Abstract
Background
Although morbid obesity rates in patients ≥65 years of age are increasing, few centers have reported weight loss surgery outcomes in elderly patients, resulting in a paucity of literature on perioperative mortality and morbidity.
Methods
A retrospective analysis was performed on 197 consecutive patients ≥65 years old who underwent weight loss surgery from January 2000 to December 2007. Primary data points included 30-day and 1-year mortality rates, length of stay (LOS), percent excess weight loss (EWL), change in daily medication use, and quality of life (QOL).
Results
The average patient's age was 67.3 years with 72.1% being female. Average preoperative weight and BMI were 131.9 kg and 48.1 kg/m2, respectively. Average preoperative daily medication use was 8.04 ± 3.67. Procedure types included Roux-en-Y gastric bypass (79.3%), adjustable gastric banding (17.2%), and vertical sleeve gastrectomy (3%). Ninety-seven percent of procedures were performed laparoscopically. Average LOS was 2.0 ± 2.1 days. Average weight, BMI, and daily medication use were significantly reduced at 6 months and 1 year (p < 0.001), with patients achieving an average EWL of 44.5% and 55.3% at 6 months and 1 year, respectively. QOL scores improved at 6 months (p < 0.001) and 1 year (p = 0.049). In all patients, the 30-day mortality rate was 0%. The 1-year mortality rate for RYGB patients was 1.3%. Complication rates were acceptable, with 7% of RYGB patients experiencing a major postoperative complication.
Conclusions
Weight loss surgery is effective in patients ≥65 years of age, producing significant EWL, reduction in daily medication use, and improvement in QOL. Surgery is also associated with a low mortality rate and an acceptable morbidity profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent data from the Centers for Disease Control and National Health and Nutrition Examination Survey show that roughly one third of American adults are obese, and the rate of morbid obesity in elderly individuals is on the rise [1]. In February 2006, the Centers for Medicare and Medicaid Services (CMS) approved coverage for bariatric surgery as reasonable and necessary for Medicare beneficiaries who meet nationally established criteria for weight loss surgery [2]. However, few centers have reported weight loss surgery outcomes in elderly patients, resulting in a paucity of literature on perioperative mortality and morbidity. The low volume of bariatric surgery performed in the elderly was confirmed by a recent study showing that only 21% of bariatric centers perform more than four procedures annually on elderly patients [3]. This study reported that only 2.7% of all bariatric operations in academic centers were performed on patients older than age 60 years, although the number of morbidly obese individuals in the Medicare population is substantial.
As the US population ages, elderly obese patients will continue to require numerous medications and a myriad of medical services to manage their weight-related comorbidities. Weight loss surgery has the potential to positively impact these patients, and it is vital to evaluate the safety and efficacy of bariatric surgery in elderly patients with larger trials. The primary purpose of this study was to examine the weight loss results, postoperative changes in daily medication use, changes in quality of life (QOL), hospital length of stay (LOS), and morbidity and mortality rates in individuals exclusively over age 65 years undergoing weight loss surgery at an American Society of Metabolic and Bariatric Surgery designated Center of Excellence.
Methods
Approval was obtained from the Institutional Review Board at Spectrum Health Hospital System in Grand Rapids, Michigan for a retrospective chart review. Our prospectively maintained bariatric database was queried for all patients age ≥65 years who underwent weight loss surgery from January 2000 through December 2007. Of these 212 patients, 14 had undergone previous weight loss surgery and were excluded from analysis. An additional patient had a partial gastrectomy and was also excluded. Information was gathered related to patient demographics, smoking status, and preoperative comorbidities, and conditions. Surgical data, including operative details, LOS, and ICU admissions, were also recorded. In addition, weight, BMI (kg/m2), waist/hip ratios (W/H), daily medication use, and QOL scores were collected preoperatively, and at 6-month and 1-year follow-up visits. Finally, information was collected concerning postoperative complications, hospital readmissions, visits to the Emergency Department (ED), postoperative esophagogastroduodenoscopies (EGDs) performed, percent excess weight loss (EWL), and 30-day and 1-year mortalities.
