Abstract
Background
Recently, the link between obesity and gut microbiota has become a focus for research. This study shed some light on the modification of postoperative gut microbial composition after bariatric surgery.
Methods
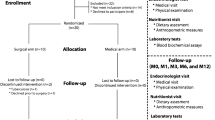
A prospective longitudinal study on healthy lean subjects and patients who underwent bariatric surgery (Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy) was carried out. Anthropometric and metabolic data, smoking, food preferences data, and stool samples were collected from lean subjects and from obese patients before and 3 and 6 months after surgery (T0, T3, and T6, respectively).
Results
We collected stool samples from 25 obese patients before surgery and 3 and 6 months thereafter and from 25 normal weight patients. After Roux-en-Y gastric bypass, Yokenella regensburgei (p < 0.05), Fusobacterium varium (p < 0.05), Veillonella dispar/atypica (p < 0.05), and Streptococcus australis/gordonii (p < 0.05) were transiently identified in the gut at T3. Roux-en-Y gastric bypass patients had a permanent increase in Akkermansia muciniphila (p < 0.05), which is associated with healthy metabolism, both at T3 and T6. There were no significant changes in gut microbiota in laparoscopic sleeve gastrectomy patients.
Conclusions
In our study, Roux-en-Y gastric bypass induced major microbial differences and greater weight loss compared with laparoscopic sleeve gastrectomy. Analyzing the microbiota composition, a proliferation of potential pathogens and the onset of beneficial bacteria was observed. The effects of these bacteria on human health are still far from clear. Understanding the mechanisms of action of these bacteria could be the keystone in developing new therapeutic strategies for obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global epidemic of obesity is a public health problem in many countries. Excess weight is a condition characterized by an imbalance between excessive accumulation of body fat, usually due to incorrect nutrition and sedentary life, and alteration of body mechanisms to regulate the energy intake, expenditure, and storage. Recently, a link between obesity and gut microbiota has become a focus for research. These bacteria play an important role in the physiological mechanism of digestion, although the exact underlying mechanism is still far from clear.
We know that the human gastrointestinal tract is colonized by about 100 trillion bacteria. The application of new advanced molecular biology techniques, such as next-generation sequencing, have improved our understanding of gut microbiota. Approximately 90% of the bacteria in the intestinal microbiota are members of the phyla of Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria.1 The first contact with bacteria occurs during birth; thereafter, weaning contributes to a change in the composition of baby gut microbiota. After that, an individual’s microbiota remains substantially unchanged, until adulthood when it changes again in relation to several factors such as diet, host genetics, medications, and health status. The composition of the intestinal microbiota is constantly changing and for this reason, it is unique and identifiable for each individual, just like fingerprints.
The most important influencing factors are long-term diet and obesity.2, 3 Studies on animal models showed that obese mice, as humans, had different gut microbiota composition compared to lean. The same modification was found also in studies on children.4 Furthermore, obese mice that lost weight after bariatric surgery had a different gut microbiota composition compared to not operated obese mice, but similar to lean mice.5 Bariatric surgery is the most lasting and effective treatment for morbid obesity. Considering the potential for obesity-related diseases to lead to both immediate and future profound health implications, bariatric surgery is performed also in obese children.6 Roux-en-Y gastric bypass (RYGB) and laparoscopic sleeve gastrectomy (LSG) induce significant reductions in BMI along with reversal in many disease-related comorbidities.7, 8
No conclusive data are yet available on the effects of surgical interventions on the gut microbiome and on the mechanisms regulating this process, neither in adult nor in pediatric obese populations.9, 10
The purpose of this study is to investigate the specific change in gut microbiota composition that may be caused by bariatric procedures and the consequently modified diet preferences post-surgery in adult obese patients. In addition, we explored the single bacterial properties and their effect on human health.
Materials and Methods
Consecutive obese patients, who underwent bariatric surgery (BS), and healthy lean subjects (normal weight (NW)) were included in this prospective longitudinal study. Informed consent was obtained from all participants and the privacy rights of human subjects were observed.
