that develop GERD after LSG. These measurements were then compared with the results of ambulatory impedance-pH reflux monitoring to assess the impact of changes to EGJ structure and function on the frequency and severity of acid reflux in this patient group.
Materials and Methods
Study Population
This prospective single center study recruited 35 morbidly obese patients selected to undergo LSG from December 2013 to November 2015 at the Digestive Surgery Unit of the Nouvel Hôpital Civil (NHC) of Strasbourg with the support of the Image-Guided Surgery Institute (Institut de Chirurgie Guidée par l’Image/IHU) of Strasbourg. The study was approved by the National Ethics Committee and registered (clinicaltrials.gov Identifier: NCT01980420).
All patients were candidates for a bariatric procedure, according to the international guidelines, after full metabolic and psychiatric assessment, had no GERD symptom nor any current antacid therapy, and signed an informed consent to undergo a laparoscopic sleeve gastrectomy and participate in this clinical before and after study [31]. Patients presenting with contraindications to MRI studies, inability to give informed consent, and pregnant or breastfeeding patients were excluded from the study.
Study Design
All patients underwent clinical examination and body mass index (BMI) calculation. Standard pre-operative assessment before LSG in our hospital includes HRM and impedance-pH reflux monitoring, an upper gastrointestinal series (UGI), and quality of life (QoL) assessment with the Gastrointestinal Quality of Life Index (GIQLI) and GERD Symptom Assessment Scale (GSAS). Additional measurements included anatomic MRI of the esophagus and stomach before and after ingestion of the 400 ml nutrient liquid Nottingham Test Meal.
Intraoperative and postoperative complications were recorded, and the patients were followed with regular outpatient visits at 8 days, 1 month, and 3 months. The final evaluation was scheduled when the patient reached a minimum of 50% reduction in excess weight. At this point, the same anatomical and functional evaluation was repeated. A schematic of the study is shown in Fig. 1.
Surgical Procedure
All operations were performed by one of three senior bariatric surgeons, respecting the same surgical sequences and steps. Patients were placed in a lithotomy position. After pneumoperitoneum induction, 5 ports were inserted in the upper abdomen.
The stages of the surgical procedure are listed below:
-
1.
Dissection of the gastrophrenic ligament to expose the external border of the left crus;
-
2.
Dissection of the gastroepiploic arcade along the greater curvature, starting 5 to 6 cm proximally to the pylorus and ending with the division of the short gastric vessels;
-
3.
Positioning of a calibration tube (32 French) along the lesser curvature of the stomach;
-
4.
Stapling and transection of the stomach with laparoscopic staplers. A mean number of 5.3 (± 0.9) cartridges were used;
-
5.
Reinforcement suture on the stapler line and withdrawal of the calibration tube;
-
6.
Extraction of the gastric resection specimen for histopathological analysis.
Objective Evaluation of EGJ Structure and Function
Physiological measurements were performed according to published standards [32,33,34].
High-Resolution Manometry
HRM studies were performed according to Chicago Classification v3.0 standard procedures with 30-s baseline recording and 10 swallows of 5 mL water in supine position after an overnight fast using a solid-state ManoScan™ catheter with 36 channels spaced at 1-cm intervals. [33] Esophageal motility and EGJ barrier function was evaluated using the ManoView™ ESO analysis program version 3.0 (Medtronic, Duluth, USA). The following parameters were obtained: (i) total lower esophageal sphincter (LES) length: distance between lower and upper LES border in end-expiration (≥ 5 mmHg above gastric pressure); (ii) intra-abdominal LES length: distance between pressure inversion point and distal LES border; (iii) mean basal LES pressure (mmHg): mean pressure during the 30-s interval; and (iv) EGJ morphology subtype: Type I: crural diaphragm (CD) and LES overlap with single pressure peak at inspiration (no hiatus hernia); Type II: LES and CD are spatially separated resulting in double peaked pressure profile limited to 1–2 cm (small hiatus hernia); Type III: separation between LES and CD > 2 cm (large hiatus hernia). Integrated relaxation pressure (IRP), distal latency (DL), and distal contractile integral (DCI) of peristaltic contractions were assessed, and the proportion of effective and ineffective esophageal contractions and manometric diagnosis were determined.