All procedures were performed by one of three board-certified surgeons, and all patients qualified for weight loss surgery as outlined by the 1991 National Institutes of Health criteria [4]. Patients underwent extensive preoperative medical evaluation, including additional cardiac testing and pulmonary function testing when medically indicated. All patients with a history of ongoing non-steroidal anti-inflammatory drug use, symptoms of gastroesophageal reflux, or positive Helicobacter pylori serum antibody testing underwent a preoperative EGD with biopsies for H. pylori, and treatment when indicated. Patients received a preoperative screening colonoscopy if needed, as indicated by national guidelines. Patients with a history of deep vein thrombosis (DVT) or pulmonary embolus were evaluated for preoperative inferior vena cava filter placement, and all patients received subcutaneous low molecular weight heparin for DVT prophylaxis preoperatively. This was maintained in the perioperative period. All patients with active tobacco use were required to quit smoking prior to their surgery. Smoking cessation was confirmed using preoperative urine nicotine levels. Patients participated in a 2-week, 800-calorie per day dietary restriction to reduce liver volume at the time of surgery. In addition, all patients met preoperatively with a nutritionist and were assessed with a complete psychological and behavioral profile by a psychologist on the bariatric team. This evaluation included the optional use of the Pearson Quality of Life Inventory, which assesses emotional health by profiling problems and strengths in 16 areas of life [5].
LOS was reported in days, from admission to the time of discharge. A patient was considered to be admitted to the ICU if they required an ICU stay at any time during their hospitalization following surgery. Daily medications were those reported by the patient, excluding any vitamin supplements and/or commercially available bowel regimens or analgesics. Death was defined as death occurring from any cause within 30 days or 1 year after weight loss surgery. Postoperative wound infections were defined as any wound that was opened, or an erythematous wound for which the patient was placed on antibiotics by their surgeon. Postoperative pneumonia was defined as sputum production with evidence of infiltrates on chest radiograph, for which the patient was placed on antibiotics.
Analysis of Data
Statistical analysis was completed using SPSS version 10. Data were expressed as a mean ± SEM. Quantitative data were compared using a paired t test. Comparisons between patients who did or did not receive the RYGB procedure for major and minor complications were performed using the Fisher's exact test. Results with a p ≤ 0.05 were considered statistically significant.
Results
A total of 197 patients were included for analysis. The average patient age was 67.3 ± 2.3 years (mean ± SD; range, 65–78), with 142 female patients (72.1%). The mean preoperative BMI was 48.1 ± 6.9 kg/m2 (range, 35.6–73.0). Average preoperative excess body weight was 72.9 ± 17.8 kg (range, 38.7–127.1). Patients had an average waist/hip ratio of 0.929 ± 0.009 (n = 194). Patients lost an average of 4.1 ± 2.8 kg (range, −2.9–15.6) during their 2-week preoperative restrictive diet (n = 191). Of those patients who filled out the QOL assessment preoperatively (n = 128), the average overall QOL percentage score was 51.2 ± 29.1% (range, 1–99%).
Preoperatively, patients presented with a significant number of obesity-related comorbidities, which had been previously diagnosed by their primary care physicians and treated accordingly. The most common comorbidities included hypertension, hypercholesterolemia or dyslipidemia, and diabetes mellitus (Table 1). Patients took an average of 8.04 ± 3.67 daily medications (range, 1–23) preoperatively. The majority of patients had a history of preoperative tobacco use (109 patients, 55.1%).
Procedure types included Roux-en-Y gastric bypass (RYGB; 157 patients, 79.3%), laparoscopic adjustable gastric banding (LAGB; 34 patients, 17.2%), and vertical sleeve gastrectomy (VSG; six patients, 3%). One patient received a partial gastrectomy instead of a planned RYGB due to intraoperative findings of a large gastrointestinal stromal tumor, and was excluded from the study. Ninety-seven percent of all procedures were performed laparoscopically, with a conversion rate of 0%. All open procedures (n = 6) were planned as open, with no documentation of laparoscopic attempt described in the operative note. Additional surgical procedures were documented in the operative note in 121 out of 197 patients (61.4%), the most common of which were adhesiolysis (78 patients, 39.6%), hiatal hernia repair and/or cruraplasty (30 patients, 15.2%), umbilical hernia repair (22 patients, 11.2%), and liver biopsy (11 patients, 5.6%).
