Abstract
Background
Recent reports have shown that sleeve gastrectomy (SG) accelerates gastric emptying (GE), but the etiology remains unclear. This study aimed to investigate the factors affecting GE before and after SG.
Methods
We enrolled 35 normal weight healthy subjects and 23 obese patients receiving SG. The normal individuals and obese patients before and 3 months after SG received oatmeal-based scintigraphy to measure GE. Gastrointestinal symptoms and circulating levels of peptide YY (PYY) were also measured.
Results
There were no differences in the GE parameters, including simple half-time at 3 h and percentage of gastric retention at 0.5, 1, 2, and 3 h between healthy controls and pre-SG obese subjects. SG led to accelerated GE, more gastrointestinal symptoms, and increased fasting PYY levels postoperatively. Based on our previously established normal GE values, 18 (78.3%) obese patients with rapid postoperative GE had higher levels of preoperative fasting glucose and glycated hemoglobin, and homeostasis model assessment of the insulin resistance index than those with normal postoperative GE. Twelve (52.2%) obese patients had preoperative diabetes mellitus (DM), and only four (17.4%) remained diabetic after SG. The post-SG gastric retention at 0.5 and 1 h was lower in patients with preoperative DM than in those without preoperative DM. Neither severity of gastrointestinal symptoms nor fasting PYY levels were associated with postoperative GE alterations.
Conclusion
Most of the obese patients had accelerated GE after SG. A higher preoperative glycemic profile was associated with rapid post-SG GE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleeve gastrectomy (SG) is one of the most popular bariatric surgeries in the world. SG reduces body weight (BW) and also improves obesity-related disorders, including type 2 diabetes mellitus (T2DM), dyslipidemia, hypertension, and obstructive sleep apnea [1, 2]. However, SG also induces several postoperative discomforts, such as nausea, vomiting, loss of appetite, and gastroesophageal reflux diseases [3]. Postoperative gastrointestinal symptoms therefore influence the quality of life after operation [4].
SG ameliorates T2DM even before major weight loss [5, 6]. The findings suggest that SG reduces blood sugar through weight-independent neuroendocrine mechanisms [5, 7]. Recent studies have shown faster gastric emptying (GE) after SG [8,9,10]. The accelerated GE causes incompletely digested nutrients to rapidly enter the intestine, thus increasing postprandial levels of several intestinal hormones. The elevation of gut hormones affects appetite and satiety [11, 12] and also improves blood sugar and insulin resistance [13].
However, not all patients after SG have accelerated GE [8, 14, 15]. GE is an integrated process, involving complex systemic and local neurohormonal and neuromuscular responses. The detailed mechanism of the change in GE after SG remains unclear. In this study, we aimed to elucidate the possible mechanisms of altered gastric motility in obesity and after SG by investigating the clinical characteristics, symptoms, gut hormones, and GE of normal weight subjects and obese patients before and after SG.
Subjects and Methods
Twenty-three obese patients, 6 men and 17 women with a mean age of 37.3 ± 11.0 years who underwent laparoscopic SG, were prospectively enrolled in this study. Among them, 12 patients had T2DM preoperatively. We further enrolled 35 healthy individuals with normal BW and BMI between 18.5 and 24 as the control group. Informed consent was obtained from all individual participants included in the study. This study was approved by the institutional review board.
Blood samples were collected with at least 8 h of overnight fasting in all the healthy individuals and obese subjects before and 3 months after surgery. Fasting glucose, insulin, and glycated hemoglobin (HbA1c) were assessed with a standard clinical automatic analyzer. The remaining serum was stored in aliquots at −80 °C. Body mass index (BMI) and waist circumference (WC) were also measured and calculated. The diagnosis of T2DM was based on the criteria of the American Diabetes Association [16]. The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as follows: fasting serum insulin concentration (mIU/mL) × fasting plasma glucose concentration (mg/dL) / 22.5. Human enzyme-linked immunosorbent assay kits were used to measure the serum levels of total peptide YY (PYY) (EZHPYYT66K, Millipore, Burlington, MA, USA) according to the manufacturer’s instructions.
