Abstract
Background
Obesity has been associated with impaired intestinal barrier function. It is not known whether bariatric surgery leads to changes in intestinal barrier function. We hypothesized that obesity is associated with disturbances in gastrointestinal barrier function, and that after bariatric surgery barrier function will improve.
Methods
Prospective single center study in which we assessed segmental gut permeability by urinary recovery of a multisugar drink in 27 morbidly obese (BMI 43.3 ± 1.1 kg/m2) and 27 age and gender matched lean subjects (BMI 22.9 ± 0.43 kg/m2). Fecal calprotectin, SCFAs, plasma cytokines, and hsCRP were assessed as inflammatory and metabolic markers. Comparisons: (a) morbidly obese subjects vs. controls and (b) 2 and 6 months postsleeve vs. presleeve gastrectomy (n = 14). In another group of 10 morbidly obese and 11 matched lean subjects colonic and ileal biopsies were obtained in order to measure gene transcription of tight junction proteins.
Results
Gastroduodenal permeability (urinary sucrose recovery) was significantly increased in obese vs. lean controls (p < 0.05). Small intestinal and colonic permeability (urinary recovery of lactulose/L-rhamnose and sucralose/erythritol, respectively) in obese subjects were not significantly different from controls. Morbidly obese subjects had a proinflammatory systemic and intestinal profile compared with lean subjects. After sleeve gastrectomy BMI decreased significantly (p < 0.001). Postsleeve gastroduodenal permeability normalized to values that do not differ from lean controls.
Conclusions
Gastroduodenal permeability, but not small intestinal or colonic permeability, is significantly increased in morbidly obese patients. After sleeve gastrectomy, gastroduodenal permeability normalized to values in the range of lean controls. Thus, the proximal gastrointestinal barrier is compromised in morbid obesity and is associated with a proinflammatory intestinal and systemic profile.
Similar content being viewed by others
Introduction
Obesity is rapidly increasing worldwide, leading to significant impairments in health status with serious comorbidities. Up to now, bariatric surgery has been the only therapy for morbid obesity with consistent beneficial long-term results [1].
Several studies have pointed to changes in gastrointestinal function, especially in intestinal barrier function in obesity. Indeed, impairment of the intestinal barrier has been observed in patients with morbid obesity and was found to be associated with an increased inflammatory response in the systemic compartment [2,3,4,5].
Bariatric surgery, either via a restrictive procedure (sleeve gastrectomy) or via a combined restrictive + malabsorptive procedure (Roux-Y Gastric Bypass; RYGB), is very effective in long-term weight management [1, 6]. Up to now, little is known on the effect of bariatric surgery on disturbed intestinal barrier function in morbid obesity.
The present study was undertaken to study in more detail intestinal barrier function in morbidly obese subjects in comparison with age and sex matched lean controls. Secondly, the morbidly obese patients were prospectively followed with measurements before and after restrictive bariatric surgery. Before and at 2 and 6 months after laparoscopic sleeve gastrectomy, tests for intestinal barrier function were performed. We evaluated several aspects of intestinal barrier and metabolic function: (1) whole gut and site-specific permeability, (2) inflammatory processes, both at local level (human beta defensin-2, calprotectin) and systemic level (plasma cytokines), (3) tight junction proteins (mRNA expression and immunohistochemistry) in biopsies, (4) intestinal metabolism (fecal short-chain fatty acids (SCFAs) concentrations), and (5) small intestinal functional enterocyte mass (plasma citrulline) and intestinal endocrine cell mass (fecal chromogranin A (CgA)).
We hypothesized that epithelial barrier function is mildly disturbed in subjects with morbid obesity and that bariatric surgery, due to weight loss, will restore intestinal barrier function to conditions not different from lean controls.
Materials and methods
This study was part of a larger prospective single center open study to evaluate epithelial barrier function, inflammation and gut microbiota composition in morbidly obese subjects vs. lean controls and to evaluate the effect of bariatric surgery on above-mentioned parameters. Gut microbiota profiling is a research topic separate from the present study.