Inclusion criteria for lean subjects were absence of any kind of morbidity and age 18 to 65 years.
Inclusion criteria for patients eligible for surgery were in line with international bariatric guidelines..11 The bariatric procedures performed were RYGB and LSG.
Exclusion criteria for both groups were the presence of inflammatory bowel disease and administration of antibiotic therapy within 1 month before the study enrollment. All BS patients were strictly followed and none of them needed antibiotic therapy until the sixth month after the operation.
During the first medical examination (T0), stool and blood samples were collected from all subject enrolled in the study and the following parameters were recorded for each patient: anthropometric (height, weight, gender, blood pressure (mmHg), and BMI (kg/m2)), metabolic (presence of type 2 diabetes mellitus (T2DM), ongoing diabetic therapy, values of baseline HbA1c%, high-density lipoprotein cholesterol [HDL], and blood triglycerides), and smoking. Metabolic syndrome (MS) was diagnosed according to the new International Diabetes Federation (IDF) consensus worldwide definition (http://www.idf.org/metabolic-syndrome). In BS, fecal samples were collected also 3 (T3) and 6 months (T6) after operation.
Three and 6 months after surgery, the following anthropometric and metabolic parameters were evaluated: final weight (FW), final BMI, percentage of excess weight loss (%EWL), improvement or remission of T2DM, and lipid profile.
A questionnaire was administered both to lean subject and to obese patients before surgery and at T3 and T6, in order to assess food preferences. The questionnaire consisted of a list of food grouped in 4 categories (carbohydrates, proteins, fats, and vegetables) and for each food, the patients had to answer to this question: “how much do you like it?” The patients were given a score from 0 to 2 corresponding to 0—not at all, 1—moderately, and 2—very much.
Sample Processing and 16S rRNA Gene Targeted Sequencing
DNA extraction was performed using the NucliSENS® easyMAG system (bioMèrieux, Marcy l’Etoile, France). DNA samples were diluted 1:10 for subsequent PCR. A real-time PCR with EVAGREEN (EvaGreen® dye, Fisher Molecular Biology, Waltham, USA), the 27FYM degenerated primer (5′-AGR GTT YGA TYM TGG CTC AG - 3′) and the U534R primer (targeting the V1–V3 region of 500 bp length) was performed. A nested PCR was performed with the primers B338F_P1-Ion-adaptor (B338F 5′-ACTCCTACGGGAGGCAGC-3′) and U534R_A-Ion-adaptor_IonXpress-barcode (U534R 5′-ATTACCGCGGCTGCTGG-3′), in order to amplify the V3-region template of 16 S rRNA gene of 200 bases for sequencing analysis. Negative controls including no template were processed with clinical samples. The Kapa 2G HiFi Hotstart ready mix 2X (Kapa Biosystems, Massachusetts, USA) was used for the amplifications and 400 ng/μL BSA (Bovine Serum Albumin) were added to the reaction mix. Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA) was used for the quantification of the amount of dsDNA.
Template preparation was performed using the Ion PGM Hi-Q View kit on Ion OneTouch™ 2 System (Life Technologies, Grand Island, NY, USA) and sequenced using the Ion PGM Hi-Q View sequencing kit (Life Technologies, NY, USA) by the Ion PGM™ System technology.
Statistical Analysis
Data were collected anonymously using Microsoft Excel 2007 (Microsoft Excel 2007, Redmond, WA, USA) and analyzed using median (min–max) or mean ± standard deviation for continuous variables and number (n) and percentage (%) for categorical variables. Categorical data were compared using Fisher’s exact test. Differences between baseline and post-surgery values were analyzed with nonparametric test (Wilcoxon and Mann–Whitney). The level of significance was defined as p < 0.05. Changes in food preferences over time were analyzed with a one-way ANOVA test.
Chao1, PD whole tree, Shannon, observed species, and Simpson metrics were used to assess alpha diversity (within-sample diversity), while beta diversity (between sample diversity comparison) was assessed with weighted and unweighted UniFrac distance matrices.