Patients who underwent RYGB had an average roux limb length of 149.8 cm (range, 100–200 cm) with 152 out of 157 patients receiving a roux limb length of 150 cm. In the majority of patients, an antecolic/antegastric orientation of the roux limb was performed (151 patients, 96.2%). Two patients (1.3%) received a retrocolic/antegastric roux limb orientation, and four patients (2.5%) received a retrocolic/retrogastric orientation, secondary to foreshortening of the small bowel mesentery.
Average LOS was 2.0 ± 2.1 days (range, 0–18). Five patients (2.5%), all of which had undergone RYGB, required admission to the ICU following their weight loss surgery.
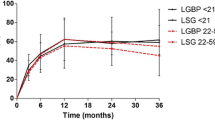
Average weight, BMI, W/H, and daily medication use were significantly reduced at 6 months and 1 year postoperatively (Table 2). QOL scores significantly improved at 6 months and at 1 year. Patients experienced an average EWL of 44.5% (range, 11.1–77.0%) at 6 months (n = 177), and an average EWL of 55.1% (range, 8.73–94.9%) at 1 year (n = 151). The greatest EWL was experienced by patients undergoing RYGB, with an EWL of 48.3% at 6 months (n = 141) and 59.8% at 1 year postoperatively (n = 125).
Twelve patients (6.1%) experienced a total of 15 major complications, including death, myocardial infarction, intestinal obstruction, pneumonia, and gastrointestinal bleeding (Table 3). In the RYGB group, 7% of patients experienced a major complication, the most common of which was gastrointestinal bleeding requiring blood transfusion and/or reoperation (four patients, 2.5%). Two patients (5.9%) in the LAGB group experienced a major complication, namely intestinal obstruction. There were significantly more obstructions in the non-RYGB group (3/40, 7.5%) than in patients who received RYGB (1/157, 0.6%). Minor complications are listed in Table 4 and included a wider range of postoperative problems such as stenosis or stricture of the gastrojejunostomy site, marginal ulcer found on EGD, incisional or port site hernias, and wound infections. Overall, a total of 48 patients (24.2%) experienced at least one minor complication. The rate of minor complications among RYGB patients was 33.1%, the most common of which was marginal ulcer (14 patients, 10.2%) and stricture or stenosis of the gastrojejunostomy site (16 patients, 8.9%). There were no mortalities in the LAGB and VSG groups, and the 30-day and 1-year mortality rates in the RYGB group were 0% and 1.3%, respectively. These two deaths occurred at 5 and 11 months postoperatively from acute cardiac events.
Reoperation rates at 30 days and 1 year were 2.5% and 5.1%, respectively. Within 30 days postoperatively, one patient required reoperation for an abdominal wall infection that led to fascial dehiscence, one patient was taken back to the operating room on the day of surgery for intraoperative evaluation of gastrointestinal bleeding, one patient required laparoscopic removal of their adjustable lap band due to acute obstruction on postoperative day 5, one patient developed a bowel obstruction from a port site hernia on postoperative day 5 requiring laparoscopic exploration with release of incarcerated small bowel and repair of the hernia, and one patient returned within 30 days postoperatively with acute cholecystitis and required laparoscopic cholecystectomy. An additional five patients required reoperation by 1-year postop: two additional patients with cholecystitis required laparoscopic cholecystectomy, two more patients underwent exploratory laparotomy and small bowel resection for acute bowel obstructions (at 4 months and 9 months postop), and one patient experienced recurrence of a hiatal hernia with associated gastric volvulus at 9 months postop, requiring re-exploration and repair with mesh.
In the first postoperative year, 43 patients (21.8%) were seen in the ED for a total of 63 visits. Thirty hospital admissions resulted from these visits, leading to a hospital readmission rate of 15.2%. Thirteen of the 30 hospital admissions were managed by the surgical service, whereas the rest were managed by medicine, cardiology, urologic or orthopedic services. Forty patients (20.3%) underwent a total of 63 upper endoscopy evaluations in the first postoperative year for symptoms such as persistent nausea, vomiting, dysphagia, or inability to sustain weight loss.
Discussion
Bariatric surgery is a safe and effective treatment for morbid obesity, with mortality rates of 0.3% reported at high-volume centers [6]. The morbidities associated with bariatric surgery have been considered to be acceptable by both patients and physicians. However, bariatric surgery continues to be performed in elderly patients at very low rates, despite the growing numbers of morbidly obese Americans over the age of 65.