24-h pH-Impedance Measurements
Ambulatory reflux monitoring was performed using a ZepHr@impedence/pH monitoring system; all studies were performed off proton-pump inhibitors (PPI) (stopped at least 7 days prior to measurement). Patients followed usual activities and diets and recorded timing of meals, symptoms, and position. Data were analyzed by proprietary software (BioVIEW® analysis software) with calculation of total percent of time with pH < 4.0 and/or the total number of reflux episodes (pathological if ≥ 4% or ≥ 80, respectively) as outlined by the Lyon Classification for GERD diagnosis [34].
Magnetic Resonance Imaging
MRI measurements of EGJ and gastric morphology were acquired on a MAGNETOM Aera 1.5 T-Siemens scanner using validated techniques described by Curcic and colleagues [28]. Operating procedures for measurements of gastric function were also based on the protocol for non-invasive assessment of gastric motor and sensory function for the Nottingham test meal (NTM) [30, 35]. Reference intervals in healthy subjects are presented in the same publication [30]. Specifically, gastric volume was measured in fasting condition first. In the second phase, liquid vanilla nutrition drink (1 kcal/mL; Fortimel®, Nutricia) was ingested over 10 min (40-ml cup/minute) until maximum satiation (maximum 400 mL). The first image acquisition was performed immediately after ingestion and subsequently every 10 min for the first 40 min and at 55, 70, and 90 min after ingestion. If required, imaging continued until emptying was complete. Measurements included total gastric volume (TGV) and gastric content volume (GCV), esophageal diameter, angle of insertion of the esophagus into the stomach (angle of His), and span width, which reflects distention of the proximal stomach and its relation to the esophagus. To acquire volume measurements a semi-automatic method was used to outline the contents and air on each image slice. This required an intensity-based method to define both high signal intensity gastric content volume (GCV) and low signal intensity air volumes using a custom-written software (IDL version 6.4; Research Systems Inc., Boulder, CO). The total gastric volume (TGV) was calculated from the sum of the air and content regions. The segmented area on each slice was multiplied by the slice thickness and summed over all contoured slices to measure the different stomach volumes (i.e. GCV and TGV). The esophagogastric insertion angle is calculated by rotating a slice plane around the esophageal axis. 2D contours were generated at 1.8° intervals to provide a complete 360° description of the angle between the esophageal axis and a tangent on the proximal fundus. In a flap valve, it is the “most acute” angle that is of interest, and this was defined as the range of rotating planes over which the insertion angle remains within 10 degrees of the nadir angle (Fig. 2) [36]. Span width represents the curvature of the parabola defined by the distribution of insertion angle measurements.
a Schematic definition of the esophagogastric insertion angle; b 2D contours were generated at regular intervals to provide a complete 360° description of the angle between the esophageal axis and a tangent on the proximal fundus. The insertion angle was defined as the range of rotating planes over which the insertion angle remains within 10 degrees of the nadir angle. Span width was measured as the range of rotating planes (α value) over which the insertion angle remains within 10 degrees of the lowest value
Correlation between Anatomical and Functional Data and between Objective Assessments and QoL
Changes in gastric geometry as assessed by MRI data were correlated to measurements of esophageal motility and function documented by HRM and reflux monitoring. The impact of these factors on patient symptoms and QoL of patients was also assessed.
Statistical Analysis
The primary outcome measure was the insertion angle of the esophagus into the proximal stomach after NTM ingestion in expiration. Previous MRI studies recruited 24 GERD patients and 24 healthy controls and reported a wider (more obtuse) insertion angle in the patient group (+7° ± 3°; p < 0.017). This was assumed to represent a clinically relevant difference in insertion angle comparing measurements before and after LSG. On this basis, to ensure statistical power was present to detect this difference in insertion angle, we aimed to obtain complete measurements in 24 patients undergoing bariatric surgery. Continuous variables were reported as mean ± standard deviation (SD) and categorical variables as numbers and percentages unless otherwise specified. Ordinal qualitative variables and quantitative variables were compared with Wilcoxon rank sum test. Paired comparison of qualitative variables was performed with Fisher’s exact test or chi-square tests. Correlations between reflux monitoring, manometry, and changes in angle of His and span width were performed using Pearson correlation coefficient (Pearson’s rho). In case of Pearson correlation coefficient < 0.2, a linear regression analysis was performed. All reported p values are two-tailed, and a p value < 0.05 was required to conclude statistical significance. SPSS software, version 20.0 (IBM-SPSS, Chicago IL), was used for the analysis.