Patients were considered candidate for bariatric surgery, when aged 18–65 years, with body mass index (BMI) > 35 kg/m2 and comorbidities or with BMI > 40 kg/m2. Exclusion criteria were severe cardiovascular, respiratory, hematological, immunological, neurological or psychiatric diseases or previous major abdominal surgery. Patients with gastrointestinal or hepatic disorders influencing gastrointestinal absorption or transit were excluded. Other exclusion criteria were alcohol consumption >20 units/week, eating disorders (all candidates for bariatric surgery were screened by a psychologist to exclude eating disorders), pregnancy, lactation, or recent use of antibiotics (<4 weeks). Patients were asked to participate in this study when visiting the outpatient department of surgery before surgery was planned. Healthy controls were recruited by poster advertisement. All subjects were informed to undergo a gut barrier function test and the withdrawal of blood samples and collection of stool samples in order to investigate barrier function and inflammation. The posters were presented at public places within the University of Maastricht campus, Maastricht University Medical Centre and Catharina Hospital Eindhoven.
Informed consent was obtained from each individual. The study protocol had been approved by Ethics Committee of the Catharina Hospital Medical Centre and the study was executed according to the revised Declaration of Helsinki (59th general assembly of the WMA, Seoul, South Korea, Oct. 2008).
Each morbidly obese subject was tested within an interval of 2 months—2 weeks prior to bariatric surgery and at 2 and at 6 months after surgery. In the morbidly obese subjects, a low caloric diet was prescribed 2 weeks prior to surgery. All baseline measurements had been performed before this diet was initiated. Weight, BMI, and weight loss were assessed during each test day in the obese group. On these test days gut permeability was measured, blood samples were drawn and fecal samples were collected.
Gut permeability test
The test we employed has been validated [7], and previously described by our group. In short, a sugar drink (in 100 mL tap water) containing 1 g sucrose (Van Gilse automatensuiker, Albert Heijn, Maastricht, the Netherlands), 1 g lactulose (Centrafarm Services, Etten-Leur, the Netherlands), 0.5 g l-rhamnose (Danisco Sweeteners, Thomson, Illinois, USA), 1 g sucralose (Tate and Lyle Sucralose Inc. Reading Great Britain) and 1 g erythritol (Cargill Nederland BV, Schiphol, the Netherlands) was ingested after an overnight fast. During the first 5 h of the test, ingestion of other food and drinks, except water, was not allowed.
All subjects were asked to collect urine for 24 h after ingesting the sugar drink. The urine output during the first 5 h was collected separately to assess small bowel permeability. In healthy control subjects, the gut permeability test was performed once.
All sugar probes used in this test are accepted and validated parameters of integrity of the intestinal barrier throughout the gut. Urine samples were obtained to measure sugar recovery. Urine aliquots were collected and stored at −80 °C until analysis. Sucrose, lactulose, l-rhamnose, sucralose, and erythritol were determined by fluorescent detection high-pressure liquid chromatography (HPLC). Sucrose excretion was used to calculate gastroduodenal permeability. The excretion ratio of lactulose/L-rhamnose (L/R) was used to calculate small bowel permeability. The ratio of sucralose/erythritol (S/E) was used to calculate large bowel permeability.
Blood and stool samples
Blood and stool samples were collected before, at 2 months, and at 6 months after surgery. From the healthy controls only one blood and one stool sample was collected. The blood samples were centrifuged at 3000 rpm at 4 °C. The supernatant was stored at −80 °C until further analysis. All stool samples were stored at −80 °C until analysis.
Plasma cytokines were determined as makers for systemic inflammation. Plasma IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α, were measured by ProcartaPlexTM multiplex immunoassay (eBioscience, USA).