Several studies have contributed to the controversy surrounding weight loss surgery in the elderly by demonstrating an increase in perioperative mortality and morbidity in this population. Flum et al. reported that mortality rates were greater for those aged 65 years or older compared with younger patients undergoing bariatric procedures, demonstrating a nearly threefold increase in the risk of early mortality in elderly patients [7]. Their study recommended only centers with high volumes consider performing bariatric procedures on elderly patients. Livingston and Langert reported a mortality rate of 3.2% in Medicare patients over age 65 years that had undergone bariatric surgery [8]. In addition, they noted a significant increase in the number of adverse outcomes in this patient population, and concluded that limiting bariatric surgery to individuals under the age of 65 years was warranted due to the increased risk.
Several recent studies have attempted to demonstrate the safety of weight loss surgery in an elderly population [9–13]. However, there has been some discrepancy in the literature as to what constitutes an “elderly” patient, with the cutoff ranging from 50 to 65 years of age. Few centers have looked exclusively at individuals age 65 years and over. Quebbemann et al. found a significant decrease in medication use and obesity-related comorbidities in patients over age 65 undergoing bariatric surgery. However, this study included just 27 patients, only 13 of who underwent RYGB [14]. Nelson et al. looked solely at outcomes in patients over age 65 years, and found a postoperative reduction in medication use and comorbidities with an in-hospital mortality rate of 1.3%. Yet this study, too, had a small cohort of only 25 patients [15].
Our study confirms, in large numbers, the recent literature suggesting that elderly patients can enjoy substantial weight loss from bariatric surgery with a low mortality rate and an acceptable morbidity profile. All patients in our study had a 30-day mortality rate of 0%, and RYGB patients had a 1-year mortality rate of 1.3%. These rates are comparable to the nationally accepted 30-day mortality rate of 0.3% recently reported by the Longitudinal Assessment of Bariatric Surgery Consortium for a younger cohort of patients undergoing bariatric surgery [6]. Our patients also had acceptable rates of serious complications and spent, on average, 2 days in the hospital postoperatively, with very few patients requiring admission to the ICU. Patients demonstrated a significant reduction in weight and BMI at their 6-month and 1-year follow-up visits, as well as a reduction in daily medication use. Also, patients reported improvement in QOL following weight loss surgery. This is a significant and notable finding in any patient undergoing bariatric surgery, regardless of age.
There is the question of how data from our study compare to outcomes reported in the literature in younger cohorts undergoing weight loss surgery. Two recent studies, one published by Podnos et al. in 2003, and another by Nguyen et al. in 2006, are large reviews of weight loss surgery in younger patients and provide some framework for comparison [16, 17]. These studies evaluated many of the same outcomes as our study, namely laparoscopic conversion rates, hospital LOS, ICU admission rates, 30-day mortality rates, and postoperative complications. Despite a significant difference in their age, our RYGB patients experienced similar rates of postoperative intestinal obstruction and GI bleed compared with their younger cohorts (Table 5). In addition, our patients spent overall fewer days on average in the hospital, fewer days in the ICU, and had a 0% postoperative anastamotic leak rate. While 30-day mortality rates were similar between the Podnos and Nguyen studies at 0.23% and 0.4%, respectively, our patients had no mortalities at 30 days, despite their advanced age.
Additional value from our study can be gleaned from our patient follow-up in the first postoperative year regarding readmissions to the hospital, visits to the ED, and EGD rates. We feel the hospital readmission rate of 15.2% for all causes is reasonable in this patient population. We found approximately one in five patients underwent upper endoscopy in the year following weight loss surgery, a figure that may be helpful in preoperative counseling for elderly patients considering weight loss surgery.
