Abstract
Background
Conflicting data have been published for bariatric surgery in older patients, with no long-term large-scale studies available. Our aim was to provide long-term (> 10 years) results on weight loss, metabolic outcomes, and quality of life in a large homogenous series of Roux-en-Y gastric bypass (RYGB) patients, according to age at baseline.
Patients and Methods
All consecutive patients who underwent primary RYGB between 1999 and 2007, and therefore eligible for 10-year follow-up, were retrospectively analyzed. According to their age at baseline, they were divided into three groups: A (< 40 years), B (40–54 years), and C (≥ 55 years). Categorical variables were compared with the χ2 test and continuous variables with ANOVA.
Results
Our series consisted of 820 patients, with a 10-year follow-up of 80.6%. Although group C (11% of all patients) had significantly more comorbidities at baseline, there was no difference in postoperative morbidity and mortality between groups. Weight loss was significantly less for group C patients up to the 7th postoperative year, but no difference remained thereafter. 10-year %total weight loss was 32.2, 32.9, and 32.3 respectively in groups A, B, and C. After 10 years, glycemic control and lipid profile improved similarly, rates of partial or complete remission of diabetes and hypertension were identical, and quality of life presented a significant improvement for all patients with no inter-group difference.
Conclusion
Our results suggest similar short- and long-term outcomes after RYGB for patients ≥ 55 years compared to younger ones; the relative benefit might even be higher for older patients, given their increased comorbidity at baseline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the worldwide aging of the population, health care providers face an ever-increasing challenge with elderly obese patients. The prevalence of metabolic syndrome in the USA has been estimated at 18.3% for patients < 40 years, and 46.7% for those > 60 years old [1], whereas life expectancy has also increased, with a 60-year-old woman in 2008 expected to live for another 24.3 years and a man for 22.8 years [2]. Thus, obesity and its related comorbidities represent a major health issue and socioeconomic burden in modern society for all age classes.
Indications for bariatric surgery vary among the different health systems, with elderly patients, sometimes as of the age of 55, often being refused surgery on the grounds of a reduced ability to lose weight and correct their long-standing comorbidities compared with younger patients [3,4,5]. In Switzerland, there is no clearly defined age limit for bariatric surgery, although the Swiss Society for the Surgery of Obesity and related diseases (SMOB) states that indication for surgery in patients > 65 years needs to take into account the risk/benefit ratio and the potential improvement in quality of life and that these patients must be treated in expert centers only. Another controversial issue is the potential over-morbidity and mortality in older patients after bariatric surgery and especially Roux-en-Y gastric bypass (RYGB) [6,7,8,9,10]. As surgical complications might also affect quality of life (QoL), which is already often severely compromised in these patients, all of these aspects need to be assessed in order to select appropriate surgical candidates. Most of the available studies include heterogeneous populations with several types of interventions, and postoperative follow-up not exceeding 5 years [3, 7, 11]. With the standardization of bariatric surgical techniques and patient management in recent years, more robust data should be made available to guide patient information as well as health care resource management.
The aim of our study was to provide long-term (≥ 10 years) results on weight loss, metabolic outcomes, and quality of life in a large homogenous series of RYGB patients, according to patients’ age at baseline.
Patients and Methods
All consecutive patients who underwent primary RYGB in one of the two participating reference centers between 1999 and 2007, and therefore eligible for the 10-year follow-up, were included. They were divided into three groups according to their age at baseline: group A (< 40 years), group B (40–54 years), and group C (≥ 55 years). Relevant demographic, metabolic, and QoL data were retrieved from a prospectively maintained institutional database. The study was approved by the local ethics committee (protocol number 304/15), and consent was obtained from all patients for the use of clinical data for research purposes. The STROBE checklist for observational studies was adhered to [12] (online appendix 1).