Results
During the study period, a total of 487 patients underwent bariatric surgical procedures, including 406 (83.4%) gastric bypasses and 81 (16.6%) LSGs. According to the prospectively defined inclusion criteria, thirty-five patients were enrolled in the study. After the screening visit, 7 patients refused surgery and 5 patients did not undergo the postoperative evaluation. Thus 23 (66%) of eligible patients completed the study. Patients’ characteristics and clinical evaluation at inclusion visit, admission, and last follow-up are reported in Table 1.
Ten patients had previous abdominal surgery; however, in all cases, LSG could be completed laparoscopically. Mean operative time was 105 ± 26 min and no intraoperative or early postoperative complications occurred. Mean hospital stay was 3.7 ± 1.13 days. There was a significant decrease in BMI from the inclusion visit to the follow-up evaluation (p < 0.0001), with a mean excess weight loss of 58.6 ± 17.8% after a mean of 7.09 (± 1.7) months.
Anatomical and Functional Objective Assessment
The results of pre- and post-operative physiological investigations are summarized in Table 2.
High-resolution manometry and reflux monitoring
Ambulatory pH-impedance studies showed an increased in mean percentage acid exposure time from before to after surgery (2.4 to. 5.1%; p = 0.04). As defined by the Lyon Consensus [34], preoperative investigation showed conclusively pathological acid exposure (pH 4 > 6%) in one case, whereas 8 patients had pathological findings at follow-up (1 (4%) vs. 8 (33%); p > 0.001). Additionally, an increase in upright refluxes was evidenced (74.8 (I.C. 58–91.5) vs. 50.2 (I.C. 40.6–59.8) pre-LSG, p = 0.006).
There was evidence of impaired EGJ structure and function after LSG. The mean intra-abdominal LES length was reduced after surgery (1.7 cm (I.C. 1.3–2.0) vs. 0.7 cm (I.C. 0.2–1.1), p = 0.001). Prior to surgery and at the intraoperative exploration of the hiatus no patients had hiatal hernia, whereas, postoperatively, 12 subjects had a negative intra-abdominal length (mean − 3.1 cm (I.C. 2.5–3.7)). This presence of a hiatus hernia was confirmed also in upper gastrointestinal imaging. These changes were accompanied by a reduction in LES basal pressure at expiration (23.0 mmHg (I.C. 16.9–30.7) vs. 12.9 mmHg (I.C. 7.2–18.7), p = 0.04). Concerning esophageal motility, no major motility disorders were present pre- or post-operatively and no differences in contractile amplitude were demonstrated. An increase of intragastric pressure was observed after LSG both at baseline and, in particular, after 10 water swallows. Specifically, the mean value of intragastric pressure at the end of the study was 21.3 (I.C.18.5–24.0) mmHg before surgery vs. 33.5 (I.C. 28.9–38.0) mmHg after the procedure (p < 0.01).
Magnetic Resonance Imaging
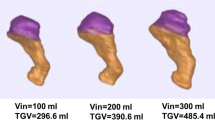
The mean period of time between preoperative and postoperative MRI evaluation was 6.8 (± 1.7) months. The test was completed in all subjects. The volume required to achieve maximum satiation was reduced after LSG (380 ± 51 vs. 188 ± 59; p < 0.001). Results are summarized in Table 3.
The esophagogastric insertion angle (angle of His) became more obtuse (effaced), from pre- to post-surgery (36° ± 11 vs. 51° ± 16; (p = 0.002)) (Fig. 3). Surgery did not significantly reduce span width (79° ± 23 vs. 69° ± 31, p = 0.22). A consistent increase in mean esophageal diameter was observed (17 ± 3 mm vs. 18 ± 4 mm (p = 0.029)). As expected, total gastric volume decreased following sleeve resection (520 ± 133.49 mL to 157.81 ± 72.9 mL; p < 0.0001)), indicating that LGB reduced gastric capacity by approximately two thirds.