High sensitivity-CRP (hsCRP) was measured on Cobas 8000 (Roche Diagnostics, Indianapolis, USA) using a latex particle-enhanced immunoturbidimetric assay following the manufacturer’s instructions. The measuring range for this assay is 0.15–20 mg/dL. Plasma citrulline, a marker of functional enterocyte mass of the small bowel, was measured to detect changes in enterocyte mass over time after bariatric surgery, and measured by HPLC [8].
Fecal human β-defensin 2, a human antimicrobial peptide produced by intestinal epithelial cells in response to (pathogenic) bacteria, was measured in stool as an indicator of host defense against microbes by enzyme-linked immunosorbent assay (ELISA, Immunodiagnostik AG, Germany).
Fecal chromogranin A (CgA), was measured as indicator of intestinal neuroendocrine cell activity. This peptide is produced by enterochromaffin cells and is co-localized in storage granules with serotonin. It was measured using a commercial radioimmunoassay (Euro-Diagnostica, Sweden) [9].
SCFAs, i.e., acetate, propionate, butyrate, valerate, and caproate, are products of microbial fermentation of nondigested oligosaccharides in the colon and were selected as indicators of gut intraluminal metabolic activity. SCFAs are an important energy source for colonocytes in humans and have been associated with various beneficial effects related to modulation of inflammation, carcinogenesis, etc [10]. Gas chromatography–mass spectrometry (GC–MS), a method previously described by Garciá-Villalba et al. was used to measure fecal SCFAs.
Fecal calprotectin was measured by ELISA (Bühlmann Laboratories, Switzerland) as indicator of intestinal inflammation, proportional to neutrophil migration towards the intestinal tract.
Colonic and distal ileal biopsies
In a separate group of ten morbidly obese, otherwise healthy, subjects (BMI > 35 kg/m2) and 11 lean subjects matched for age and gender with a stable bodyweight for 3 months were included in this second study. The morbidly obese and control subjects underwent colonoscopy either for surveillance or for diagnostic purposes at the Gastroenterology-Hepatology department of the Catharina Hospital in Eindhoven. Starting from 2 days before colonoscopy subjects were asked to follow a fiber restricted diet. In the interval of 8–12 h before the colonoscopy subjects had to drink a bowel preparation solution consisting of macrogol (either Klean Prep or Moviprep, Norgine B.V., Amsterdam, The Netherlands) and in this time interval subjects were asked to refrain from food intake. Dietary restrictions and bowel preparations were identical in both groups. During colonoscopy (with or without conscious sedation with midazolam), eight biopsies were taken at standardized locations: four biopsies in the ascending colon (5–10 cm distal from the ileocecal valve) and four biopsies in the terminal ileum using a standard biopsy forceps. After tissue sampling, tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
Gene transcription of tight junction proteins
Gene transcription of tight junctions (TJ) and associated proteins, i.e., occludin, claudin-3, claudin-4, zonula occludens 1 (ZO-1), and myosin light-chain kinase (MLCK) were determined in mucosal biopsies. Nucleic acid extraction and purification, RNA isolation, reverse transcription and quantitative real-time polymerase chain reaction were performed as previously described [11, 12].
Data were presented as normalized expression ratios i.e., expression of target genes normalized to reference gene glyceraldehyde-3-phosphate dehydrogenase.
Statistical considerations
Data on gut permeability in obesity are scarce and inconclusive. Therefore the sample size calculation of the primary outcome (i.e., intestinal permeability in vivo) was based on the effect size of indomethacin on urinary L/R excretion ratio [13]. With a difference in means of 0.018, power of 0.80, standard deviation of 0.02 and α of 0.05, the minimum sample size is 20 subjects for obese patients and for healthy control subjects. However, we decided to include 27 obese patients and 27 healthy controls, considering possible drop-outs after inclusion.