Surgical technique was standardized as described previously [13], with a 15-mL gastric pouch and a retrocolic 100–150-cm Roux alimentary limb, a 21-mm circular stapled gastrojejunostomy, and a linear stapled jejunojejunostomy. Patients not seen for more than 12 months at the 10-year term were considered lost from follow-up.
Postoperative morbidity was graded according to the Clavien-Dindo classification [14]. Weight loss was expressed as the percentage of total body weight loss from baseline (%TBWL) and percentage of excess BMI (body mass index) loss (%EBMIL), with BMI = 25 kg/m2 considered as ideal body weight. For long-term metabolic outcomes, the absolute values of glucose, triglycerides, total cholesterol, HDL- and LDL-cholesterol fractions, and also the evolution of obesity-related comorbidities (diabetes, hypertension) were taken into account.
For hypertension, remission was defined as normal arterial pressure (systolic < 140 mmHg, diastolic < 90 mmHg) without treatment, improvement as reduction in pharmaceutical treatment needed to keep normal arterial pressure, and worsening as the need to add treatment or the development of de novo hypertension. For diabetes, remission was defined as a normal glycemia (≤ 5.6 mmol/L) without any treatment, improvement (partial remission) as normal glycemia with reduced treatment or glycemia < 7 mmol/L with same treatment (with preoperative glycemia > 7), and worsening as increased need for treatment or de novo diabetes. Unfortunately, HbA1c was measured only rarely at the beginning of our experience, so it could not be used to assess evolution of diabetes.
Quality of life (QoL) was measured with the validated Moorehead-Ardeldt score [15].
Statistical Analysis
Among the three patient groups, categorical variables were expressed as frequencies (%) and compared with the chi-square or Fisher’s exact test as appropriate. Continuous variables expressed as mean ± SD (standard deviation) were compared with the ANOVA (analysis of variance) tests. Apart from this group analysis, further analysis was also performed in order to possibly identify factors affecting results (weight loss, operative morbidity, remission of comorbidities, and quality of life), where age and BMI at baseline were considered as continuous variables using Pearson’s correlation, and sex and presence of diabetes as dichotomic variables using chi-square test. Significance threshold was set at p < 0.05 and all tests were two-sided. Statistical analysis was performed with the Systat® 13.2 statistical package.
Results
Overall, 820 patients (621 female and 199 male) who underwent RYGB for severe obesity were included. One patient died in the immediate postoperative period as the consequence of a leak from the gastric remnant staple line, and another 27 (3,3%) died before the 10-year limit from various causes unrelated to RYGB. A total of 792 were therefore eligible for follow-up at 10 years, with clinical data available in 638 of them, representing a 10-year follow-up rate of 80.6%. Groups A, B, and C consisted of 396 (48.3%), 337 (41.1%), and 87 (10.6%) patients respectively, with similar 10-year follow-up rates (Fig. 1). Table 1 shows anthropometric data at baseline; there was no significant difference between groups regarding BMI and proportion of superobese patients. Most comorbidities were significantly more prevalent in group C patients than in younger ones, except GERD, which was more common in group A (Table 2).
Postoperative Morbidity-Mortality
Intraoperative characteristics did not differ, with a mean operative time of 141 ± 34, 149 ± 38, and 149 ± 46 min for groups A, B, and C (p = 0.65). Overall postoperative morbidity was 12.1%, 14.8%, and 14.9% for the three groups respectively (p = 0.50), while major complications (> Clavien IIIA) were observed in 3.5%, 2.9%, and 4.6% of groups A, B, and C patients (p = 0.74). Postoperative (30 days) mortality was 0.2% for group A (1 patient) and 0% for groups B and C (p = 0.38). Preoperative age, sex, BMI, or the presence of diabetes, hypertension, or sleep apnea syndrome did not influence overall or serious morbidity rates.