Differences in microbial community composition were investigated using QIIME 1.9.1 by the analysis of similarity (ANOSIM, 999 permutations), ADONIS, Kruskal–Wallis, and parametric t test. The p value was corrected for False Discovery Rate (FDR). The observation_metadata_correlation.py (Fisher_z_transform) and the BIOENV (Spearman’s rank correlation) scripts were used to investigate the correlation between single and combination of clinical features to the microbial changes, respectively.
Results
From March 2016 to May 2017, a total of 100 stool samples were prospectively collected. We enrolled 25 patients in BS and 25 in NW group. Each BS patient provided a stool sample before surgery and three and six months thereafter; consequently, we collected 75 stool samples from BS and 25 from the NW patients. None of the BS patients had dropped out of the study. Table 1 shows the patients demographics. Smokers were 7/25 (28%) and 3/25 (12%) in the surgical and control group, respectively.
In NW patients, 20 (80%) were females and five (20%) were males. Patients had a median age of 45 years, with mean age of 44.2 years (± 9.3 years) within a range between 19 and 59 years. Mean baseline weight and BMI were 63.1 ± 10.5 kg and 22.7 ± 3.2 kg/m2, respectively.
In BS, nine (36%) patients underwent RYGB and 16 (64%) LSG; 21 (84%) were females and four (16%) were males. Patients had a median age of 45 years, with mean age of 45.5 years (± 8.8 years) within a range between 20 and 62 years. Mean baseline weight and BMI were 122.8 ± 16.4 kg and 44.5 ± 5.5 kg/m2, respectively. Mean baseline excess weight and excess BMI were 53.7 ± 15.1 kg and 19.5 ± 5.5 kg/m2, respectively. In patients who underwent RYGB, mean baseline weight and BMI were 130.72 ± 20.20 kg and 37.19 ± 6.89 kg/m2; mean baseline excess weight and excess BMI were 33.92 ± 19.2 kg and 19.59 ± 3.96 kg/m2. In patients who underwent LSG, mean baseline weight and BMI were 103.69 ± 20.26 kg and 37.22 ± 6.92 kg/m2; mean baseline excess weight and excess BMI were 33.97 ± 19.27 kg and 19.79 ± 4.09 kg/m2.
Metabolic syndrome (MS), hypertension, hypercholesterolemia, and T2DM were found in 13 (52%), 12 (48%), 17 (68%), and eight (32%) patients, respectively, while obesity without comorbidities was found in six patients (24%).
At T3, weight and BMI were 99.9 ± 13.2 kg and 36.3 ± 4.6 kg/m2, respectively. Mean excess weight and excess BMI were 30.9 ± 12.4 kg and 11.3 ± 4.6 kg/m2, respectively. In patients who underwent RYGB, mean baseline weight and BMI were 103.46 ± 20.05 kg and 36.98 ± 6.82 kg/m2; mean baseline excess weight and excess BMI were 33.43 ± 19.04 kg and 12.22 ± 6.77 kg/m2. In patients who underwent LSG, mean baseline weight and BMI were 103.24 ± 20.16 kg and 37.06 ± 6.93 kg/m2; mean baseline excess weight and excess BMI were 33.5 ± 19.26 kg and 12.30 ± 6.76 kg/m2.
At T6, weight and BMI were 89.3 ± 12.6 kg and 32.4 ± 4.6 kg/m2, respectively. Mean excess weight and excess BMI were 20.2 ± 12.4 kg and 7.4 ± 4.6 kg/m2, respectively. In patients who underwent RYGB, mean baseline weight and BMI were 103.3 ± 20.14 kg and 36.83 ± 6.95 kg/m2; mean baseline excess weight and excess BMI were 33.03 ± 19.37 kg and 12.14 ± 6.81 kg/m2. In patients who underwent LSG, mean baseline weight and BMI were 102.8 ± 20.36 kg and 36.9 ± 7.04 kg/m2; mean baseline excess weight and excess BMI were 33.05 ± 19.57 kg and 12.18 ± 6.83 kg/m2. Six months after surgery, mean weight reduction was 33.5 ± 9.7 kg and mean BMI reduction was 12.1 ± 3.2 kg/m2, compared to baseline. At this time, mean percentage of excess weight loss was 64.4 ± 17.2; 20 (80%) patients lost more than 50% EWL while five (20%) patients did not reach this target. Six months after surgery, a statistically significant reduction in weight was observed (p < 0.001).