Correlation between MRI Measurements and Physiological and Clinical Data
EGJ Geometry and Functional Outcomes
An increase in the esophagogastric insertion angle (angle of His) was observed in 78% (18/23) of patients after LSG. This change in local anatomy was associated with reduction in abdominal (p = 0.002, Pearson = −0.76) and total LES length (p = 0.005, Pearson = − 0.51) and EGJ tone (p = 0.01, Pearson = 0.59). In addition, IRP and EGJ relaxation increased after surgery (p = 0.01, Pearson = 0.59) indicating reduced viscous resistance to flow across the EGJ reflux barrier. Individuals with an increased insertion angle had a significant increase in total upright (p = 0.016, Pearson = 0.52) and total acid reflux events (p = 0.043, Pearson = 0.45). In the remaining 5 patients (22%) in whom the insertion angle was not altered or reduced, no correlation was found with any of the manometric or reflux monitoring results. Overall LSG had no effect on span width; however, a reduction in span width was observed in 15 patients (82° ± 25 vs. 54° ± 17) postoperatively, a finding that indicates a reduction in contact between the gastric fundus and the intra-abdominal esophagus. In this subgroup, an increase in the number of upright (p = 0.004, Pearson = −0.61) and total reflux events (p = 0.01, Pearson = −0.54) was present. Additionally, a linear correlation was present between the decrease in span width and EGJ tone (p = 0.006; Pearson = −0.62). Of note, 12/15 (80%) of patients with decreased span also had an increase in the esophagogastric angle. In the remaining 8 patients in whom span width did not decrease after LSG, there was no correlation of this metric with pH impedance monitoring and manometry parameters.
Gastric Volume and Pressure and Gastroesophageal Reflux
Gastric volume reduction had a complex relationship with gastroesophageal reflux as assessed by pH-impedance monitoring. Overall, there was no association between gastric volume and acid exposure; however, patients with the largest reduction in gastric volume reduction (≥ 80%; n = 7 patients) had an increase in supine non-acid refluxes (p = 0.008, Pearson = 0.54) and often had increased reflux symptoms (see below). This group also had the largest increase in intra-gastric pressure after LSG as assessed by HRM and, similar to volume reduction, a correlation was present between this measurement and the frequency of non-acid reflux (p = 0.04, Pearson = 0.45). Finally, it was noted that individuals in this group had a greater postoperative mean weight loss as compared with all other patients (32.3 ± 3.8 kg vs. 22.1 ± 5.5 kg p = 0.001).
Quality of Life
A significant increase in the GIQLI score was observed after surgery (114 ± 10.1 vs. 103 ± 14.5, p = 0.004). Analyzing the subdomains of the GIQLI score, despite the overall increase in score, a worsening in gastrointestinal symptoms was identified (65.9 ± 6.6 vs. 58.5 ± 5.9, p = 0.001). No change was noted in the GSAS score (0.49 ± 0.3 vs. 0.54 ± 0.23). None of the physiological measurements influenced GIQLI and GSAS values; however, 6 of 7 patients with > 80% of gastric volume reduction after LSG had an increased GSAS.
Three out of 23 patients (13%) were under PPI medications at the last follow-up.
Discussion
In this study, LSG was an effective bariatric procedure, inducing a mean excess weight loss of 59% after a mean follow-up of 7 months. The only frequent complication of this procedure was an increase in the number of patients with pathological gastroesophageal reflux after surgery. Comprehensive assessment by MRI and HRM identified important changes to the structure and function of the upper gastrointestinal tract that are associated with increased gastroesophageal reflux after this form of bariatric surgery.
The majority of subjects that underwent ambulatory reflux monitoring after LSG showed an increase in esophageal acid exposure time, number of reflux events, and number of refluxes longer than 5 min, all changes that have been linked to the occurrence of reflux esophagitis and reflux symptoms [37,38,39]. Prior to LSG, only one obese patient had pathological acid exposure; whereas, despite weight loss, eight individuals had a de novo diagnosis of GERD after surgery based on physiological measurement. The literature reports highly variable frequency of GERD after this form of bariatric surgery [8], reflecting the inconsistency of operating techniques and methods of assessment. Various mechanisms have been proposed to explain increased reflux after LSG; however, few previous publications reported objective measurements from a prospectively recruited population of bariatric patients. This study applied validated imaging and physiological measurements to provide a comprehensive assessment of the upper gastrointestinal tract before and after surgery. Evidence is presented that LSG had a negative impact on the structure and function of the EGJ reflux barrier. Additionally, patients with a very large reduction (> 80%) of gastric capacity after surgery had an increase number of reflux events and symptoms.