Weight Loss
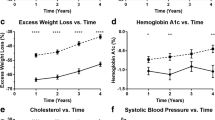
Mean BMI at 10 postoperative years was 32.7 kg/m2 for group A, 32.6 kg /m2 for group B, and 32.1 kg/m2 for group C patients (p = 0.79). Most patients reached their lowest mean BMI during the 2nd postoperative year (29.0 kg/m2 for group A, 30.2 kg/m2 for group B, and 30.7 kg/m2 for group C) (Fig. 2). The %TBWL after 2 years was 36.8%, 33.6%, and 33.1% (p < 0.001) for groups A, B, and C (Fig. 3), while the 2-year %EBMIL was 83.3%, 77.1%, and 71.1% (p < 0.001) respectively (Fig. 4). While weight loss was better during the first 7 postoperative years in younger patients, no difference remained as of the 8th postoperative year. Indeed, 10 years after surgery, %TBWL and %EBMIL were 28.7% and 65.8% respectively in group A, 28.1% and 64.6% in group B, and 29.6% and 67.9% in group C. %TBWL did not correlate with preoperative age, BMI, or glycemia. %TBWL was significantly higher in females compared with males (29 vs 26.7%, p = 0.02), but did not differ between patients with or without diabetes at baseline.
Obesity-Related Comorbidities
After 10 years, complete or partial remission of pre-existing hypertension was observed in 91.7%, 84.1%, and 80% of groups A, B, and C patients (p = 0.2) (Fig. 5a), whereas worsening or de novo hypertension was seen in 6.7%, 3.4%, and 8% of patients respectively. Long-term results were even more favorable for diabetes: 95.7%, 93.7%, and 91.7% of groups A, B, and C patients maintained complete or partial remission at 10 postoperative years (Fig. 5b), whereas 2.5%, 2.15%, and 4.5% respectively presented worsening or de novo diabetes. There was no relationship between diabetes remission/improvement and preoperative BMI, age, or sex. For hypertension, preoperative BMI was lower in patients who achieved remission/improvement (45.5 vs 48.9 kg/m2, p = 0.01), but there was no other predictor.
Metabolic Results
Baseline fasting glucose levels were significantly higher for group C patients and remained so during the 1st postoperative year as well as at the 10-year time point (Fig. 6). Within each group, nadir glucose levels were reached during the 1st postoperative year. Although group C patients maintained a higher mean glycemia than groups A and B patients, they were those whose mean value improved most at all timepoints. Lipid profile improved in all groups, and there was no significant difference between them at the 10-year mark, even though group C patients had significantly higher total cholesterol, LDL-cholesterol, and triglyceride values at baseline (Fig. 7). Group C patients improved their lipid values more when compared to younger patients after 10 years. On average, patients whose total cholesterol level normalized or significantly improved were almost 4 years older (42 versus 38.1, p = 0.01) than those in whom no or only minor change was observed. Modifications in triglyceride levels did not correlate with any of the analyzed variables.
Long-Term Quality of Life
All patients presented a significant and similar improvement in quality of life (QoL) compared to baseline. The 10-year mean Moorehead-Ardeldt scores were 1.67 for group A, 1.66 for group B, and 1.64 for group C (p = 0.99). There was no association between preoperative BMI, age, sex, or presence of diabetes at baseline and improvement of quality of life.
Discussion
In this RYBG series of 820 patients with a ≥ 10-year follow-up, 10-year results after RYGB were the same irrespective of age at baseline. Patients ≥ 55 years had similar postoperative morbidity and mortality as younger ones, despite significantly more comorbidities at baseline. Weight loss results were significantly better for younger patients during the first postoperative years, but no difference remained thereafter. Remission rates for type 2 diabetes and hypertension as well as the lipid profile were similar at 10 years in all groups. Lower preoperative BMI was associated with better improvement of hypertension and older age with better improvement of total cholesterol. Finally, QoL presented a significant improvement, similar to all patient groups.