Among patients with metabolic syndrome, a remission was observed in 7/13 (53.8%) patients; of the 12 patients with hypertension, remission was recorded in three (25%) cases, four (33.3%) patients were treated with a lower dosages of therapy, and in the remaining cases, medication was unchanged. In 12/17 (70.6%) cases, hypercholesterolemia was solved, while in 4/17 (23.5%) patients, there was an improvement and in 1/17 (5.8%) case, it worsened. Of the eight diabetic patients with HbA1c>6%, remission of T2DM was recorded in five cases (62.5%); one patient (12.5%) showed an improvement in glycemic control and in two cases, the HbA1c was unchanged (25%).
In LSG patients, by applying the one-way ANOVA test, three and six months after surgery, we noticed a statistically significant reduction in preference of carbohydrates (p < 0.01) compared to baseline; no differences in preferences were recorded between T3 and T6 (Fig. 1). Preferences for proteins, vegetables, and fats were unchanged during the study period. In RYGB patients, food preferences were unchanged during the study period.
The sequencing of fecal microbial community yielded 4,785,753 high-quality reads (Q > 20), with an average of 47857 reads/sample (range 6821–236,750). We identified 11,288 operation taxonomy units (OTUs) across all the samples. For further analysis, all the samples were rarefied to the lowest number of reads observed (6800).
Regarding alpha diversity, no significant difference was observed according to the five alpha diversity metrics used (Table 2).
Considering the beta diversity, based on the unweighted UniFrac beta diversity distance matrix, the percentage of microbial difference explained by the sample grouping, expressed by the R value (effect size), was 0.10 with a p value of 0.003. Based on the weighted UniFrac beta diversity distance matrix, the effect size was 0.08 and the p value was 0.017. These tests showed the role of surgery in microbiota variation. The impact of surgery on microbial variation was quantified using the Adonis statistical method. The R value (effect size) of the surgery on the unweighted UniFrac beta diversity distance matrix was 0.08 with a p value of 0.001. The R value of the surgery on the weighted UniFrac beta diversity distance matrix was 0.13 with a p value of 0.001. In order to identify the bacterial identities (phyla/species), a comparison between obese (T0) and NW patients fecal microbiome, both at the phylum and at the species level, was performed and no statistical difference was observed. Comparing the samples between NW patients and each time point of the two different surgery techniques (T3-SLG, T6-LSG and T3-RYGB, T6-RYGB), we found that RYGB introduced many statistical differences in the bacterial identities (Table 3).
At the phylum level, Bacteroidetes specifically contributed to the differences between T3-RYGB and T3-LSG. At T3-RYGB, the number of Bacteroidetes was lower than the value observed in the T3-LSG group. The variation in Firmicutes (mainly Clostridia), Fusobacteria, and Verrucomicrobia explained the difference between NW and T3/T6-RYGB groups. Firmicutes is slightly decreased in T3-RYGB compared to NW patients. The main differences among Firmicutes phylum probably stemmed from a variation in the relative abundance of Clostridia which, at T6-RYGB, were still lower than the value observed in NW. Fusobacteria and Verrucomicrobia were absent in NW, slightly represented in obese patients, while at T6-RYGB, their number increased. Gammaproteobacteria were statistically different among all the groups. Gammaproteobacteria readily and significantly increased three months after RYGB procedure and decreased at six months. Nonetheless, at T6-RYGB, the relative abundance of Gammaproteobacteria remained permanently higher than the T0-RYGB value (Fig. 2).