All healthy individuals and obese subjects before and 3 months after SG received oatmeal-based scintigraphy to measure their functions of GE [17]. In addition, each participant was screened with the Gastroparesis Cardinal Symptom Index (GCSI) to evaluate the severity of gastrointestinal symptoms [18].
Surgical Techniques
All procedures were performed laparoscopically via a 5-port technique. The greater omentum was dissected using the 5-mm laparoscopic LigaSure™ (Covidien-Medtronic, Minneapolis, MN, USA) from 4 cm proximal to the pyloric ring to the angle of His. Sleeve calibration was performed by a 36-French bougie inserted along the lesser curvature. Then, the stomach was transected with sequential firings of linear green, gold, and blue 60-mm staplers (Echelon Flex™, Ethicon Endo-Surgery, Ethicon US, LLC) starting approximately 4 cm proximal to the pylorus and ending approximately 2 cm distal to the left of the esophagus. The staple-line of the remnant gastric tube was oversewn with 3–0 V-Loc (Covidien-Medtronic, Minneapolis, MN, USA) to prevent leakage and hemorrhage.
Gastric Emptying Study
Gastric scintigraphy examination was performed based on our previously established test meal and scintigraphic protocol [17]. In brief, after an overnight fast of at least 12 h, one instant oatmeal packet (43 g, Quaker Oats Co., Chicago, IL, USA) was mixed with 200 mL hot water and 1 mCi (37 MBq) of 99mTc diethylenetriaminepentacetic acid. Participants ingested the test meal within 10 min in a sitting position and then lay supine on the imaging table. All images were collected by a gamma-camera from 30° left anterior oblique positions, with participants in the supine position. Dynamic images with 30 s per frame were acquired for 90 min at first, and then static images were taken for 30 s at 2 and 3 h. The participants were allowed to change positions between each imaging acquisition.
The image counts at the regions of interest in the stomach were reviewed and corrected for time decay. Data points were connected by straight lines, and time activity curves were then obtained. A simple half-time (T1/2) of GE, the time of emptying 50% of the original gastric contents, was calculated according to the dynamic and static images within 3 h. The percentages of gastric retention were measured at 0.5, 1, 2, and 3 h after oatmeal ingestion. The results of GE were categorized as normal, delayed, or rapid in reference to the normal values established in our previous study [17].
Gastroparesis Cardinal Symptom Index
All subjects completed the questionnaire of GCSI to assess their symptoms during the preceding 2 weeks before the GE study. GCSI is a reliable tool to evaluate the severity of gastrointestinal symptoms in patients with T2DM and gastroparesis [17, 18]. The GCSI questionnaire contains nine individual gastroparesis symptoms and can be further categorized into three subgroups: nausea/vomiting (three items), post-prandial fullness/early satiety (four items), and bloating (two items). Each symptom severity was measured using a 0–5 scale, where no symptoms = 0, very mild = 1, mild = 2, moderate = 3, severe = 4, and very severe = 5. A total score is the sum of nine individual symptoms scores, with a range of 0–45.
Statistical Analysis
The quantitative data were summarized as the means ± standard deviation (SD), and the categorical variables were summarized as numbers and percentages. Student’s t tests were applied for differences in continuously quantitative results. Paired t tests were used to compare the preoperative and postoperative quantitative variables. For comparing categorical data, the χ2 test was applied. The Fisher’s exact test was used when the expected frequency was less than 5. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics software version 19.0 (IBM, Armonk, NY, USA).
Results
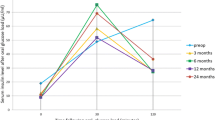
The demographic and laboratory data of the normal weight individuals and the obese patients before and 3 months after SG are shown in Table 1. There were no differences in age, gender, and circulating levels of PYY between the normal weight and pre-SG obese subjects. The baseline values of BW, BMI, WC, and glycemic profiles were significantly higher in the obese group than in the normal weight group. Twelve (52.2%) obese patients had T2DM at baseline, and only 4 (17.4%) remained diabetic 3 months after SG. The values of BW, BMI, WC, and glycemic profiles were significantly lower after the operation. In contrast, the serum levels of fasting PYY were higher after SG. The patients had more gastrointestinal symptoms and higher total GCSI scores after SG.