There is no uniformity in the literature regarding the definition of older patients, with reported cut-offs between 50 and 75 years of age [2, 16,17,18]. In the present study, 55 years was set as the limit for older patients for 2 reasons. First, the small number of highly selected patients > 60 years undergoing surgery limits meaningful statistical comparisons. Second, one previous study reported significantly worse results in terms of weight loss after 5 years in patients ≥ 55 years of age [3] and we sought to verify this conclusion in a larger group of patients in the long term.
Conflicting results have been published regarding the impact of age on postoperative complications and mortality after bariatric surgery, and there is still a common belief that risks are higher and results worse in older patients. This apprehension is largely founded upon older registry studies [19, 20] where many different “weight loss procedures” are jointly reported and no distinction exists between laparoscopic and open surgery, limiting extrapolation of results to current practice. Dorman et al. in a registry study of various bariatric procedures reported a trend for higher mortality in patients older than 70 years compared to those between 35 and 49 years (0.6 versus 0.1%, p = 0.15); of note, only 20% of their patients had their surgery done laparoscopically [9]. Even when considering RYGB alone, as one of the most commonly performed procedures worldwide and the standard procedure in our practice, published data remain conflicting. In a systematic review of > 1800 RYBG patients, Chow et al. reported low mortality (0.14%) and complication (21.1%) rates total in patients > 65 years [16]. On the other hand, Giordano et al. reported significantly higher postoperative morbidity (12.9 vs 6.8%, p = 0.02) and mortality (2 vs 0.14%, p = 0.04) for older patients in a meta-analysis of comparative laparoscopic RYGB studies [6]. A French study also found higher rates of postoperative surgical (12.3 vs 3.8%, p = 0.03) and medical (7.0 vs 0.8%, p < 0.01) complications for patients > 60 years after laparoscopic RYGB, but not after sleeve gastrectomy or adjustable gastric banding [7]. Another large Australian study showed higher surgical (7.5 vs 4.4%, p < 0.001) and medical (4.6 vs 2%, p < 0.001) complication rates for patients > 55 years [18]. A recently published registry-based study found increased rates for leaks, readmissions, and reoperations after RYGB when compared to sleeve gastrectomy in patients aged 60 years or more [11]. In the present analysis, no difference was found in overall morbidity (9.3 vs 8.3%, p = 0.37), major morbidity (2.5 vs 1.9%, p = 0.56), or mortality (0.3 vs 0%, p = 1) for patients aged more or less than 55 years. The same holds true if we consider our entire experience with primary RYGB (2212 patients, with 972 < 40, 930 between 40 and 54, and 311 ≥ 55 years): 8.3, 10.3, and 7.7% overall morbidity (p = 0.21), 2.9, 2.0, and 1.9% major morbidity (p = 0.41), and 0.1, 0, and 0% mortality for groups A, B, and C respectively. Even considering only patients > 60 years, there is no higher overall or major morbidity in our experience.
Other concerns often raised for bariatric surgery in older patients are the inherent decrease of lipolytic ability, lower basal energy expenditure, and lower capacity to lose weight after 40 years [4,5,6]. This physiological trend has been reflected in some studies, where older patients had poorer 1- and 5- postoperative year weight loss compared to younger patients with RYGB [3, 6, 7, 21]. Despite that, most series report encouraging short-term (1-year) weight loss for older patients with 54–77%EWL [2, 10, 15, 21,22,23] and 22–34% TBWL [10, 24], with RYGB offering better weight loss in the elderly population compared to sleeve gastrectomy or adjustable gastric banding [7, 8, 25].To our knowledge, our study is the first to report 10-year weight loss in relation to patients’ age. We observed a slightly inferior TBWL and EBMIL for patients > 55 years compared to younger patients up to the 7th postoperative year, but no difference was observed beyond this limit and up to 12 years after surgery. Overall, there was no correlation between age and long-term weight loss. Older patients reached 29.5 %TBWL and 67.5% %EBMIL at 10 postoperative years, which are comparable with recent long-term data from Adams et al. [26] (29.6% TBWL 12 years after RYGB), although no correlation is made with patients’ age in that study.