Data were statistically analyzed using Graphpad Prism 6.0. A p value of p < 0.05 was considered as statistically significant. Results were expressed as median ± range. The following statistical tests were used: repeated measures analysis of variance and Friedman test (nonparametric) in completers, Mann Whitney and unpaired t-test comparing lean and morbidly obese subjects, and Pearson correlations. Gene transcription p values were corrected for multiple testing by the false-discovery-rate (FDR) of Benjamini–Hochberg.
Results
Subjects
A total of 27 morbidly obese subjects (BMI 43.3 ± 1.08 kg/m2) and 27 lean (BMI 22.9 ± 0.43 kg/m2) age and gender matched subjects participated in the baseline valuation. From this group a subgroup of 14 morbidly obese subjects (BMI 45.4 ± 6.2 kg/m2) was also willing to participate in postoperative follow-up. Baseline characteristics (BMI, weight loss, age, and gender) did not differ between the initial 27 morbidly obese subjects and the 14 morbidly obese subjects in the repeated measurements. The subgroup underwent three tests: one test preoperatively (baseline) and two tests postoperatively, at 2 and at 6 months, respectively. Significant weight loss was observed after the bariatric procedure. BMI decreased significantly from 45.4 ± 6.2 kg/m2 to 38.7 ± 5.8 kg/m2 after 2 months (p < 0.001) and to 34.3 ± 5.3 kg/m2 (p = 0.001 vs. baseline) at 6 months after surgery. After 12 months BMI was 32.5 ± 4.7 kg/m2 (p < 0.001).
Gut permeability
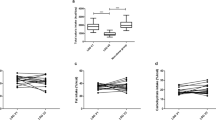
Gastroduodenal permeability (sucrose) was significantly increased in morbidly obese subjects vs. lean controls. The 5-h urinary sucrose recovery was 0.51% [0.01–5.14] vs. 0.20% [0.02–2.35], respectively, (p < 0.05), as depicted in Fig. 1. In the 14 subjects in whom permeability data were available both before and 2 and 6 months after the surgical procedure, gastroduodenal permeability (sucrose) decreased to values not significantly different from controls. However, within the group of morbidly obese subjects the reduction was not statistically significant (p = 0.09), as depicted in Table 1.
In morbidly obese subjects neither small intestinal nor colonic permeability, as assessed by 5-h urinary recovery of lactulose and l-rhamnose, and by the 24-h urinary recovery of sucralose and erythritol, respectively, were significantly different from controls.
In the postoperative state, L/R ratio was significantly (p < 0.05) increased 6 months after sleeve gastrectomy compared with preoperative values (Table 1). Colonic permeability (S/E ratio) did not significantly change 2 and 6 months following surgery compared with baseline although erythritol excretion at 2 months after surgery was significant different compared with baseline (Table 1).
Biomarkers in plasma
Baseline plasma citrulline concentration was significantly (p < 0.0001) lower in the morbidly obese (median 27.9 [13.5–51.9] µmol/L) vs. the lean subjects (median 40.7 [28.2–62.0] µmol/L). No significant change over time was observed in the morbidly obese subjects 2 and 6 months after sleeve gastrectomy compared with baseline (respectively 30.6 [23.0–36.3] µmol/L after 2 months and 28.5 [21.3–40.4] µmol/L after 6 months).
Plasma hsCRP was significantly higher in morbidly obese subjects vs. controls (Table 2) and hsCRP values decreased significantly 6 months after sleeve gastrectomy (p < 0.002; Table 3). Plasma TNF-α was significantly higher in morbidly obese subjects compared with lean subjects: median 5.36 [0.08–9.56] µmol/L vs. median 0.08 [0.08–9.56] µmol/L, respectively (Table 2). Plasma TNF-α did not change significantly after surgery. IL-6 and IL-10/12 ratio were significantly lower in morbidly obese vs. lean subjects but these parameters did not change after bariatric surgery.