The fecal bacterial phyla from patients belonging to normal weight (NW) patients and to each time point (before surgery, after three and six months from surgery) of the Roux-en-Y-bypass (RYGB) and sleeve gastrectomy (LSG). The script of plot_taxa_summary.py of QIIME was executed after the rarefaction to 6800 sequences/samples
At the species level, at T3/T6-RYGB, Veillonella atypica, Veillonella dispar, Streptococcus australis, Streptococcus gordonii, Yokenella regensburgei, and Fusobacterium were significantly different compared to NW. Veillonella atypica and Veillonella dispar were detected at T3-RYGB and decreased at T6-RYGB. The same trend was observed for Streptococcus gordonii and Streptococcus australis. A different trend was observed for Yokenella regensburgei and Akkermansia muciniphila, which increased at T3-RYGB and remained steady at T6-RYGB. Among Fusobacteria, Fusobacterium varium was detected at T3-RYGB and slightly increased at T6-RYGB (Fig. 3).
Comparing the two different surgical technique, we found that at phylum level, RYGB induced statistical variation in Proteobacteria, Firmicutes, Fusobacteria, Verrucomicrobia, and Bacteroidetes, and at species level in Veillonella atypical, Veillonella dispar, Streptococcus gordonii, Streptococcus australis, Yokenella regensburgei, and Fusobacterium varium. Conversely, the microbial composition was not statistically affected by LSG.
Correlation Between Specific Changes in Gut Microbiome and Clinical Data
At univariate analysis, none of the bacteria was statistically associated with the clinical data considered. Using multivariate analysis and applying the BIOENV method, considering ρ ≥ 0.1, surgery, hypercholesterolemia, and fats were the most correlated variables to the unweighted and weighted distance matrices (Fig. 4).
The output of the BIOENV rank-correlation procedure. Correlations between clinical features and both weighted and unweighted UniFrac distance matrices are shown. These results were generated from the jackknifed PCoA results using the compare_categories. py script (metric BIOENV) of QIIME, the first five features with the highest Spearman’s rank correlation coefficient (ρ) were graphed. Abbreviations: F = fats, FV = fruits and vegetables, GH = glycated hemoglobin, HC = hypercholesterolemia, HT = hypertension, MS = metabolic syndrome, P = proteins, S = surgery
According to the unweighted and weighted distance matrix generated by the samples belonging to a specific surgery procedure, we evaluated the effect of clinical data on the microbial composition. Among patients who underwent LSG, the microbial composition was mainly correlated with eating habits and showed a beneficial effect on hypertension and metabolic syndrome. Among patients who underwent RYGB, the microbial composition was correlated with the %EWL induced by surgery and to an improvement of hypertension and hypercholesterolemia (Figs. 5 and 6).
The output of the BIOENV rank-correlation procedure on SLG samples. Correlations between clinical features and both weighted and unweighted UniFrac distance matrices generated from the samples belonging to controls and to patients who underwent SLG are shown. These results were generated from the jackknifed PCoA results using the compare_categories. py script (metric BIOENV) of QIIME, the first five features with the highest Spearman’s rank correlation coefficient (ρ) were graphed. Abbreviations: D = diabetes, F = fats, FV = fruits and vegetables, GH = glycated hemoglobin, HC = hypercholesterolemia, HT = hypertension, MS = metabolic syndrome, P = proteins
The output of the BIOENV rank-correlation procedure on RYGB samples. Correlations between clinical features and both weighted and unweighted UniFrac distance matrices generated from the samples belonging to NW patients and patients who underwent RYGB are shown. These results were generated from the jackknifed PCoA results using the compare_categories. py script (metric BIOENV) of QIIME, the first five features with the highest Spearman’s rank correlation coefficient (ρ) were graphed. Abbreviations: %EWL = % excess weight loss, F = fats, FV = fruits and vegetables, GH = glycated hemoglobin, HC = hypercholesterolemia, HT = hypertension, S = surgery
Discussion
Bariatric surgery is the most effective treatment for obesity, although the mechanistic explanation underpinning weight loss is still incomplete.