Obesity-related comorbidities are the most serious consequence of obesity within the aging population as they increase risk of death and disability as well as health-care dependency. In the present study, we observed very high long-term rates of complete or partial resolution of hypertension (80%) and diabetes (91.7%), as well as major improvements of the lipid profile in older patients, comparable to those observed in younger ones. In fact, patients who most improved their total cholesterol were older than patients in whom little or no change was observed. These findings are in line with previous studies, where older patients can achieve a similar improvement in comorbidities, and especially diabetes, as younger patients after successful bariatric surgery [6, 20, 22, 27]. Considering the long-standing end-organ damages caused by obesity and diabetes in older patients, these results strongly suggest that this organ-damaging process can be reversed, or at least slowed down, if complete or partial remission of comorbidities is achieved, even when surgery is proposed after 55 years [28]. Indeed, 12 months after bariatric surgery, a significant reduction has been reported in carotid artery atherosclerosis and the overall cardiovascular risk for patients > 50 years compared to younger ones [29]. In our study, lipid profile showed a sustained improvement during the 10-year follow-up, with the cholesterol/HDL ratio, a surrogate marker for cardiovascular risk, being at or very close to its nadir at 10 years for all patients. As previous data from our institution suggest, improvement of the lipid profile in itself reduced the long-term cardiovascular risk [30].
Obesity has a significant long-term impact on patients’ well-being, QoL, and loss of autonomy. Few studies report QoL data after bariatric surgery; Almerie et al. recently reported a significant improvement in all aspects of QoL for patients > 60 years 2 years after RYGB [11], whereas two previous studies had also suggested a significant QoL improvement 6 months after surgery [31, 32]. As our patients are followed on a yearly basis with a specific QoL assessment at 10 postoperative years, we were able to demonstrate that patients > 65 years upon 10-year follow-up presented a sustained significant improvement in QoL, comparable to that observed in younger patients.
Our study has several limitations. First, and even though patient data were gathered prospectively, analysis is retrospective with some missing data especially in laboratory values, comorbidity evolution, and QoL. Second, although our follow-up rate is one of the highest in the literature at 10 postoperative years, some patients are lost from follow-up, potentially confounding outcome assessment. Finally, one might argue that 55 years is hardly a cut-off for “elderly” patients in modern society. As mentioned earlier, this threshold was chosen based on previously published studies and in order to have an adequate older patient group for meaningful comparisons.
In conclusion, our results provide considerable evidence for not excluding patients from bariatric surgery programs, and especially RYGB, based on age alone. Older patients can expect similar 10-year outcomes as younger ones with even a higher relative benefit, as their baseline health status is significantly worse.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- EBMIL:
-
Excess BMI loss
- TBWL:
-
Total body weight loss
- QoL:
-
Quality of life
- RYGB:
-
Roux-en-Y gastric bypass
References
Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–4.
Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging. 2015;10:1627–35.
Scozzari G, Passera R, Benvenga R, et al. Age as a long-term prognostic factor in bariatric surgery. Ann Surg. 2012;256(5):724–8. discussion 728-9
Printen KJME. Gastric bypass for morbid obesity in patients more than fifty years of age. Surg Gynecol Obs. 1977;144(2):192–4.
Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010 Jan;9(1):1–11.
Giordano S, Victorzon M. Laparoscopic Roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107(1):6–13.
Ritz P, Topart P, Benchetrit S, Tuyeras G, Lepage B, Mouiel J, Becouarn G., Pattou F., Chevallier J.M. Benefits and risks of bariatric surgery in patients aged more than 60 years. Surg Obes Relat Dis 2014
Kaplan U, Penner S, Farrokhyar F, et al. Bariatric surgery in the elderly is associated with similar surgical risks and significant long-term health benefits. Obes Surg. 2018;28(8):2165–70.