Comparison of pre- and postoperative MRI of the EGJ and stomach detailed anatomical changes induced by LSG. The physiological and clinical relevance of these findings were confirmed by correlation with established biomarkers from high-resolution manometry and reflux monitoring. The primary finding confirmed the primary study hypothesis that LSG increased (i.e. made more obtuse) the esophagogastric insertion angle in the majority (78%) of patients. This anatomical change was associated with a decrease in the intra-abdominal length and resting pressure of the LES measured by HRM. Structural disruption of the EGJ reflux barrier was associated with dissociation of the intrinsic and extrinsic components (i.e. LES and diaphragmatic crus, respectively), and hiatus hernia was present in 12 subjects. In addition, the anatomical relation (“span width”) of the intra-abdominal esophagus and the proximal stomach was altered. This finding was also associated with an increase in acid reflux events, especially in the upright position. Previous work that applied the same methodology to assess the functional anatomy of the EGJ and proximal stomach have reported similar findings in non-bariatric GERD patients, in particular the esophagogastric insertion angle was more obtuse in this group than healthy controls. [29] Further studies showed that the insertion angle was maintained by tonic contraction in the clasp and sling fibers of the proximal stomach and that an acute insertion angle in GERD patients could be restored by administration of baclofen (GABAB agonist known to suppress reflux events) [40]. Biophysical analysis on 3D models of anatomy from MRI support the hypothesis that a functional “flap valve-like” mechanism contributes to reflux protection [36]. In this model, the acute insertion angle between the esophagus and proximal stomach facilitates compression of the intra-abdominal esophagus by the fundus as it distends during the meal. [36] These predictions are consistent with direct observations not only in the original comparison between healthy controls and GERD patients [29] but also in the current study comparing patients before and after bariatric surgery. The structure and function of the EGJ reflux barrier is often altered by LSG. Surgical studies have shown that division of the gastric sling fibers reduces the LES tone in animal models [41, 42], and this has been reported also following partial resection of these fibers during LSG in humans [43]. The authors of several trials considered that “destruction of the angle of His” was partially accountable for increased incidence of postoperative GERD after LSG [19, 20]. In previous work, this assessment was based on qualitative interpretation of endoscopic or radiologic images. The current study provides objective and quantitative evidence in support of this mechanism of disease in this patient group.
Reduction in gastric capacity and compliance (distensibility) has also been proposed as a factor facilitating GERD after LSG. This study demonstrated a large reduction in gastric capacity in all patients after the bariatric operation. On average, the volume retained in the stomach after ingestion of the test meal reduced by 60–70%; however, seven patients had > 80% reduction of gastric capacity. The latter group had increased reflux events on pH-impedance studies and symptoms as assessed by the validated GSAS questionnaire. It was noted that this effect was independent of effects on EGJ structure and function (i.e. no association between gastric volume reduction and other measurements from the reflux barrier). These results are consistent with observations by other authors. Measurements of gastric volume and pressure using an electronic barostat before and after LSG have shown that the residual stomach (sleeve) is “10-times” less distensible than the resected portion [44]. As expected from physical principles (Boyle’s Law), HRM studies have shown that intra-gastric pressure is increased in LSG patients with much reduced gastric volume [45]. This observation was confirmed by the current study and, again, linked to increased reflux events. In another, prospective trial intragastric pressure after water swallows was much higher in 16/32 (50%) patients with de novo GERD after LSG compared with those without this complication (mean 49 vs. 25 mmHg, p = 0.03) [46]. These mechanistic observations suggest that increased reflux occurs in patients with a small volume sleeve because intra-gastric pressure frequently exceeds resting pressure in the EGJ reflux barrier. The fact that the reflux events that increased most in number were non-acid in this group may be due to the reduction in secretion capacity after LSG.
Overall, patients reported significantly better quality of life after surgery, which is expected in this patient population whose main goal is weight loss. This study was not powered to assess the clinical relevance of these findings in terms of reflux symptoms and it is known that the link between the objective severity of acid exposure and the subjective outcome in terms of symptoms is weak [37]. Notwithstanding these considerations, when specifically considering the gastrointestinal symptoms domain of the GIQLI score, a worsening value was observed postoperatively. By contrast, GSAS scores used in the assessment of gastrointestinal symptoms did not significantly increase after LSG.
This prospective mechanistic trial did not include a large number of patients; however, power calculations indicated that 24 subjects were sufficient to address the study hypothesis.
In addition, given the short follow-up period, we were not able to evaluate the presence of further adaptive mechanisms that could have influenced functional outcomes over the time. However, larger series [5] reported a post-LSG GERD incidence up to 31.8% after 5 years, not significantly different in comparison with the 33% rate that we reported in our case series. On these bases, we can speculate that, despite the putative adaptive processes that could eventually take place over a longer time lapse, these mechanisms are probably insufficient to guarantee a reduction of post-LSG GERD incidence over time.