Fecal markers
Fecal calprotectin levels were low, both in controls and in morbidly obese subjects (Table 2). However, fecal calprotectin levels in morbidly obese were significantly higher compared with lean subjects. After sleeve gastrectomy, calprotectin levels increased significantly (p < 0.05) over baseline in the morbidly obese subjects (Table 3).
The other fecal parameters are listed in Table 4. Baseline CgA, beta defensin, acetate, propionate, butyrate, and valerate did not differ between lean and obese subjects. Neither did total SCFAs concentration and ratios (e.g., butyrate/total SCFA ratio, proprionate/total SCFA, or acetate/total SCFA differ between lean and obese subjects. Apart from calprotectin, no 2 and 6 months postsleeve gastrectomy data are available for the other fecal parameters listed in Table 4.
Correlations
Correlation analyses of permeability parameters (sucrose recovery, lactulose recovery, and sucralose recovery) with BMI, fecal calprotectin and hsCRP were performed. Sucrose recovery (r = 0.360, p = 0.008) and hsCRP (r = 0.685, p < 0.0001) were significantly correlated with BMI as depicted in Fig. 2. Fecal calprotectin significantly correlated with sucrose recovery (r = 0.424, p = 0.003), but not with lactulose recovery (r = 0.140, p = 0.349) or sucralose recovery (r = −0.169, p = 0.250). No significant correlation was found between hsCRP and permeability (all p ≥ 0.236).
Tight junction gene expression in ileal and colon biopsies
No significant differences in TJ gene expressions were found between lean and morbidly obese in ileal biopsies (Table 5). In colon biopsies MLCK, claudin-3, and claudin-4 expression were significantly higher in morbidly obese compared with lean subjects, both before and after FDR correction for multiple testing. Gene expression of occludin and ZO-1 did not differ between lean and morbidly obese subjects in colonic biopsies.
Discussion
We evaluated gastrointestinal barrier function in morbidly obese subjects vs. controls and more closely analysed the effect of sleeve gastrectomy on intestinal barrier function. In morbidly obese subjects, gastroduodenal permeability was significantly increased, but not small intestinal or colonic permeability. Morbidly obese subjects were characterized by a proinflammatory plasma and stool profile. The SCFA metabolic profile was not different between morbidly obese and lean subjects. After sleeve gastrectomy, gastroduodenal permeability decreased to levels not different from lean controls.
Gastroduodenal permeability, measured by urinary sucrose recovery, was significantly increased in morbidly obese subjects while small intestinal and colonic permeability were not different from lean controls. Our data confirm previous findings from Brignardello et al. [14] and Carswell et al. [15] on small intestinal permeability in which they found an increased proximal gut permeability in morbidly obese subjects. Moreover, BMI was found to be significantly correlated with sucrose recovery, supporting this observation. After sleeve gastrectomy, gastroduodenal permeability decreased to values no longer significantly different from those found in lean controls.
Paracellular translocation of potential noxious substances can lead to intestinal and/or systemic inflammation as suggested by association studies in IBD [16,17,18].
We performed correlation analyses of paracellular permeability parameters (i.e., sucrose, lactulose, and sucralose recovery) with intestinal (fecal calprotectin) and systemic inflammation (hsCRP). Fecal calprotectin was significantly correlated with sucrose recovery, but not with lactulose or sucralose recovery. These observations show that intestinal but not systemic inflammation is associated with increased proximal permeability in obesity.