The GE scintigraphy results are presented in Table 2. The GE parameters of normal weight subjects, including T1/2 values measured based on 3 h of static images and gastric retention at 0.5, 1, 2, and 3 h, were not significantly different from those of the pre-SG obese patients. However, the preoperative obese patients displayed a wider range of parameters than the normal weight subjects. Three obese patients (13%) had delayed GE, and another three (13%) had rapid GE according to our normal values [17]. The values of T1/2 and gastric retention at 0.5, 1, and 2 h were decreased 3 months after SG. Eighteen patients (78.3%) had rapid emptying, while only 5 patients (21.7%) had normal GE after the operation. Representative scintigraphic images and time-activity curves of normal preoperative and rapid postoperative GE are shown in Fig. 1.
Representative scintigraphic images and time-activity curves obtained from an obese and diabetic subject with normal preoperative and rapid postoperative gastric emptying. a Preoperative and b postoperative static image series obtained hourly from 0 to 3 h. The region of interest (ROI) is also shown. c Preoperative and d postoperative time-activity curve obtained from 0 to 3 h. The T1/2 (3 h) was 52.14 and 35.22 min, respectively
Table 3 summarizes the pre- and postoperative characteristics and preoperative GE parameters of the obese patients with normal and rapid GE 3 months after SG. The patients with rapid postoperative GE had higher levels of fasting glucose, HbA1c, and HOM-IR than those with normal postoperative GE. There were no statistically significant differences in age, gender, other preoperative demographic and laboratory data listed in Table 3 or in the total GCSI scores between the two groups. There were also no statistically significant differences in the postoperative values listed in Table 3 and preoperative GE parameters between the patients with normal and rapid postoperative GE.
Before SG, the obese patients with T2DM had similar age, gender, BMI, WC, and serum levels of insulin and total GCSI scores as those without T2DM (Table 4). The T2DM patients had higher levels of fasting glucose, HbA1c, HOMA-IR, and circulating levels of PYY than non-T2DM patients. There was also no difference in preoperative GE parameters between patients with and without preoperative T2DM. Compared with preoperative non-T2DM patients, those with preoperative T2DM retained higher fasting glucose and HbA1c levels and had similar BW, BMI, WC, insulin, HOMA-IR, PYY, and total GCSI scores. Interestingly, the postoperative gastric retention at 0.5 and 1 h of patients with pre-SG T2DM was lower than in patients without pre-SG T2DM. Eleven (91.7%) patients with preoperative T2DM had rapid postoperative GE, while only seven (63.6%) with preoperative T2DM had rapid postoperative GE.
Discussions
In this prospective research comparing normal weight subjects with obese patients before and after SG, our data demonstrated SG reduced BW, improved T2DM, and increased circulating levels of PYY 3 months after the operation. SG also induced more postoperative gastrointestinal symptoms and increased the total GCSI scores. There were no differences in the oatmeal-based GE parameters between normal weight and pre-SG obese subjects. Most of the obese patients had accelerated GE 3 months after SG. The patients with rapid postoperative GE had higher preoperative blood sugar profiles than those with normal postoperative GE. Post-SG GE parameters, including gastric retention percentages at 0.5 and 1 h, were lower in patients with preoperative T2DM than in those without T2DM.