Dorman RB, Abraham AA, Al-Refaie WB, et al. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg. 2012;16(1):35–44.
Janik MR, Mustafa RR, Rogula TG, et al. Safety of laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass in elderly patients - analysis of the MBSAQIP data. Surg Obes Realt Dis. 2018;14:1276–83.
Almerie MQ, Rao VSR, Peter MB, et al. The impact of laparoscopic gastric bypass on comorbidities and quality of life in the older obese patients (age > 60): our UK experience. Obes Surg. 2018; Dec;28(12):3890–4.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Duvoisin C, Favre L, Allemann P, et al. Roux-en-Y gastric bypass. Ann Surg. 2018;268(6):1019–25.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240(2):205–13.
Moorehead MK, Ardelt-Gattinger E, Lechner H, et al. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes Surg. 2003;13(5):684–92.
Chow A, Switzer NJ, Gill RS, et al. Roux-en-Y gastric bypass in the elderly: a systematic review. Obes Surg. 2016;26(3):626–30.
Lynch J, Belgaumkar A. Bariatric surgery is effective and safe in patients over 55: a systematic review and meta-analysis. Obes Surg. 2012;22(9):1507–16.
Morgan DJR, Ho KM. Incidence and outcomes after bariatric surgery in older patients: a state-wide data-linked cohort study. ANZ J Surg. 2017;87(6):471–6.
Livingston EH, Langert J. The impact of age and Medicare status on bariatric surgical outcomes. Arch Surg. 2006;141(11):1115–20. discussion 1121
Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294(15):1903–8.
Sugerman HJ, DeMaria EJ, Kellum JM, et al. Effects of bariatric surgery in older patients. Ann Surg. 2004;240(2):243–7.
Thereaux J, Poitou C, Barsamian C, et al. Midterm outcomes of gastric bypass for elderly (aged ≥ 60 yr) patients: a comparative study. Surg Obes Relat Dis. 2015;11(4):836–41.
Robert M, Pasquer A, Espalieu P, et al. Gastric bypass for obesity in the elderly: is it as appropriate as for young and middle-aged populations? Obes Surg. 2014;24(10):1662–9.
Nor Hanipah Z, Punchai S, Karas LA, et al. The outcome of bariatric surgery in patients aged 75 years and older. Obes Surg. 2018;28(6):1498–503.
Moon RC, Kreimer F, Teixeira AF, et al. Morbidity rates and weight loss after Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric banding in patients older than 60 years old: which procedure to choose? Obes Surg. 2016;26(4):730–6.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61.
Gebhart A, Young MT, Nguyen NT. Bariatric surgery in the elderly: 2009-2013. Surg Obes Relat Dis. 2015;11(2):393–8.
Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570–82.
Jonker FHW, van Houten VAA, Wijngaarden LH, et al. Age-related effects of bariatric surgery on early atherosclerosis and cardiovascular risk reduction. Obes Surg. 2018;28(4):1040–6.
Gero D, Favre L, Allemann P, et al. Laparoscopic Roux-en-Y gastric bypass improves lipid profile and decreases cardiovascular risk: a 5-year longitudinal cohort study of 1048 patients. Obes Surg. 2018;28(3):805–11.
O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010;20(9):1199–205.
Quebbemann B, Engstrom D, Siegfried T, et al. Bariatric surgery in patients older than 65 years is safe and effective. Surg Obes Relat Dis. 2005;1(4):389–92. discussion 392-3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 100 kb)
Rights and permissions
About this article
Cite this article
Mantziari, S., Dayer, A., Duvoisin, C. et al. Long-Term Weight Loss, Metabolic Outcomes, and Quality of Life at 10 Years After Roux-en-Y Gastric Bypass Are Independent of Patients’ Age at Baseline. OBES SURG 30, 1181–1188 (2020). https://doi.org/10.1007/s11695-019-04181-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04181-z