In our study, T6 data showed significant weight loss and metabolic improvement. At this time, 80% of patients lost more than 50% EWL and, from a metabolic point of view, good preliminary results were reached. More than 50% of patients suffering from metabolic syndrome had a remission and among patients with HbA1c>6%, remission of T2DM was recorded in 62.5% of cases after surgery.
Incorrect eating habits play an important role in the onset of obesity. The results from the food preferences questionnaire administered to obese patient before surgery showed an unbalanced diet with excess carbohydrates and fatty foods. After surgery, an important improvement in eating habits was recorded with a statistically significant reduction in preferences for carbohydrates and fats (p < 0.001). The weight loss induced by bariatric surgery and the modification in eating habits affected the microbiota composition. Several authors showed that the gut microbiome is involved in the process of weight loss after surgery rather than being a neat demarcation line between lean and obese subjects.12,13,14,15 Recent findings have assessed that some of the previously obesity-related microbiome alterations, such as the decrease in microbial diversity and the increase in Firmicutes/Bacteroidetes ratio, can be explained by the presence of obese-related comorbidities, such as type 2 diabetes.16 Our results confirm those previous results showing that the alpha diversity is not statistically different comparing obese with normal weight patients neither before nor after bariatric surgery. Thus, the Firmicutes/Bacteroidetes ratio is not an effective marker of eubiotic or dysbiotic condition.
After bariatric surgery, the changes in microbiota composition were more evident, as reported in literature.14, 17 In particular, RYGB induced more microbial differences and greater weight loss compared with LSG,18 as recently documented in animal models.19
At the moment, it is still unclear whether the bacterial modification induces beneficial or detrimental long-term effects on human health.
In our study, two potentially harmful bacteria related with colon disease were found after bariatric surgery: Yokenella regensburgei and Fusobacterium varium. Yokenella regensburgei (Proteobacteria) is an opportunistic human pathogen, often difficult to differentiate from Hafnia alvei with standard procedures.20 It was already identified in the colon bacterial community where it seems to elicit an inflammatory response. In our samples, a high level of Yokenella regensburgei appeared at T3-RYGB and remained high at T6. In one patient only, a persistent increased of the Fusobacterium varium after RYGB was found. In several studies, it was found to be associated with colorectal carcinoma.21 Although the presence of these two species has been linked with inflammatory or neoplastic colon disease, on the other hand, a protective effect of bariatric surgery on colon cancer onset was highlighted in several studies.22 Thus, the relationship between the clinical impact of these pathogens and host response is still unclear. In particular, our study with a limited cohort cannot provide definitive results on the role that these bacteria play on the host and on the potential onset of neoplastic disease.
Another aspect to consider is some non-resident microbial species selection due to the anatomical changes after RYGB. The gastro-jejunal anastomosis affects gastrointestinal pH and increases the amount of oxygen concentration in the lower tract of the gut. Increased gastrointestinal pH favors the proliferation of bacteria normally inhabiting the oral cavity; the presence of partially digested food in the distal bowel tract is responsible for the spread of specific bacteria which are able to metabolize the residual carbohydrates. Increased oxygen concentration that diffuses deeper into the luminal gut environment favors the presence of facultative anaerobes.5, 23, 24 Indeed, at T3-RYGB, the species Veillonella dispar/atypica and Streptococcus australis/gordonii, normally inhabiting the oral mucosae,25 spread. We noticed their decrease at T6-RYGB is probably due to an adaptive attitude of the bowel. Thus, the colonization of the gut niche by oral bacteria could be considered as a transient effect of the bariatric surgery.