While our study demonstrates both the effectiveness and the safety of bariatric surgery in patients over the age of 65, it does not attempt to suggest which type of weight loss surgery is best suited for the elderly. Our RYGB patients realized a greater EWL than patients undergoing alternative types of weight loss surgery. However, the purpose of this study was not to compare the types of weight loss surgery, but to examine all patients undergoing weight loss surgery at our institution with age ≥65. Fewer of our patients underwent both adjustable laparoscopic band placement and vertical sleeve gastrectomy, and we acknowledge that the numbers we reported concerning these two groups of surgical patients may not be representative of a larger sample. In addition, sleeve gastrectomy is not currently covered by CMS, and our data related to the sleeve gastrectomy patients should be viewed carefully due to the low study numbers. Further studies need to be undertaken to determine which type of weight loss surgery offers elderly patients the greatest benefit and the lowest risk profile. Finally, the QOL assessments used at our center were voluntary, and were associated with a nominal fee for our patients. This could clearly be a source of bias in our study, as patients who were unhappy with their weight loss outcomes may have been less likely to keep their follow-up appointments or to participate in postoperative QOL assessments.
Conclusion
This study demonstrates the safety and efficacy of weight loss surgery as a treatment for morbid obesity in patients ≥65 years of age. Despite their advanced age, our patients experienced a clinically significant reduction in weight, with an average of 55% EWL at 1 year, as well as a reduction in daily medication use. Significant improvements were also seen in QOL postoperatively. We report comparable complication and mortality rates to studies examining weight loss surgery results in younger patients, which we attribute to thorough preoperative evaluation in this traditionally higher-risk cohort. Age should not be a barrier for patients who desire weight loss surgery.
References
Ogden CL, Carrol MD, McDowell MA, Flegal KM. Obesity among adults in the United States—no change since 2003–2004,” NCHS data brief no. 1, Hyatsville, MD, National Center for Health Statistics, 2007.
Decision Memo for Bariatric Surgery for the Treatment of Morbid Obesity (CAG-00250R). Available via: www.cms.hhs.gov Accessed Nov 11, 2007.
Varela JE, Wilson SE, Nguyen N. Outcomes of bariatric surgery in the elderly. Am Surg. 2006;72:865–9.
National Institutes of Health Consensus Development Conference Panel. National Institutes of Health conference: gastrointestinal surgery for severe obesity. Ann Intern Med. 1991;171:74–9.
Quality of Life Inventory, Pearson Education, Inc., 2008. Available via: http://psychcorp.pearsonassessments.com. Accessed Feb 21, 2009.
Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. NEJM. 2009;361(5):445–54.
Flum DR, Salem L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–8.
Livingston EH, Langert J. The impact of age and Medicare status on bariatric surgical outcomes. Arch Surg. 2006;141:1115–20.
Fatima J, Houghton SG, Igbal CW. Bariatric surgery at the extremes of age. J Gastrointest Surg. 2006;10:1392–6.
Hallowell PT, Stelatto TA. Avoidance of complications in older patients and Medicare recipients undergoing gastric bypass. Arch Surg. 2007;142:506–12.
Trieu HT, Gonzalvo JP, Szomstein S. Safety and outcomes of laparoscopic gastric bypass surgery in patients 60 years of age and older. Surg Obes Relat Dis. 2007;3:383–6.
Dunkle-Blatter SE, St. Jean MR. Outcomes among elderly bariatric patients at a high-volume center. Surg Obes Relat Dis. 2007;3:163–70.
Hazzan D, Chin EH. Laparoscopic bariatric surgery can be safe for treatment of morbid obesity in patients older than 60 years. Surg Obes Relat Dis. 2006;2:613–6.
Quebbemann B, Engstrom D. Bariatric surgery in patients older than 65 years is safe and effective. Surg Obes Relat Dis. 2005;1:389–93.
Nelson LG, Lopez PP, et al. Outcomes of bariatric surgery in patients ≥65 years. Surg Obes Relat Dis. 2006;2:384–8.
Podnos Y, Jimenez J, et al. Complications after laparoscopic gastric bypass: a review of 3,464 cases. Arch Surg. 2003;138:957–61.
Nguyen NT et al. Result of a national audit of bariatric surgery performed at academic centers. Arch Surg. 2006;141:445–50.
Acknowledgements
Allen Shoemaker, Ph.D. for assistance with statistical analysis.
Conflict of Interest Disclosure
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
O’Keefe, K.L., Kemmeter, P.R. & Kemmeter, K.D. Bariatric Surgery Outcomes in Patients Aged 65 Years and Older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. OBES SURG 20, 1199–1205 (2010). https://doi.org/10.1007/s11695-010-0201-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0201-4