Other limitations include incomplete blinding of surgical investigators; however, objective end-points were derived by automatic analysis for HRM and reflux studies, and the MRI data was analyzed and interpreted by experts not involved in clinical care (JC, MF). Strengths of this work include acquisition of novel quantitative measurements from MRI that describe the structure and function of the EGJ and stomach in more detail than that achievable by other techniques.
To our knowledge, this is the first study to provide quantitative measurements detailing changes in the functional anatomy of the EGJ and stomach after LSG. Key findings included an important increase in the esophagogastric insertion angle (angle of His), decrease in span width, and reduction in gastric capacity. The physiological relevance of these measurements was confirmed by comparison with the results of HRM and ambulatory pH-impedance studies. Analysis of this information provides new evidence that GERD after LSG is caused by (i) disruption of EGJ structure and function and (ii) excessive reduction in gastric volume (i.e. excessively large sleeve resection of gastric tissue). No preoperative clinical or manometric parameters were predictive of postoperative GERD, suggesting that operator dependent factors were causative. The same conclusion was reached by the authors of another mechanistic study based on an analysis of HRM and pH-data [46]. Based on these observations, recommendations can be made to reduce the prevalence of reflux after LSG. First, to avoid impairment of EGJ barrier function measures should be taken to avoid damage of the clasp and sling fibers. Specifically, resection of the fundus during LSG should not involve the cardia [11]. Second, to avoid excessive pressurization of the stomach no more than 75% of gastric capacity should be resected during LSG. More precise guidance will require measurements in a larger cohort of patients to define specific cut-off values for the angle of His and reduction in gastric volume that would achieve both appropriate weight loss and also avoid de novo GERD after LSG. If this information were available from intra-operative imaging or the use of a “clever bougie” (e.g. Endo-FLIP®) during the procedure, then it may be possible to tailor the operation and optimize outcomes according to patient-specific esophagogastric anatomy and function.
References
Akkary E, Duffy A, Bell R. Deciphering the sleeve: technique, indications, efficacy, and safety of sleeve gastrectomy. Obes Surg. 2008;18(10):1323–9.
Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;15(2):CD003641.
Rebibo L, Dhahri A, Badaoui R, et al. Laparoscopic sleeve gastrectomy as day-case surgery (without overnight hospitalization). Surg Obes Relat Dis. 2015;11(2):335–42.
Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–20. discussion 20-2
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–65.
Benaiges D, Goday A, Ramon JM, et al. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7(5):575–80.
Boza C, Gamboa C, Salinas J, et al. Laparoscopic roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg Obes Relat Dis. 2012;8(3):243–9.
Gagner M, Deitel M, Kalberer TL, et al. The second international consensus summit for sleeve gastrectomy, March 19-21, 2009. Surg Obes Relat Dis. 2009;5(4):476–85.
Boza C, Daroch D, Barros D, et al. Long-term outcomes of laparoscopic sleeve gastrectomy as a primary bariatric procedure. Surg Obes Relat Dis. 2014;10(6):1129–33.
Braghetto I, Csendes A, Korn O, et al. Gastroesophageal reflux disease after sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20(3):148–53.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Rebecchi F, Allaix ME, Giaccone C, et al. Gastroesophageal reflux disease and laparoscopic sleeve gastrectomy: a physiopathologic evaluation. Ann Surg. 2014;260(5):909–14. discussion 14-5
Samakar K, McKenzie TJ, Tavakkoli A, et al. The effect of laparoscopic sleeve gastrectomy with concomitant hiatal hernia repair on gastroesophageal reflux disease in the morbidly obese. Obes Surg. 2016;26(1):61–6.
Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of Barrett's esophagus: results of a multicenter study. Obes Surg. 2019;29(5):1462–9.
Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett's esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–74.
Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, sleeve dilation, and Barrett's esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017;27(12):3092–101.
Sohn S, Fischer J, Booth M. Adenocarcinoma of the gastro-oesophageal junction after sleeve gastrectomy: a case report. ANZ J Surg. 2017;87(10):E163–E4.
Santoro S. Technical aspects in sleeve gastrectomy. Obes Surg. 2007;17(11):1534–5.