Urinary L/R ratio increased significantly 6 months after sleeve gastrectomy. Assuming that permeability is affected due to early postoperative changes (e.g., inflammation) a rise would have been expected at 2 months (or earlier) instead of 6 months post operatively. When analyzing urinary recovery of individual sugars in more detail, it is apparent that in the morbidly obese subjects, urinary excretion of lactulose was not affected by sleeve gastrectomy while l-rhamnose excretion decreased. Likewise, sucralose excretion was not affected by sleeve gastrectomy while erythritol excretion decreased. Thus, the recovery of disaccharides (paracellular transport route) was not affected while recovery of monosaccharides was reduced (transcellular transport route) after sleeve gastrectomy. Structural differences exist between sleeve gastrectomy (stomach volume reduced to 15–20%, small intestine unaltered although significant changes in gut peptide secretion occur [19]) and RYGB (small stomach pouch created and connected to a jejunal limb thereby bypassing part of the proximal bowel: bile and pancreatic juice flow are partially diverted from nutrient flow). Despite these structural differences, Savassi-Rocha et al. have made similar observations in patients after RYGB compared with sleeve gastrectomy, with urinary lactulose excretion not being affected 1 month after RYGB, while mannitol excretion (monosaccharide; transcellular transport route) decreased significantly [20]. A possible explanation for this decrease in recovery of monosaccharides may be related to an accelerated gastrointestinal transit after sleeve gastrectomy or after RYGB resulting in a reduction in exposure time of the intestinal area to monosaccharides for transcellular transport. Indeed, several authors have previously reported that intestinal transit is accelerated after sleeve gastrectomy [21,22,23]. Moreover, a positive correlation has been found between intestinal transit time and the amount of urinary mannitol excretion [12, 24]. In our study we cannot exclude potential impact of confounders such as intestinal transit time, on the observed changes in intestinal permeability.
Our hypothesis on increased small and large bowel permeability in morbid obesity was not confirmed. In fact, the intestinal barrier proved to be undisturbed. Further evidence for an intact intestinal barrier was provided by a normal gene expression profile of several TJ proteins in ileal biopsies of morbidly obese subjects.
However, gene expressions of TJ MLCK, claudin-3 and claudin-4 in colonic biopsies were significantly higher in the morbidly obese subgroup. Little et al. determined ZO-1 and occludin in duodenum tissue of lean and obese subjects, and found significant lower ZO-1 in obese vs. lean subjects but no significant differences in occludin [25]. However that study did not report on claudins, nor on MLCK. An explanation for higher MLCK, claudin-3 and claudin-4 gene expressions in morbidly obese vs. lean subjects is not readily available since no differences were seen in functional (multisugar test) and metabolic (SCFAs) parameters. It is well known that the intestinal barrier is not static, but constantly remodeling in order to selectively regulate intestinal permeability. As a consequence, it is assumed that the differences we found are a compensatory mechanism in order to maintain a well-functioning intestinal barrier.
As stated before, we cannot exclude the effect of an acceleration in gastrointestinal transit time on permeability findings, nor can we exclude alterations in gut peptide secretion to have influenced permeability measurements. It has been shown that in obesity postprandial responses of both PYY and GLP-1 may become affected [26]. These peptides are known to influence gastrointestinal transport.
Obesity has been associated with low-grade intestinal and systemic inflammation. In line with previous studies [27] we found a significant correlation between BMI and hsCRP. Moreover, hsCRP and TNF-α plasma levels were significantly higher in morbidly obese subjects compared with the controls in our study. We observed a significant reduction in hsCRP but not in the other systemic inflammatory markers. However, Illan-Gomez et al. observed a significant decrease in both hsCRP and IL-6 after bariatric surgery and weight loss [28].
The fecal calprotectin levels we observed were low, well below threshold level for intestinal inflammation. Although below threshold, fecal calprotectin was significantly higher in the morbidly obese subjects compared with the lean controls. Our findings confirm those of Poullis et al. and Verdam et al. [29, 30].
The increase in fecal calprotectin we observed after sleeve gastrectomy is not in line with studies reporting a decrease in fecal calprotectin after diet induced weight loss [31]. It is not known whether fecal calprotectin levels were affected by the surgical procedure or other factors such as changes in intestinal transit [15] or more active enterocyte turnover [32, 33].