Gastric motility is a complex and well-integrated process. Many factors, such as the central nervous system, local proximal and distal gastric neuronal control, the nature and energy density of the meal, and circulating neurohormonal substances, influence the rate of GE. Other than ingesting and transporting food, GE plays an important role in the regulation of satiety and appetite [19]. The role of GE in obesity is still not well understood. Alterations in gastric motility have been observed in obese subjects, and most studies have reported that GE of solids is faster in obesity [20, 21]. However, the GE of liquid in obese patients appears to not be significantly different from that of normal controls [20, 22]. The current oatmeal-based study—using a semisolid food—disclosed no differences in the GE parameters between normal weight and pre-SG obese subjects. Our results are similar to the findings of Buchholz et al. with semisolid food [23]. The discrepancy of GE in obesity could be due to the different types and energy densities of the food, the inclusion criteria of patients, and the measurement techniques.
Most published reports, except for one study, have shown that GE is accelerated after SG [10, 24]. In the study from Bernstine et al., GE with a semisolid meal was not changed 3 months after SG. Bernstine et al. created the sleeve by using a larger (48-Fr) bougie at a distance of 6 cm from the pylorus [15]. Therefore, the sleeve was larger than that in other studies and in our studies. Baumann et al. examined gastric motility by time-resolved magnetic resonance imaging in five obese women and observed that the stomach was functionally divided into a sleeve without propulsive peristalsis and an accelerated antrum after SG [25]. In humans, the gastric pacemaker is found at the greater curvature of the mid- to upper corpus [26, 27]. SG removes the fundus and most of the body along the greater curvature, including the normal gastric pacemaker, and disrupts the normal pacing activity in the stomach. Berry et al. evaluated the patients before and after SG by laparoscopic high-resolution electric mapping and disclosed that patients after LSG developed a distal unifocal ectopic pacemaker with retrograde propagation or bioelectrical quiescence [28]. The disrupted gastric pacing is one of the possible mechanisms of the alteration of gastric motility after SG.
Vives et al. evaluated the effect of partial antrum resection on GE by starting the resection in SG 3 or 8 cm from the pylorus. The results showed that GE was significantly increased in both groups but was greater in the 3-cm group. Furthermore, the distance did not influence the GE of preoperative diabetic patients. In pre-SG non-diabetic patients, the speed in non-diabetic patients was significantly higher for the 3-cm group [29]. The findings suggested that antral preservation and preoperative DM status might affect post-SG GE.
The most interesting finding of the present study is that the patients with preoperative T2DM had lower GE parameters after SG than those without preoperative T2DM. Abnormal GE, either delayed or rapid, has been observed in some diabetes patients [30,31,32,33]. Patients with T2DM had neuropathy, myopathy, and alteration of gut hormones, such as glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic peptide, PYY, and ghrelin [30, 32, 34]. GLP-1, PYY, leptin, and ghrelin have been shown to affect gastric motility [35,36,37,38,39]. In addition, SG also reduces systemic levels of several obesity-associated gut hormones, such as PYY, ghrelin, leptin and GLP-1 [13], and changes neural activities [40, 41]. Although our data showed no differences in preoperative GE between patients with and without preoperative T2DM, differences in postoperative GE between the two groups might have been due to the diabetic and SG-associated disruptions of the harmony controlling GE, including through the changes of gut hormones or neural activities.
PYY has been shown to affect gastric motility. Intravenous infusion of PYY1-36 and PYY3-36 both inhibit GE in humans [38, 39]. However, reports of fasting and postprandial PYY levels in obese and lean people are inconsistent [42,43,44]. Studies from Karamanakos and Dimitriadis both disclosed that postprandial PYY levels are increased after SG. On the other hand, Karamanakos reported increased fasting PYY levels, but Dimitriadis disclosed similar fasting PYY levels after SG [11, 43]. Fasting and postprandial circulating PYY might have different physiopathological effects. The present study revealed that the fasting PYY levels were not different between lean and obese subjects, were decreased after SG and were higher in obese individuals with T2DM than those with non-T2DM. Fasting PYY levels were not associated with GE before or after SG. Since gastric motility is a complex process in humans, PYY might only play a small role in GE of obese people after SG.