Another important gut microbiota modification observed in our data involves Firmicutes and Proteobacteria. These phyla are able to convert choline in trimethylamine (TMA), which in turn is converted into trimethylamine-N-oxide (TMAO) in the liver. Strong evidences revealed that circulating TMAO is linked to atherogenesis and major adverse cardiovascular events with a not yet clarified mechanism.26 Thus, it would seem that the RYGB, affecting the proliferation of these bacteria, could increase the cardiovascular risks of the patients. In particular, our data showed that Firmicutes transitory increased at T3 and decreased at T6, Gammaproteobacteria permanently increased after RYGB. These data were reported previously in two studies and in both cases, TMAO increased; this is a topic for further investigation and analysis.27,28,29 On the other hand, it is commonly assumed that RYGB improves hypercholesterolemia, which in turn is strictly related to atherogenesis and adverse cardiovascular events and induces weight loss. Thus, RYGB, acting with different mechanisms, reduces the overall cardiovascular risk score of the patients and improves general health. Probably, the transitory increased of Firmicutes and the permanently high level of Proteobacteria are counteracted by the positive effect of RYGB on the lipid and glucose metabolism, blood pressure, and weight loss. A correlation between high blood TMAO levels and an increased colorectal cancer risk should also be noted.30, 31 Considering that the overall cancer risk has been found to be reduced after RYGB,22 the effect of the increased TMAO on the colorectal cancer development needs further investigation.
The positive effect of the RYGB could be explained even by the proliferation of some beneficial species detected only after surgery. In our samples, among Verrucomicrobia phylum, Akkermansia muciniphila was found only after RYGB. Akkermansia muciniphila is an expression of a healthy metabolic status because it is strongly associated with markers of lipid metabolism and negatively associated with inflammation in adipose tissue, circulating glucose, leptin, triglycerides, and insulin.32, 33
Conclusion
Weight loss and metabolic improvement are the results of complex mechanisms involving hormonal, anatomical, and gut microbiological modifications. The beneficial effect of bariatric surgery, in terms of weight loss and the improvement or remission of obesity-related comorbidity, and the specific bacteria properties are well-known. But a gap of knowledge about how these bacteria interact with each other and the interaction between the gut microbiota and the host still exist. The cross-talk between the microbiota and the host and the pathways linking all these factors are still unclear and are topics of study. Bariatric surgery and changes in food habits induce a significant gut microbiota alteration. In our study, the proliferation of potential pathogens and the onset of beneficial bacterial was observed. Data interpretation is the real challenge. Long follow-ups of obese patients who underwent bariatric surgery show the effectiveness of the surgical procedures; it is possible that surgery affects the microbiota composition selecting beneficial bacteria which outweigh any potential pathogen-induced risks for human health. Further studies are needed to discover the relationship between the gut microbiota and the host and the effects of the bacteria on human health. Understanding the mechanisms of action of some bacterial species that improve human health reducing the risk of fat accumulation or increasing its disposal could be the keystone in developing therapeutic strategies not only for obese adult patients but also as prevention of obesity among adolescents.
References
Kallus SJ, Brandt LJ. The intestinal microbiota and obesity. J Clin Gastroenterol 2012;46(1):16–24.
Rothe M, Blaut M. Evolution of the gut microbiota and the influence of diet. Benef Microbes 2013;4(1):31–7.
Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes 2012;3(3):186–202.
Hou YP, He QQ, Ouyang HM, Peng HS, Wang Q, Li J, Lv XF, Zheng YN, Li SC, Liu HL, Yin AH. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. Biomed Res Int 2017;2017:7585989
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab 2015;22(2):228–38
Michalsky M, Reichard K, Inge T; American Society for Metabolic and Bariatric Surgery. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis 2012; 8:1–7
Alqahtani AR, Antonisamy B, Alamri H, Elahmedi M, Zimmerman VA. Laparoscopic sleeve gastrectomy in 108 obese children and adolescents aged 5 to 21 years. Ann Surg 2012; 256:266–273
Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018;319(3):241–254.
Campisciano G, Palmisano S, Cason C, Giuricin M, Silvestri M, Guerra M, Macor D, De Manzini N, Crocé LS, Comar M. Gut microbiota characterisation in obese patients before and after bariatric surgery. Benef Microbes. 2018;9(3):367–373
Campisciano G, Cason C, Palmisano S, Giuricin M, Rizzardi A, Croce LS, De Manzini N, Comar M. Bariatric surgery drives major rearrangements of the intestinal microbiota including the biofilm composition. Front Biosci (Elite Ed). 2018;10:495–505.