Fecal SCFA levels in the morbidly obese subjects were not significantly different from controls. In several studies, a role for SCFAs as contributor to obesity has been suggested. In line with this assumption, some obese humans were found to have higher fecal SCFA concentrations [34]. Our data are not in line with these observations. SCFA concentrations have been related to gut health. In that respect, our findings that fecal SCFA concentrations are not affected by obesity should be considered as positive result.
Plasma citrulline is known to reflect small intestinal mass. Plasma citrulline levels were significantly reduced in our morbidly obese subjects. Our findings are in line with those of Takashina et al. [35] Verdam et al. on the other hand found evidence for increased plasma citrulline levels in obese subjects, in particular in the subgroup with hyperglycemia [8].
A strength of our study lies in consecutive measurements over time both before and after sleeve gastrectomy. A weakness of our study, on the other hand, is that we cannot determine whether the changes we observed after surgery are due to weight loss, the surgical procedure, or both. Other factors such as inflammation, gut microbiota composition, and transit time may have attributed to the observed changes in permeability.
The multisugar permeability test has proven to be an elegant noninvasive test to measure gastrointestinal permeability. It has been validated previously. We are the first to report on this test in the evaluation of gastrointestinal permeability before and after sleeve gastrectomy.
In conclusion, we have shown that gastroduodenal permeability, but not small intestinal and colonic permeability, is significantly increased in morbidly obese subjects. Morbidly obese persons are characterized by a proinflammatory plasma and stool profile. The SCFA metabolic profile was not different in morbidly obese vs. lean subjects. After sleeve gastrectomy gastroduodenal permeability decreased to levels in the range of lean controls.
References
Pekkarinen T, Mustonen H, Sane T, Jaser N, Juuti A, Leivonen M. Long-term effect of gastric bypass and sleeve gastrectomy on severe obesity: do preoperative weight loss and binge eating behavior predict the outcome of bariatric surgery? Obes Surg. 2016;26:2161–7.
Arismendi E, Rivas E, Agusti A, Rios J, Barreiro E, Vidal J, et al. The systemic inflammome of severe obesity before and after bariatric surgery. PLoS ONE. 2014;9:e107859.
Damms-Machado A, Louis S, Schnitzer A, Volynets V, Rings A, Basrai M, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105:127–35.
Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol. 2018;246:217–30.
Rainone V, Schneider L, Saulle I, Ricci C, Biasin M, Al-Daghri NM, et al. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes. 2016;40:1026–33.
Busetto L, Dixon J, De Luca M, Shikora S, Pories W, Angrisani L. Bariatric surgery in class I obesity: a position statement from the international federation for the surgery of obesity and metabolic disorders (IFSO). Obes Surg. 2014;24:487–519.
van Wijck K, Verlinden TJ, van Eijk HM, Dekker J, Buurman WA, Dejong CH, et al. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr. 2013;32:245–51.
Verdam FJ, Greve JW, Roosta S, van Eijk H, Bouvy N, Buurman WA, et al. Small intestinal alterations in severely obese hyperglycemic subjects. J Clin Endocrinol Metab. 2011;96:E379–83.
Mujagic Z, Jonkers D, Ludidi S, Keszthelyi D, Hesselink MA, Weerts Z et al. Biomarkers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil 2017;29:e13137.
Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28:42–66.
Pijls KE, Jonkers DM, Elizalde M, Drittij-Reijnders MJ, Haenen GR, Bast A, et al. Is intestinal oxidative stress involved in patients with compensated liver cirrhosis? Ann Hepatol. 2016;15:402–9.
Keszthelyi D, Troost FJ, Jonkers DM, van Donkelaar EL, Dekker J, Buurman WA, et al. Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine? Am J Clin Nutr. 2012;95:603–8.
Troost FJ, Saris WH, Brummer RJ. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur J Clin Nutr. 2003;57:1579–85.
Brignardello J, Morales P, Diaz E, Romero J, Brunser O, Gotteland M. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–14.