The current data showed more gastrointestinal symptoms and higher GCSI scores in obese patients after SG. There were no differences in the symptoms between patients with and without preoperative T2DM either before or after SG, and the symptoms were not associated with GE parameters. Our results are similar to Burgerhart’s report [45]. Recent studies have suggested that GCSI is a reliable tool to evaluate the severity of gastrointestinal symptoms in patients with T2DM and gastroparesis [17, 18]. However, most of the patients after SG had rapid GE rather than gastroparesis. Postoperative gastrointestinal discomfort may be due to the reduced gastric volume and distensibility, increased intragastric pressure, alterations of gastrointestinal motility, gut hormones, and central and visceral neural activities [46,47,48]. Therefore, the association of GE and gastrointestinal symptoms is disrupted after the operation and is different between the patients with T2DM gastroparesis and those after SG. Our data suggest that GSCI is not a good indicator of GE in obese patients before and after SG.
The strength of the current study is that we compared the comprehensive clinical characteristics, hormonal factors, and GE parameters of normal weight individuals and obese patients before and after SG. Moreover, we classified the results of GE according to our established normal values in the Chinese healthy controls [17]. It is very important to separate normal from disease status. Nevertheless, our study may have several limitations. The numbers of patients were relatively small, with few patients in each subgroup, which might have reduced the power of statistical analyses. Furthermore, we only focused on semisolid meals, without data for liquids or solids. Some patients keep semisolid food for several months after SG. Therefore, we assume the oatmeal-based gastric scintigraphy could represent the daily GE 3 months after SG. Lastly, the patients were still on the rapid weight loss process 3 months after the operation. Therefore, it is difficult to separate the pathophysiologic changes of SG from those from weight reduction. Further prospective studies with more patients and longer postoperative time points would be helpful to clarify the mechanisms among obesity, GE, and SG.
Conclusions
There were no differences in the oatmeal-based GE parameters between normal weight and pre-SG obese subjects. Most of the obese patients had accelerated GE 3 months after SG. A higher preoperative glycemic profile was associated with rapid postoperative GE.
References
Gagner M. The future of sleeve gastrectomy. Eur Endocrinol. 2016;12(1):37–8.
Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis. 2017;13(2):170–80.
El Labban S, Safadi B, Olabi A. The effect of Roux-en-Y gastric bypass and sleeve gastrectomy surgery on dietary intake, food preferences, and gastrointestinal symptoms in post-surgical morbidly obese Lebanese subjects: a cross-sectional pilot study. Obes Surg. 2015;25(12):2393–9.
Andersen JR. Quality of life following laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(1):76–8.
Vidal J, Ibarzabal A, Nicolau J, et al. Short-term effects of sleeve gastrectomy on type 2 diabetes mellitus in severely obese subjects. Obes Surg. 2007;17(8):1069–74.
Rizzello M, Abbatini F, Casella G, et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20(1):50–5.
Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35(6 Pt 2):518–23.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21.
Shah S, Shah P, Todkar J, et al. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6(2):152–7.
Sioka E, Tzovaras G, Perivoliotis K, et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract. 2018;2018:4135813.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7.
Sista F, Abruzzese V, Clementi M, et al. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis. 2017;13(1):7–14.
Vigneshwaran B, Wahal A, Aggarwal S, et al. Impact of sleeve gastrectomy on type 2 diabetes mellitus, gastric emptying time, glucagon-like peptide 1 (GLP-1), ghrelin and leptin in non-morbidly obese subjects with BMI 30-35.0 kg/m(2): a prospective study. Obes Surg. 2016;26(12):2817–23.
Pomerri F, Foletto M, Allegro G, et al. Laparoscopic sleeve gastrectomy–radiological assessment of fundus size and sleeve voiding. Obes Surg. 2011;21(7):858–63.
Bernstine H, Tzioni-Yehoshua R, Groshar D, et al. Gastric emptying is not affected by sleeve gastrectomy–scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obes Surg. 2009;19(3):293–8.
American Diabetes A.2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–27.
Tseng PH, Wu YW, Lee YC, et al. Normal values and symptom correlation of a simplified oatmeal-based gastric emptying study in the Chinese population. J Gastroenterol Hepatol. 2014;29(11):1873–82.
Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis cardinal symptom index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13(4):833–44.
Xing J, Chen JD. Alterations of gastrointestinal motility in obesity. Obes Res. 2004;12(11):1723–32.
Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–46 e4.
Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131(6):1717–24.
Seimon RV, Brennan IM, Russo A, et al. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am J Physiol Endocrinol Metab. 2013;304(3):E294–300.
Buchholz V, Berkenstadt H, Goitein D, et al. Gastric emptying is not prolonged in obese patients. Surg Obes Relat Dis. 2013;9(5):714–7.
Melissas J, Leventi A, Klinaki I, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258(6):976–82.
Baumann T, Kuesters S, Grueneberger J, et al. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy–preliminary results. Obes Surg. 2011;21(1):95–101.
Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133(1):29–33.
O'Grady G, Du P, Cheng LK, et al. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G585–92.
Berry R, Cheng LK, Du P, et al. Patterns of abnormal gastric pacemaking after sleeve gastrectomy defined by laparoscopic high-resolution electrical mapping. Obes Surg. 2017;27(8):1929–37.
Vives M, Molina A, Danus M, et al. Analysis of gastric physiology after laparoscopic sleeve gastrectomy (LSG) with or without antral preservation in relation to metabolic response: a randomised study. Obes Surg. 2017;27(11):2836–44.
Phillips LK, Rayner CK, Jones KL, et al. Measurement of gastric emptying in diabetes. J Diabetes Complicat. 2014;28(6):894–903.
Chang J, Rayner CK, Jones KL, et al. Diabetic gastroparesis and its impact on glycemia. Endocrinol Metab Clin N Am. 2010;39(4):745–62.
Marathe CS, Rayner CK, Jones KL, et al. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396–405.
Ariga H, Imai K, Chen C, et al. Does ghrelin explain accelerated gastric emptying in the early stages of diabetes mellitus? Am J Phys Regul Integr Comp Phys. 2008;294(6):R1807–12.
Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–13.
Falken Y, Hellstrom PM, Sanger GJ, et al. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol Motil. 2010;22(6):e192–200.
Halim MA, Degerblad M, Sundbom M, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. 2018;103(2):575–85.
Kiely JM, Noh JH, Graewin SJ, et al. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124(1):98–103.
Savage AP, Adrian TE, Carolan G, et al. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28(2):166–70.
Witte AB, Gryback P, Holst JJ, et al. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul Pept. 2009;158(1–3):57–62.
Ballsmider LA, Vaughn AC, David M, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985.
Liu L, Ji G, Li G, et al. Structural changes in brain regions involved in executive-control and self-referential processing after sleeve gastrectomy in obese patients. Brain Imaging Behav. 2018; https://doi.org/10.1007/s11682-018-9904-2.
Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8.
Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257(4):647–54.
Cahill F, Ji Y, Wadden D, et al. The association of serum total peptide YY (PYY) with obesity and body fat measures in the CODING study. PLoS One. 2014;9(4):e95235.
Burgerhart JS, van Rutte PW, Edelbroek MA, et al. Association between postprandial symptoms and gastric emptying after sleeve gastrectomy. Obes Surg. 2015;25(2):209–14.
Farre R, Vanheel H, Vanuytsel T, et al. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. 2013;145(3):566–73.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg. 2008;18(9):1083–8.
Mion F, Tolone S, Garros A, et al. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–56.
Funding
This project was supported in part by grants from the National Taiwan University Hospital (NTUH 105-M3266 and NTUH 107-S3776) and E-Da Hospital – National Taiwan University Hospital Joint Research Program (NTUH 106-EDN19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All the contributing authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yang, PJ., Cheng, MF., Yang, WS. et al. A Higher Preoperative Glycemic Profile Is Associated with Rapid Gastric Emptying After Sleeve Gastrectomy for Obese Subjects. OBES SURG 29, 569–578 (2019). https://doi.org/10.1007/s11695-018-3558-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3558-4