Gastrointestinal Surgery for Severe Obesity. NIH Consensus Statement. 1991 Mar 25–27; 9:1–20
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023
Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol 2012; 7: 91–109
. Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9(10):590–8.
Anhê MS, Varin TV, Schertzer JD, Marette A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can J Diabetes 2017;41:439–447
Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets 2016; 16: 99–106
Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: a Systematic Review and Meta-analysis. Obes Surg 2017; 27:1345–1357
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg 2017; 27:917–925
Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, Yao Q. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. Obes Surg. 2017;27(2):295–302.
Stock I, Sherwood KJ, Wiedemann B. Antimicrobial susceptibility patterns, β-lactamases, and biochemical identification of Yokenella regensburgei strains. Diagnostic Microbiology and Infectious Disease 2004; 48: 5–15
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol Rep 2016; 35: 325–333
Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, Halverson RC, Simper SC, Hopkins PN, Hunt SC. Cancer incidence and mortality after gastric bypass surgery. Obesity 2009;17(4):796–802
Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, Brach T, Liang S, Feng Q, Jørgensen NB, Bojsen-Møller KN, Dirksen C, Burgdorf KS, Holst JJ, Madsbad S, Wang J, Pedersen O, Hansen T, Arumugam M. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med 2016;8:67
O’May GA, Reynolds N, Macfarlane GT. Effect of pH on an in vitro model of gastric microbiota in enteral nutrition patients. Appl Environ Microbiol 2005;71:4777–83
Zaura E, Brandt BW, Prodan A, Teixeira de Mattos MJ, Imangaliyev S, Kool J, Buijs MJ, Jagers FL, Hennequin-Hoenderdos NL, Slot DE, Nicu EA, Lagerweij MD, Janus MM, Fernandez-Gutierrez MM, Levin E, Krom BP, Brand HS, Veerman EC, Kleerebezem M, Loos BG, van der Weijden GA, Crielaard W, Keijser BJ. On the ecosystemic network of saliva in healthy young adults. The ISME Journal 2017; 11: 1218–1231
Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12
Narath SH, Mautner SI, Svehlikova E, Schultes B, Pieber TR, Sinner FM, Gander E, Libiseller G, Schimek MG, Sourij H, Magnes C. An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS One 2016;11(9): e0161425.
Trøseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, Lappegård KT. Major increase in microbiota-dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord. 2016;14(4):197–201
Bernd Schultes. Increased Trimethylamine-N-Oxide (TMAO) Levels After Roux-en Y Gastric Bypass Surgery—Should We Worry About It? Obes Surg 2017; 27:2170–2173
Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TY, Miller JW, Green R, Lane DS, Beresford SA, Caudill MA. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res 2014;74(24):7442–52
Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics. 2015;16(Suppl 7):S4.
Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 2015; 5:16643
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–436.
Acknowledgments
We would like to acknowledge Dr. Jill Woodcock for translating this article.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the present research and reviewed the entire manuscript. All authors have also approved the final version of the manuscript and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
S.P.: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
G.C.: Participated substantially in execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
M.S.: Participated substantially in conception, design, and execution of the study and in the analysis and critical revision of data.
M.G.: Participated substantially in conception, design, and execution of the study and in the analysis and critical revision of data.
M.G.: Participated substantially in the analysis and interpretation of data.
B.C.: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data.
M.C.: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
N.d.M.: Participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The abstract has been accepted for presentation in the Scientific Forum program at the American College of Surgeons Clinical Congress, October 21–25, 2018 Boston, MA, and published on Journal of American College of Surgeon 2018; 227 (number 4 supplement 1): S15-S16.
Rights and permissions
About this article
Cite this article
Palmisano, S., Campisciano, G., Silvestri, M. et al. Changes in Gut Microbiota Composition after Bariatric Surgery: a New Balance to Decode. J Gastrointest Surg 24, 1736–1746 (2020). https://doi.org/10.1007/s11605-019-04321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04321-x