Carswell KA, Vincent RP, Belgaumkar AP, Sherwood RA, Amiel SA, Patel AG, et al. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24:796–805.
Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology. 2017;153:723–31 e1.
Michielan A, D’Inca R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157.
Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci. 2012;1258:159–65.
McCarty TR, Jirapinyo P, Thompson CC. Effect of sleeve gastrectomy on ghrelin, GLP-1, PYY, and GIP gut hormones: a systematic review and meta-analysis. Ann Surg. 2019.
Savassi-Rocha AL, Diniz MT, Vilela EG, Diniz Mde F, Sanches SR, da Cunha AS, et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes Surg. 2014;24:184–90.
Trung VN, Yamamoto H, Furukawa A, Yamaguchi T, Murata S, Yoshimura M, et al. Enhanced intestinal motility during oral glucose tolerance test after laparoscopic sleeve gastrectomy: preliminary results using cine magnetic resonance imaging. PLoS ONE. 2013;8:e65739.
Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258:976–82.
Mans E, Serra-Prat M, Palomera E, Sunol X, Clave P. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am J Clin Nutr. 2015;102:540–7.
Madsen JL, Scharff O, Rabol A, Krogsgaard OW. Relationship between small-intestinal transit rate and intestinal absorption of (14)C-labelled mannitol and (51)Cr-labelled ethylenediaminetetraacetic acid in healthy subjects. Scand J Gastroenterol. 1996;31:254–9.
Little TJ, Cvijanovic N, DiPatrizio NV, Argueta DA, Rayner CK, Feinle-Bisset C, et al. Plasma endocannabinoid levels in lean, overweight, and obese humans: relationships to intestinal permeability markers, inflammation, and incretin secretion. Am J Physiol Endocrinol Metab. 2018;315:E489–95.
Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–54.
Rojano-Rodriguez ME, Valenzuela-Salazar C, Cardenas-Lailson LE, Romero Loera LS, Torres-Olalde M, Moreno-Portillo M. C-reactive protein level in morbidly obese patients before and after bariatric surgery. Rev Gastroenterol Mex. 2014;79:90–5.
Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragon-Alonso A, Pascual-Diaz M, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–5.
Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279–84.
Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21:E607–15.
Kant P, Fazakerley R, Hull MA. Faecal calprotectin levels before and after weight loss in obese and overweight subjects. Int J Obes. 2013;37:317–9.
Park HK, Sinar DR, Sloss RR, Whitley TW, Silverman JF. Histologic and endoscopic studies before and after gastric bypass surgery. Arch Pathol Lab Med. 1986;110:1164–7.
Sinar DR, Flickinger EG, Park HK, Sloss RR. Retrograde endoscopy of the bypassed stomach segment after gastric bypass surgery: unexpected lesions. South Med J. 1985;78:255–8.
Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TM. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38:1525–31.
Takashina C, Tsujino I, Watanabe T, Sakaue S, Ikeda D, Yamada A, et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr Metab (Lond). 2016;13:5.
Acknowledgements
We would like to thank Anton van der Stokker, analyst at the laboratory of the Catharina hospital in Eindhoven, for analyzing hsCRP and for collecting and preserving all specimens before analysis. We would like to thank the department of Surgery in Maastricht for analyzing urine specimens.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wilbrink, J., Bernards, N., Mujagic, Z. et al. Intestinal barrier function in morbid obesity: results of a prospective study on the effect of sleeve gastrectomy. Int J Obes 44, 368–376 (2020). https://doi.org/10.1038/s41366-019-0492-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0492-z
- Springer Nature Limited
This article is cited by
-
Paracellular permeability and tight junction regulation in gut health and disease
Nature Reviews Gastroenterology & Hepatology (2023)
-
Serum Versus Fecal Calprotectin Levels in Patients with Severe Obesity Before and 6 Months After Roux-Y-Gastric Bypass: Report of the Prospective Leaky-Gut Study
Obesity Surgery (2023)