Hamoui N, Anthone GJ, Kaufman HS, et al. Sleeve gastrectomy in the high-risk patient. Obes Surg. 2006;16(11):1445–9.
Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6.
Klaus A, Weiss H. Is preoperative manometry in restrictive bariatric procedures necessary? Obes Surg. 2008;18(8):1039–42.
Laffin M, Chau J, Gill RS, et al. Sleeve gastrectomy and gastroesophageal reflux disease. J Obes. 2013;2013:741097.
Fujiwara Y, Nakagawa K, Tanaka T, et al. Relationship between gastroesophageal reflux and gastric emptying after distal gastrectomy. Am J Gastroenterol. 1996;91(1):75–9.
Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44(5):541–7.
Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29(12)
Jasper D, Freitas-Queiroz N, Hollenstein M, Misselwitz B, Layer P, Navarro-Rodriguez T, et al. Prolonged measurement improves the assessment of the barrier function of the esophago-gastric junction by high-resolution manometry. Neurogastroenterol Motil. 2017;29(2).
Schwizer W, Steingoetter A, Fox M. Magnetic resonance imaging for the assessment of gastrointestinal function. Scand J Gastroenterol. 2006;41(11):1245–60.
Curcic J, Fox M, Kaufman E, et al. Gastroesophageal junction: structure and function as assessed by using MR imaging. Radiology. 2010;257(1):115–24.
Curcic J, Roy S, Schwizer A, et al. Abnormal structure and function of the esophagogastric junction and proximal stomach in gastroesophageal reflux disease. Am J Gastroenterol. 2014;109(5):658–67.
Parker H, Hoad CL, Tucker E, et al. Gastric motor and sensory function in health assessed by magnetic resonance imaging: establishment of reference intervals for the Nottingham test meal in healthy subjects. Neurogastroenterol Motil. 2018;30(12):e13463.
NIH conference. Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med 1991;115(12):956–961.
Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc. 2014;28(6):1753–73.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–74.
Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67(7):1351–62.
Parker HL, Tucker E, Hoad CL, et al. Development and validation of a large, modular test meal with liquid and solid components for assessment of gastric motor and sensory function by non-invasive imaging. Neurogastroenterol Motil. 2016;28(4):554–68.
Roy S, Fox MR, Curcic J, et al. The gastro-esophageal reflux barrier: biophysical analysis on 3D models of anatomy from magnetic resonance imaging. Neurogastroenterol Motil. 2012;24(7):616–25. e269
Fox M, Forgacs I. Gastro-oesophageal reflux disease. BMJ. 2006;332:88–93.
Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol. 1992;87(9):1102–11.
Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–80.
Curcic J, Schwizer A, Kaufman E, et al. Effects of baclofen on the functional anatomy of the oesophago-gastric junction and proximal stomach in healthy volunteers and patients with GERD assessed by magnetic resonance imaging and high-resolution manometry: a randomised controlled double-blind study. Aliment Pharmacol Ther. 2014;40(10):1230–40.
Korn O, Csendes A, Burdiles P, et al. Anatomic dilatation of the cardia and competence of the lower esophageal sphincter: a clinical and experimental study. J Gastrointest Surg. 2000;4(4):398–406.
Stein HJ, DeMeester TR, Peters JH, et al. Technique, indications, and clinical use of ambulatory 24-hour gastric pH monitoring in a surgical practice. Surgery. 1994;116(4):758–66. discussion 66-7
Braghetto I, Lanzarini E, Korn O, et al. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. 2010;20(3):357–62.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy--volume and pressure assessment. Obes Surg. 2008;18(9):1083–8.
Mion F, Tolone S, Garros A, et al. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–56.
Coupaye M, Gorbatchef C, Calabrese D, et al. Gastroesophageal reflux after sleeve gastrectomy: a prospective mechanistic study. Obes Surg. 2018;28(3):838–45.
Acknowledgments
The authors would like to thank associate Professor Dr. Andreas Steingötter for his technical advice and support with acquisition and analysis of Magnetic Resonance Imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quero, G., Fiorillo, C., Dallemagne, B. et al. The Causes of Gastroesophageal Reflux after Laparoscopic Sleeve Gastrectomy: Quantitative Assessment of the Structure and Function of the Esophagogastric Junction by Magnetic Resonance Imaging and High-Resolution Manometry. OBES SURG 30, 2108–2117 (2020). https://doi.org/10.1007/s11695-020-04438-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04438-y